The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature
Abstract
:1. Introduction
2. Methods
2.1. Information Sources and Search Strategies
2.2. Eligibility Criteria
- Type of study: case-control studies, cohort studies, or case series.
- Period of publication: no restriction.
- Language: only English-language studies were included.
- Data search: January 2003–December 2023.
- Participants: women with ovarian cancer who underwent NACT with available samples before and after chemotherapy, as well as immune cell infiltration evaluation.
- Comparators: (i) sTILs and ieTILs before and after NACT; (ii) PD-1, PD-L1 expression and other immune cell populations before and after NACT.
- Outcomes: prognostic impact of sTILs and ieTILs, PD-1/PD-L1, and other immune cell populations before and after NACT in terms of PFS and OS.
2.3. Study Selection and Data Extraction
3. Results and Discussion
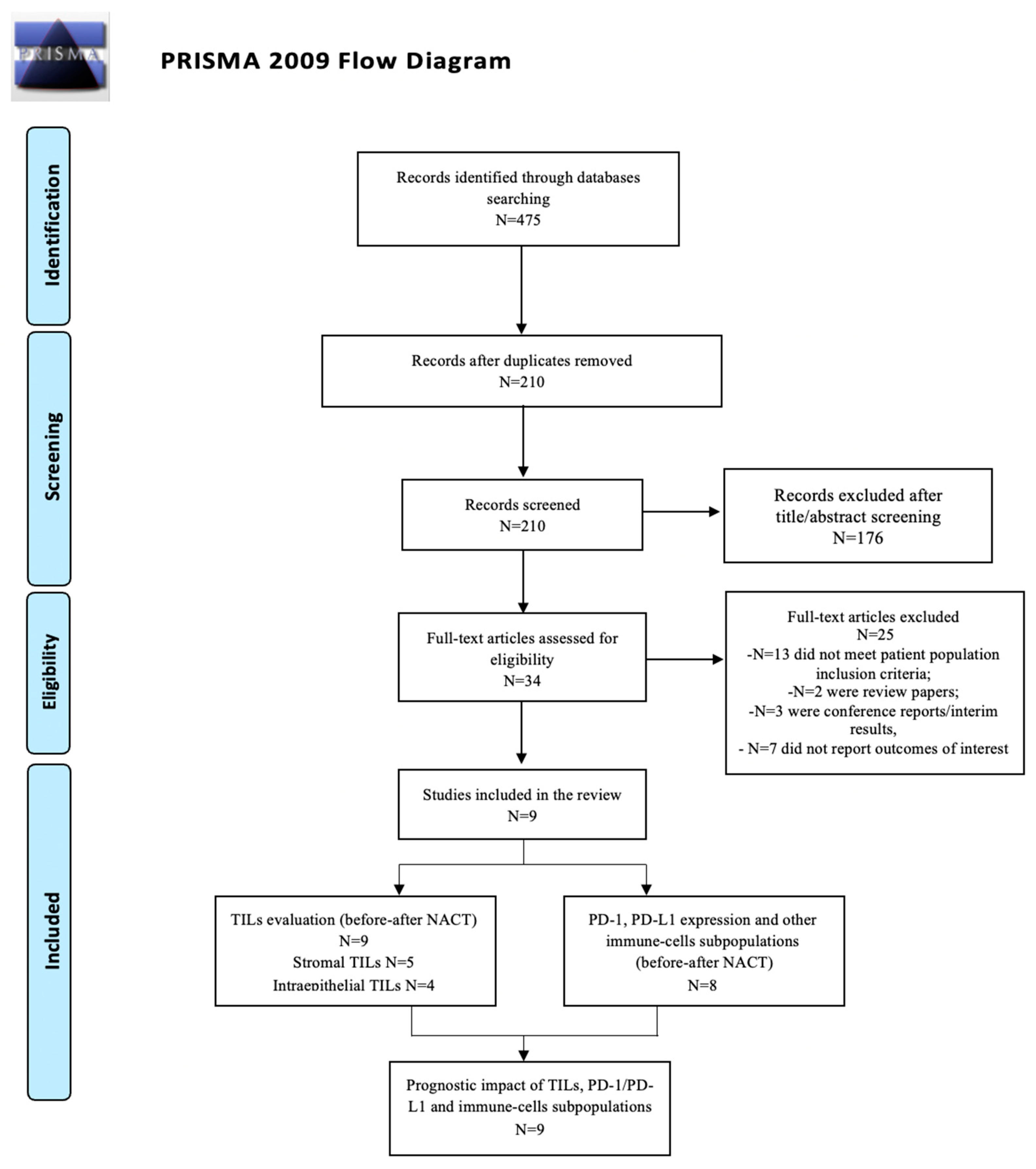
3.1. Study Selection
Study and Patient Characteristics Overview
3.2. Tumor-Infiltrating Lymphocytes (TILs)
3.2.1. Stromal TILs (sTILs)
3.2.2. Intraepithelial TILs (ieTILs)
3.3. PD-1, PD-L1 Expression and Other Cytokine Expression
3.4. Discussion
Combination of Immune Checkpoint Inhibitors and Chemotherapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.Y.; Kim, S.; Kim, Y.T.; Lim, M.C.; Lee, B.; Jung, K.W.; Kim, J.W.; Park, S.Y.; Won, Y.J. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer 2018, 18, 601. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomized, controlled, noninferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Ledermann, J.A. EGCEa: Updated treatment recommendations for newly diagnosed epithelial ovarian carcinoma from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. SOLO1 Investigators. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Menzies, A.M.; Scolyer, R.A. Neoadjuvant Checkpoint Immunotherapy and Melanoma: The Time Is Now. J. Clin. Oncol. 2023, 41, 3236–3248. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.P. Relapsed or refractory classical Hodgkin lymphoma: Which immunotherapy, and when? Lancet Oncol. 2021, 22, 417–419. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597, Erratum in J. Clin. Oncol. 2022, 40, 1265. [Google Scholar] [CrossRef]
- Ward Grados, D.F.; Ahmadi, H.; Griffith, T.S.; Warlick, C.A. Immunotherapy for Bladder Cancer: Latest Advances and Ongoing Clinical Trials. Immunol. Investig. 2022, 51, 2226–2251. [Google Scholar] [CrossRef]
- Odunsi, K.; Jungbluth, A.A.; Stockert, E.; Qian, F.; Gnjatic, S.; Tammela, J.; Intengan, M.; Beck, A.; Keitz, B.; Santiago, D.; et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003, 63, 6076–6083. [Google Scholar] [PubMed]
- Tomšová, M.; Melichar, B.; Sedláková, I.; Šteiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.; Hasenburg, A.; Riener, M.