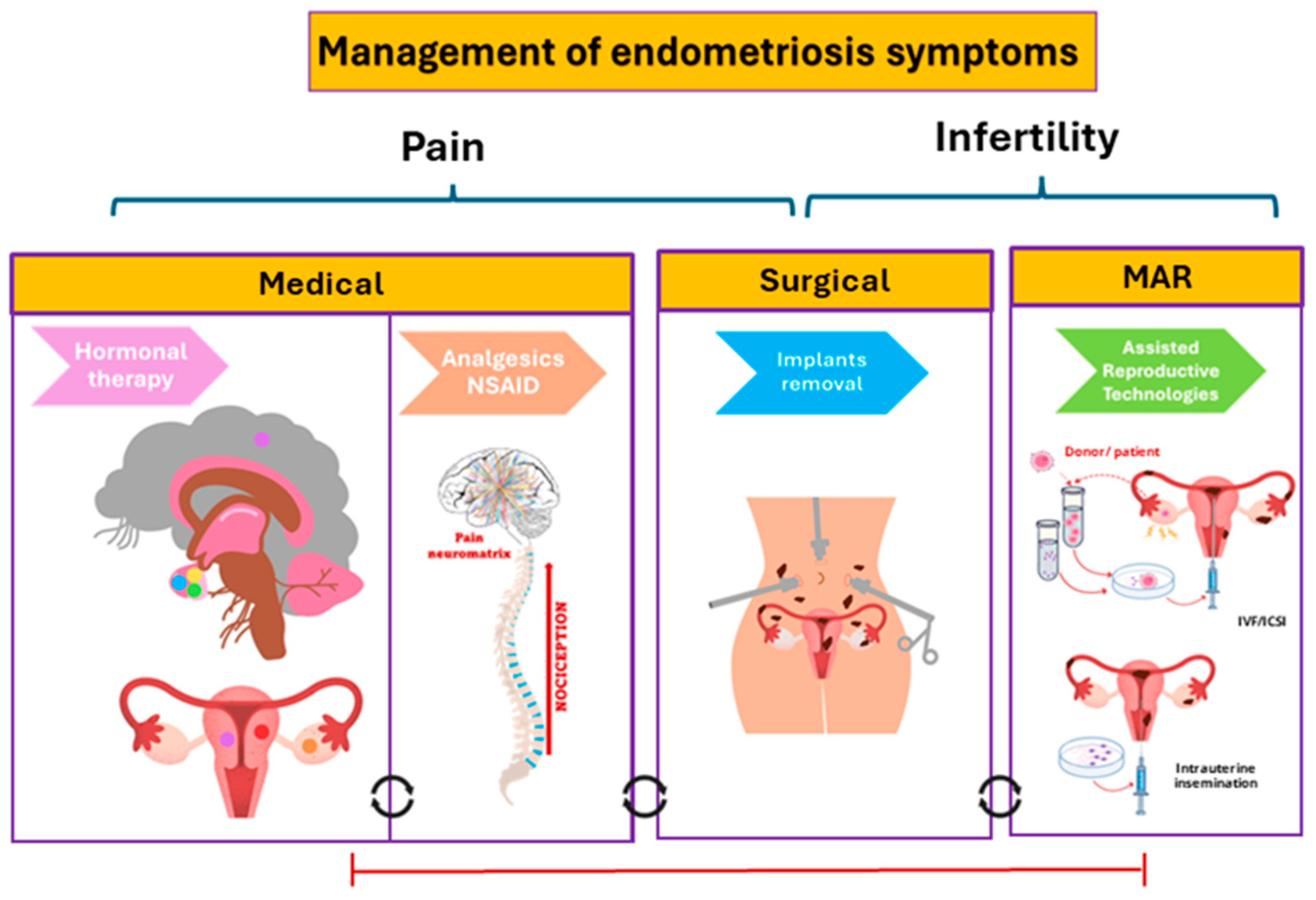
New Potential Pharmacological Options for Endometriosis Associated Pain
Abstract
1. Introduction
2. Immunotherapy
| Drug Name and Structure | Target | Model | Effects | Reference |
|---|---|---|---|---|
| IL-33 Ab | IL-33 | Women (n = 26) In vitro ESC Mice In vivo 50 μg + Erastin 300 μL 10 days | ↓Development EM ↓Ferropoptosis tolerance eESC ↑M1 macrophage polarization | [13] |
| IL-8 Ab AMY109 | IL-8 | Monkeys In vivo 2 or 10 mg/Kg/4 weeks subcutaneous 6 months | ↓Nodular lesions, ↓volume adhesions, neutrophils migration inhibition | [14] |
| IL-6R Ab Tocilizumab | IL-6R | Rats In vivo 8 mg/Kg/2 tw Intraperitoneally 4 weeks | Suppress volume endometriosic lesions | [15] |
| CTLA-4 Ab | CTLA-4 | Mice In vitro peritoneal fluid 0.1 mg/mL 14 days | Proliferation and invasion of ectopic endometrial cells suppression | [17] |
| CD47 Ab siRNA | CD47 | Women (n = 23) (10 EAOC, 13 eESC) In vitro Mice In vivo Intraperitoneal 0, 0.5, 2 and 4 µg/mL 12 h | Inhibition of growth and cellular migration, ↑apoptosis | [19] |
| CD20 Ab Rituximab | CD20 | Rats In vivo 10 mg/Kg Intraperitoneal 14 days | ↓Endometriotic implant size | [20] |
| EP4 antagonist | PGE2R | Women (n = 35) In vitro Normal uterus tissues, ovarian EM tissues, adenomyosis tissues and peritoneal fluid | ↓cAMP levels, ↓expression growth factor | [21] |
| Lipoic acid | cAMP, protein kinase A signaling/ NF-kB | Women In vitro ESC 1–10 mM | ↓NALP-3 ↓IL-1β, IL-18 ↓ICAM-1, ↓activity MMP2, 9 ↓invasion | [23] |
| TGR5 agonist INT-777 | TGR5 | Women In vitro ESC 5 µM/24 h 10 µM/48 h | Agonist, ↓proinflammatory cytokines, ↓adhesion molecules, Inhibits NF-κB | [24] |
| Sunitinib | p-VEGFR-PI3K-AKT-YBX1-Snail signalling pathway | Women (n = 3) In vitro eESC 0.1 µM 24 h | Receptors inhibitor, Kinases inhibition, ↓tumor migration, ↓tumor invasion | [25] |
| Gal3C | Galectin3 | Mice In vivo 50 µg/day Intraperitoneal 15 days | ↓Implantation, ↓size of lesions, ↓expression of VEGF and VEGFR-2, ↓inflammation, ↓COX-2, ↓TGFβ1 | [26] |
| BSA-GOx-NP | Neutrophils | Mice In vivo 5 μL 10 mg/mL Intraperitoneal 14 days | ↑Neutrophils | [27] |
3. Natural Compounds with Antioxidant and Anti-Inflammatory Activity
| Drug Name and Structure | Target | Model/Dose | Effects | Reference |
|---|---|---|---|---|
| Gui Zhi Fu Ling Wan (GZFLW) | PI3K/Akt pathway | Mice In vivo 0.54–1.08 g/Kg oral | ↓Uterine contractions ↓NO, ↓PGF2α, ↓Ca2+ | [29] |
| Rats In vivo 1.89 g/mL | Inhibits inflammation, ↓volume of lesions, ↓sensitivity to pain | [30] | ||
| Women (n = 90) In vivo 2 caps Gynoclear© /3 td oral 12 months | ↓Dyspareunia, ↓fatigue, ↑QoL | [31] | ||
| Sulphoraphane | PI3K/Akt pathway Nrf2/ARE pathway | Rats In vivo 5–30 mg/Kg intragastrical 3 weeks | ↓Endometriotic foci, ↓adhesion score, ↓ IL-6, IL-10, TNF-α, IFN-γ, and VEGF | [32] |
| Rats In vivo 5,15,30,60 mg/Kg/day intraperitoneal 28 days | Inhibit ectopic endometrial tissue growth, ↓pain | [33] | ||