-O.; Ju, U.; Wang, C.; Shen, Y.; Orlowska-Volk, M.; Fisch, P.; Wang, Z.; Gitsch, G.; et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: Relevance of clonal selection of T lymphocytes. Br. J. Cancer 2009, 101, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.C.; Komdeur, F.L.; Workel, H.H.; Klip, H.G.; Plat, A.; Kooi, N.M.; Wisman, G.B.A.; Mourits, M.J.; Arts, H.J.; Oonk, M.H.; et al. Treatment regimen, surgical outcome, and T-cell differentiation influence prognostic benefit of tumor-infiltrating lymphocytes in high-grade serous ovarian cancer. Clin. Cancer Res. 2016, 22, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Dangaj Laniti, D.; Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. Nat. Rev. Cancer. 2022, 22, 640–656. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Pénault-Llorca, F.; et al. The evaluation of tumorinfiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group. 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Pölcher, M.; Braun, M.; Friedrichs, N.; Rudlowski, C.; Bercht, E.; Fimmers, R.; Sauerwald, A.; Keyver-Paik, M.-D.; Kübler, K.; Büttner, R.; et al. Foxp3+ cell infiltration and granzyme B+/Foxp3+ cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol. Immunother. 2010, 59, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Montfort, A.; Pearce, O.M.; Topping, J.; Chakravarty, P.; Everitt, G.L.; Clear, A.; McDermott, J.R.; Ennis, D.; Dowe, T.; et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin. Cancer Res. 2016, 22, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.S.; Sanii, S.; Kroeger, D.R.; Milne, K.; Talhouk, A.; Chiu, D.S.; Rahimi, K.; Shaw, P.A.; Clarke, B.A.; Nelson, B.H. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin. Cancer Res. 2017, 23, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, S.J.L.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C.; et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PDL1) expression in epithelial ovarian cancer (OC). Ann. Oncol. 2017, 28, 651–657. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Lee, Y.J.; Kim, S.H.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Molberg, K.; Strickland, A.L.; Castrillon, D.H.; Carrick, K.; Jiang, Q.; Niu, S.; Rivera-Colon, G.; Gwin, K.; Hinson, S.; et al. PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Different Types of Tubo-ovarian Carcinoma and Their Prognostic Value in High-grade Serous Carcinoma. Am. J. Surg. Pathol. 2020, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.; Genestie, C.; Blanc-Durand, F.; Gouy, S.; Dunant, A.; Maulard, A.; Drusch, F.; Cheaib, B.; Michels, J.; Bentivegna, E.; et al. Neoadjuvant chemotherapy alters the balance of effector to suppressor immune cells in advanced ovarian cancer. Cancer Immunol. Immunother. 2021, 70, 519–531. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Genestie, C.; Galende, E.Y.; Gouy, S.; Morice, P.; Pautier, P.; Maulard, A.; Mesnage, S.; Le Formal, A.; Brizais, C.; et al. Distribution of novel immune-checkpoint targets in ovarian cancer tumor microenvironment: A dynamic landscape. Gynecol. Oncol. 2021, 160, 279–284. [Google Scholar] [CrossRef]