| Danefukang extrac (DEFK) | Not described | Women (n = 174) In vivo 15 g/2td/15 days Mifepristone 12.5 mg–6.25 mg/day/10 days 3 months | ↑QoL, ↓depression, ↓anxiety, ↓TNF-α, ↓IL-6, ↓Ca125 | [34] |
| Fisetin | NLRP3 | Rats In vivo 40 mg/kg/day oral 14 days | ↓Endometriotic implantation, ↓mast cell infiltration, ↓fibrosis, ↓histological alterations, ↓neutrophil infiltration, ↓cytokine release, ↓oxidative stress, ↓nitrotyrosine, ↓poly ADP ribose expressions, ↑apoptosis in endometrial lesions | [35] |
| Quince seed Mucilage | MAPK pathway Caspase-3 | Rats In vivo 2 cc/day vaginal +50 mg/kg/day Hesperidin 21 days | ↓Inflammation, ↓apoptotic process, ↓mitochondrial damage, restoring the uterine mucosa | [36] |
| Ferulic acid | Not described | Women (n = 27) In vitro ESC and peritoneal macrophages. 500 µM Rats In vivo 4 g/Kg once oral 2 h | ↓Inflammatory cytokines, ↓angiogenic factor (VEGF) | [38] |
| Resveratrol | MAPK pathway | Women (n = 22) In vitro ESC 100 µmol/L | ↓MCP-1, ↓IL-6, ↓IL-8, ↓RANTES | [39] |
| Baicalein | MAPK pathway Nrf2/ARE pathway | Women (n = 8) In vitro 20 µM ectopic endometrium, peritoneal and cyst fluid | Ferroptosis inhibition Restauration phagocytosis | [42] |
| 1,25 (OH)2 D3 | VDR | Women (n = 0) ESC and WEC In vitro | ↓TNF-α, ↓inflammatory cytokines | [43] |
| Ascorbic acid | Not described | Women (n = 60) In vivo 1000 mg + 800 IU vitamin E/day oral 8 weeks | ↓malondialdehyde, ↓ROS, ↓dysmenorrhea, ↓dyspareunia, ↓CPP | [44] |
| Omega fatty acids | Not described | Women (n = 30) In vivo 1000 mg/2 td 8 weeks | ↓PP, ↑QoL | [46] |
4. Repurposed Drugs
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, K.; Heinemann, K.; Imthurn, B.; Marions, L.; Moehner, S.; Gerlinger, C.; Serrani, M.; Faustmann, T. Real world data on symptomology and diagnostic approaches of 27,840 women living with endometriosis. Sci. Rep. 2021, 14, 20404. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Horne, A.W.; Missmer, S.A. Time for global health policy and research leaders to prioritize endometriosis. Nat. Commun. 2023, 14, 8028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gong, T.T.; Wang, H.Y.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national endometriosis trends from 1990 to 2017. Ann. N. Y. Acad. Sci. 2021, 1484, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Y.; Shams-Beyranvand, M.; Khateri, S.; Gharahjeh, S.; Tehrani, S.; Varse, F.; Tiyuri, A.; Najmi, Z. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 2021, 154, 446–454. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Z.; Fei, F.; Zhou, S. An overview and comprehensive analysis of interdisciplinary clinical research in endometriosis based on trial registry. iScience 2024, 27, 109298. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Oskotsky, T.T.; Falako, S.; Opoku-Anane, J.; Sirota, M. Endometriosis in the era of precision medicine and impact on sexual and reproductive health across the lifespan and in diverse populations. FASEB J. 2023, 37, e23130. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- França, P.R.C.; Lontra, A.C.P.; Fernandes, P.D. Endometriosis: A Disease with Few Direct Treatment Options. Molecules 2022, 23, 4034. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Womens Health 2021, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Donati, A.; Ottolini, F.; Frassineti, A.; Fiorini, J.; Nebuloni, V.; Frattaruolo, M.P.; Roberto, A.; Mosconi, P.; Somigliana, E. A stepped-care approach to symptomatic endometriosis management: A participatory research initiative. Fertil. Steril. 2018, 109, 1086–1096. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The Role of Peritoneal Macrophages in Endometriosis. Int. J. Mol. Sci. 2021, 6, 10792. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, Z.; Jiang, J.; Feng, X.; Liu, J.; Zhang, Z.; Wang, H.; Wang, N.; Gou, Y.; Li, Z.; et al. Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis. Cell Death Dis. 2023, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto-Kakiuchi, A.; Sato, I.; Nakano, K.; Ohmori, H.; Kayukawa, Y.; Tanimura, H.; Yamamoto, S.; Sakamoto, Y.; Nakamura, G.; Maeda, A.; et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci. Transl. Med. 2023, 22, 5858. [Google Scholar] [CrossRef] [PubMed]
- El-Zayadi, A.A.; Mohamed, S.A.; Arafa, M.; Mohammed, S.M.; Zayed, A.; Abdelhafez, M.S.; Badawy, A.M. Anti-IL-6 receptor monoclonal antibody as a new treatment of endometriosis. Immunol. Res. 2020, 68, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Abramiuk, M.; Bębnowska, D.; Hrynkiewicz, R.; Polak, P.N.G.; Kotarski, J.; Roliński, J.; Grywalska, E. CLTA-4 Expression is Associated with the Maintenance of Chronic Inflammation in Endometriosis and Infertility. Cells 2021, 25, 487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, P.; Liu, L.; Ma, G.; Ma, J.; Liu, X.; Liu, Y.; Lin, W.; Zhu, Y. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model. Eur. J. Pharm. Sci. 2017, 1, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; He, H.; Wu, Y.H.; Zou, L.J.; She, X.L.; Xia, X.M.; Wu, X.Q. Eutopic endometrium from patients with endometriosis modulates the expression of CD36 and SIRP-α in peritoneal macrophages. J. Obstet. Gynaecol. Res. 2019, 45, 1045–1057. [Google Scholar] [CrossRef]
- Li, J.; Yan, S.; Li, Q.; Huang, Y.; Ji, M.; Jiao, X.; Yuan, M.; Wang, G. Macrophage-associated immune checkpoint CD47 blocking ameliorates endometriosis. Mol. Hum. Reprod. 2022, 29, 10. [Google Scholar] [CrossRef]
- Dogan, A.C.; Dogan, M.; Togrul, C.; Ozkan, N.T. The effects of Rituximab on experimental endometriosis model in rats. J. Reprod. Immunol. 2023, 156, 103814. [Google Scholar] [CrossRef]
- Makabe, T.; Koga, K.; Nagabukuro, H.; Asada, M.; Satake, E.; Taguchi, A.; Takeuchi, A.; Miyashita, M.; Harada, M.; Hirata, T.; et al. Use of selective PGE2 receptor antagonists on human endometriotic stromal cells and peritoneal macrophages. Mol. Hum. Reprod. 2021, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.E.; Spain, R.I.; Kim, E.; Salinthone, S. Lipoic acid modulates inflammatory responses of monocytes and monocyte-derived macrophages from healthy and relapsing-remitting multiple sclerosis patients. Immunol. Cell Biol. 2021, 99, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Di Nicuolo, F.; Castellani, R.; De Cicco Nardone, A.; Barbaro, G.; Paciullo, C.; Pontecorvi, A.; Scambia, G.; Di Simone, N. Alpha-Lipoic Acid Plays a Role in Endometriosis: New Evidence on Inflammasome-Mediated Interleukin Production, Cellular Adhesion and Invasion. Molecules 2021, 26, 288. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Tang, N.; Wang, J.; Zhang, Y.; Chang, J.; Liu, Z.; Liu, H. TGR5 agonist INT-777 mitigates inflammatory response in human endometriotic stromal cells: A therapeutic implication for endometriosis. Int. Immunopharmacol. 2019, 71, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Tong, Y.; Chen, Y.; Chen, Y. Sunitinib Reduced the Migration of Ectopic Endometrial Cells via p-VEGFR-PI3K-AKT-YBX1-Snail Signaling Pathway. Anal. Cell. Pathol. 2022, 30, 6042518. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.M.; Machado, D.E.; Perini, J.A.; Alessandra-Perini, J.; Meireles da Costa, N.O.; Wiecikowski, A.F.D.R.O.; Cabral, K.M.D.S.; Takiya, C.M.; Carvalho, R.S.; Nasciutti, L.E. Galectin-3 plays an important role in endometriosis development and is a target to endometriosis treatment. Mol. Cell. Endocrinol. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Xue, N.; Zhu, X.; Li, F.; Dai, Q.; Qing, X.; Chen, D.; Liu, X.; Wei, Z.; et al. Highly specific neutrophil-mediated delivery of albumin nanoparticles to ectopic lesion for endometriosis therapy. J. Nanobiotechnol. 2023, 8, 81. [Google Scholar] [CrossRef]
- Nagayasu, M.; Imanaka, S.; Kimura, M.; Maruyama, S.; Kobayashi, H. Nonhormonal Treatment for Endometriosis Focusing on Redox Imbalance. Gynecol. Obstet. Investig. 2021, 86, 1–12. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Zong, S.; Wang, Z.; Zhou, J.; Xu, Z.; Ding, G.; Xiao, W.; Kou, J. Traditional Chinese medicine Guizhi Fuling capsule used for therapy of dysmenorrhea via attenuating uterus contraction. J. Ethnopharmacol. 2016, 15, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ji, W.; Lu, M.; Wang, Z.; Jia, X.; Wang, D.; Cao, P.; Hu, C.; Sun, X.; Wang, Z. Systemic pharmacological verification of Guizhi Fuling decoction in treating endometriosis-associated pain. J. Ethnopharmacol. 2022, 28, 115540. [Google Scholar] [CrossRef]
- Armour, M.; Al-Dabbas, M.A.; Ee, C.; Smith, C.A.; Ussher, J.; Arentz, S.; Lawson, K.; Abbott, J. The effectiveness of a modified Gui Zhi Fu Ling Wan formulation (Gynoclear™) for the treatment of endometriosis: A study protocol for a placebo-controlled, double-blind, randomised controlled trial. Trials 2021, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hong, Y.; Lv, Y. Sulforaphane Attenuates Endometriosis in Rat Models Through Inhibiting PI3K/Akt Signaling Pathway. Dose Response 2019, 11, 1559325819855538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Lu, X.; Meng, J.; Qin, X.; Jiang, J. Anti-nociceptive and anti-inflammatory effects of sulforaphane on sciatic endometriosis in a rat model. Neurosci. Lett. 2020, 1, 134858. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.C.; Zhou, X.F.; Hou, C.M.; Li, W.P. Effect of danefukang on symptoms and biomarkers in women with endometriosis. Taiwan J. Obstet. Gynecol. 2019, 58, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Marino, Y.; Fusco, R.; Siracusa, R.; Cordaro, M.; D’Amico, R.; Macrì, F.; Raffone, E.; Impellizzeri, D.; Cuzzocrea, S.; et al. Fisetin, a Natural Polyphenol, Ameliorates Endometriosis Modulating Mast Cells Derived NLRP-3 Inflammasome Pathway and Oxidative Stress. Int. J. Mol. Sci. 2023, 7, 5076. [Google Scholar] [CrossRef] [PubMed]
- Ermiş, I.S.; Deveci, E.; Aşır, F. Effects of Quince Gel and Hesperidin Mixture on Experimental Endometriosis. Molecules 2023, 8, 5945. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Barradas, T.; Araujo Cardoso, S.; de Castro Grimaldi, P.; Lohan-Codeço, M.; Escorsim Machado, D.; Medina de Mattos, R.; Eurico Nasciutti, L.; Palumbo, A., Jr. Development, characterization and evidence of anti-endometriotic activity of Phytocannabinoid-Rich nanoemulsions. Int. J. Pharm. 2023, 25, 123049. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Koga, K.; Tokita, Y.; Matsumoto, T.; Satake, E.; Taguchi, A.; Makabe, T.; Miyashita, M.; Takamura, M.; Harada, M.; et al. The effects of tokishakuyakusan, a traditional Japanese medicine (kampo), ferulic acid and paeoniflorin, on human endometriotic stromal cells and peritoneal macrophages. J. Reprod. Immunol. 2020, 139, 103104. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A.A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 10, 110. [Google Scholar] [CrossRef]
- Ni, C.; Li, D. Ferroptosis and oxidative stress in endometriosis: A systematic review of the literature. Medicine 2024, 15, 37421. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.H.; Li, S.Q.; Ke, J.Y.; Wang, Y.; Zhao, M.Z.; Li, J.; Li, M.Q.; Zhu, Z.L. Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Curr. Issues Mol. Biol. 2022, 7, 6189–6204. [Google Scholar] [CrossRef]
- Ghanavatinejad, A.; Rashidi, N.; Mirahmadian, M.; Rezania, S.; Mosalaei, M.; Ghasemi, J.; Zarnani, A.H. Vitamin D3 Controls TLR4- and TLR2-Mediated Inflammatory Responses of Endometrial Cells. Gynecol. Obstet. Investig. 2021, 86, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.E.X.G.; Medeiros, F.D.C.; Rocha, H.A.L.; Silva, K.S.D. Effects of omega-6/3 and omega-9/6 nutraceuticals on pain and fertility in peritoneal endometriosis in rats. Acta Cir. Bras. 2019, 34, e201900405. [Google Scholar] [CrossRef]
- Abokhrais, I.M.; Denison, F.C.; Whitaker, L.H.R.; Saunders, P.T.K.; Doust, A.; Williams, L.J.; Horne, A.W. A two-arm parallel double-blind randomised controlled pilot trial of the efficacy of Omega-3 polyunsaturated fatty acids for the treatment of women with endometriosis-associated pain (PurFECT1). PLoS ONE 2020, 15, e0227695, Erratum in PLoS ONE 2020, 15, e0230055. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Muto, H.; Masumoto, H.; Ogawa, K.; Koshiba, A.; Mori, T.; Itoh, K.; Teramukai, S.; Matsuda, K.; et al. Levofloxacin or gonadotropin releasing hormone agonist treatment decreases intrauterine microbial colonization in human endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 103–116. [Google Scholar] [CrossRef]
- Machado, D.E.; Saggioro, E.M.; Sales, S.F., Jr.; Alessandra-Perini, J.; de Campos Gomes Diniz, L.; Coelho, W.S.; Zancan, P.; Perini, J.A. Clotrimazole is effective, safe and tolerable for the treatment of endometriosis and functions by downregulating inducible nitric oxide synthase and modulating oxidative stress biomarkers. Mol. Cell. Endocrinol. 2023, 15, 111883. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, C.; Held, K.; Ciprietti, M.; De Clercq, K.; Kerselaers, S.; Marchand, A.; Chaltin, P.; Voets, T.; Vriens, J. Loratadine, an antihistaminic drug, suppresses the proliferation of endometrial stromal cells by inhibition of TRPV2. Eur. J. Pharmacol. 2022, 5, 175086. [Google Scholar] [CrossRef]
- Akyol, A.; Kavak, E.; Akyol, H.; Pala, Ş.; Gürsu, F. The Non-Ergot Derived Dopamine Agonist Quinagolide as an Anti-Endometriotic Agent. Gynecol. Obstet. Investig. 2017, 82, 527–532. [Google Scholar] [CrossRef]
- Iampietro, C.; Brossa, A.; Canosa, S.; Tritta, S.; Croston, G.E.; Reinheimer, T.M.; Bonelli, F.; Carosso, A.R.; Gennarelli, G.; Cosma, S.; et al. Quinagolide Treatment Reduces Invasive and Angiogenic Properties of Endometrial Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 4, 1775. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.; Suslova, E.; Tkachenko, N.; Molotkov, A.; Kogan, I. Dopamine agonists as genital endometriosis target therapy. Gynecol. Endocrinol. 2020, 36, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Scaramuzzino, S.; Viscardi, M.F.; Viggiani, V.; Piccioni, M.G.; Cacciamani, L.; Merlino, L.; Angeloni, A.; Muzii, L.; Porpora, M.G. Efficacy of N-Acetylcysteine on Endometriosis-Related Pain, Size Reduction of Ovarian Endometriomas, and Fertility Outcomes. Int. J. Environ. Res. Public Health 2023, 7, 4686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, M.; Winuthayanon, S.; MacLean, J.A., 2nd; Hayashi, K. Niclosamide targets the dynamic progression of macrophages for the resolution of endometriosis in a mouse model. Commun. Biol. 2022, 11, 1225. [Google Scholar] [CrossRef]
- Murphy, A.R.; Campo, H.; Kim, J.J. Strategies for modelling endometrial diseases. Nat. Rev. Endocrinol. 2022, 18, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Song, H.; Shi, G. Anti-TNF-α treatment for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2013, 3, CD008088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xiao, Y.; Ruan, J.; Tian, Q.; Cheng, Q.; Chang, K.; Yi, X. Distinct subtypes of endometriosis identified based on stromal-immune microenvironment and gene expression: Implications for hormone therapy. Front. Immunol. 2023, 14, 1133672. [Google Scholar] [CrossRef]
- Zhou, J.; Chern, B.S.M.; Barton-Smith, P.; Phoon, J.W.L.; Tan, T.Y.; Viardot-Foucault, V.; Ku, C.W.; Tan, H.H.; Chan, J.K.Y.; Lee, Y.H. Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes. Int. J. Mol. Sci. 2020, 21, 3515. [Google Scholar] [CrossRef]

| Drug Name and Structure | Target | Model/Dose | Effects | Reference |
|---|---|---|---|---|
Levofloxacin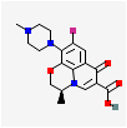 | Bacterial topoisomerase IV and DNA gyrase | Women (n = 53) In vivo 500 mg/24 post-surgery/oral or +1.88 mg GnRHa/month intramuscular 3 months | Inhibition bacterial topoisomerase IV and DNA gyrase, ↓tissue inflammation, ↓cell proliferation, ↓angiogenesis endometriotic, ↓lesions pain | [47] |
Clotrimazole | CYP53 enzyme | Rats In vivo 200 mg/kg oral 15 days | CYP53 enzyme inhibition, ↓inflammation, ↓endometric lesions, ↓distribution of iNOS, ↑antioxidant system | [48] |
Loratadine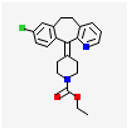 | TRPV2 | Women In vitro Cell culture ESC | Antagonist, ↓cell proliferation/ migration, ↓inflammation | [49] |
Quinagolide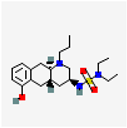 | Dopamine D2 receptor | Rats In vivo 200 µg/Kg/day oral 4 weeks | ↓ Endometriotic implants, ↓IL-6, ↓VEGF | [50] |
| Women (n = 10) In vitro ESC 24 h | Agonist, ↓lesion size, ↓invasion and differentiation, ↓AKT signaling pathway | [51] | ||
Cabergoline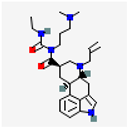 | Dopamine D2 receptor | Women (n = 227) In vivo 0.25–0.5 mg/twice/week oral cabergoline + hormone therapy 6 months | ↓Pain syndrome | [52] |
N-acetyl-L-cysteine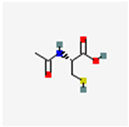 | COX2 pathway | Women (n = 120) In vivo 600 mg/day/3 consecutive days/ week oral 3 months | ↓Dysmenorrhea, ↓dyspareunia, ↓CPP, ↓endometrioma size, ↓serum Ca125 levels, improve fertility | [53] |
Niclosamide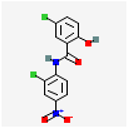 | Wnt/β-catenin signal pathway Macrophage activity | Mice In vivo 200 mg/Kg/day/3 weeks oral 6 weeks | Reverse macrophage transcriptomic changes in EM Rescue LPM and B cell communication after EM induction | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Izquierdo, L.; Marín-Sánchez, P.; García-Peñarrubia, P.; Martínez-Esparza, M. New Potential Pharmacological Options for Endometriosis Associated Pain. Int. J. Mol. Sci. 2024, 25, 7068. https://doi.org/10.3390/ijms25137068
García-Izquierdo L, Marín-Sánchez P, García-Peñarrubia P, Martínez-Esparza M. New Potential Pharmacological Options for Endometriosis Associated Pain. International Journal of Molecular Sciences. 2024; 25(13):7068. https://doi.org/10.3390/ijms25137068
Chicago/Turabian StyleGarcía-Izquierdo, Laura, Pilar Marín-Sánchez, Pilar García-Peñarrubia, and María Martínez-Esparza. 2024. "New Potential Pharmacological Options for Endometriosis Associated Pain" International Journal of Molecular Sciences 25, no. 13: 7068. https://doi.org/10.3390/ijms25137068
APA StyleGarcía-Izquierdo, L., Marín-Sánchez, P., García-Peñarrubia, P., & Martínez-Esparza, M. (2024). New Potential Pharmacological Options for Endometriosis Associated Pain. International Journal of Molecular Sciences, 25(13), 7068. https://doi.org/10.3390/ijms25137068