- Lee, Y.J.; Woo, H.Y.; Kim, Y.N.; Park, J.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T.; Park, E.; Joung, J.G.; et al. Dynamics of the Tumor Immune Microenvironment during Neoadjuvant Chemotherapy of High-Grade Serous Ovarian Cancer. Cancers 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Palova-Jelinkova, L.; Klapp, V.; Holicek, P.; Lanickova, T.; Kasikova, L.; Drozenova, J.; Cibula, D.; Álvarez-Abril, B.; García-Martínez, E.; et al. Immunological control of ovarian carcinoma by chemotherapy and targeted anticancer agents. Trends Cancer. 2022, 8, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.; Ray-Coquard, I.L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021, 22, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, A.; Cybulska, P.; Mager, K.L.; Koplev, S.; Cast, O.; Couturier, D.L.; Memon, D.; Selenica, P.; Nikolovski, I.; Mazaheri, Y.; et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020, 52, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.; Luxenberg, D.; Ling, V.; Wang, I.; Marquette, K.; Lowe, D.; Khan, N.; Veldman, G.; Jacobs, K.A.; Valge-Archer, V.E.; et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokinedriven proliferation: Attenuation of ICOS, IL-4, and IL-21, But Not CD28, IL-7, and IL-15 responses. J. Immunol. 2003, 170, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumorassociated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Zhang, L.; Gajewski, T.F.; Kline, J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009, 114, 1545–1552. [Google Scholar] [CrossRef]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 2021, 39, 1842–1855. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.E.; Pujade-Lauraine, E.; Oaknin, A.; Belin, L.; Leitner, K.; Cibula, D.; Denys, H.; Rosengarten, O.; Rodrigues, M.; de Gregorio, N.; et al. Atezolizumab Combined With Bevacizumab and Platinum-Based Therapy for Platinum-Sensitive Ovarian Cancer: Placebo-Controlled Randomized Phase III ATALANTE/ENGOT-ov29 Trial. J. Clin. Oncol. 2023, 41, 4768–4778. [Google Scholar] [CrossRef] [PubMed]
- Tdlmr, A.L.; Lortholary, A.; Asselain, B.; Alexandre, J.; Floquet, A.; Savoye, A.M.; Chardin, L.; Delanoy, N.; Gavoille, C.; You, B.; et al. Phase Ib INEOV neoadjuvant trial of the anti-PDL1, durvalumab (D) +/− anti-CTLA4 tremelimumab (T) with platinum chemotherapy for patients (pts) with unresectable ovarian cancer (OC): A GINECO study. Ann. Oncol. 2021, 32, S731. [Google Scholar]
| Study Design | Number of Patients | Number of Patients with Paired Pre- and Post-NACT | Histotype | Site of Origin | TILs Assessment | Type of CHT | Number of Cycles | FU | |
|---|---|---|---|---|---|---|---|---|---|
| Polcher et al. 2010 [23] | Prospective | 93 | 30 | Serous/serous-papillary High-grade endometrioid (EC) | Intraperitoneal biopsies | Hematoxylin staining (HS) Immunohistochemistry (IHC) | Platinum-/ taxane-based NAC | 3 cycles | 24 months |
| Bohm et al. 2016 [24] | Prospective | 60 | 54 | HGSC | Omental biopsies and plasma samples | IHC, flow cytometry (FC), electrochemiluminescence assays, and RNA sequencing (RS) | Taxane/platinum combinations | 3 or >4 | NA |
| Lo et al. 2016 [25] | Retrospective | 150 | 26 (in 18 patients, paired anatomic extra-pelvic biopsies were available) | HGSC | Omentum, pelvic site, uterus, colon, abdomen, cul-de-sac (paired only extra-pelvic biopsies) | IHC (Aperio, Pannoramic MIDI, Vectra) | Platinum-/ taxane-based NAC | A mean of 4 cycles | NA |
| Mesnage et al. 2016 [26] | Retrospective | 150 | 83 | High-grade serous (HGSC), EC, clear cell (CC), low-grade serous (LGSC), mucinous carcinoma (MC) | NA | Hematoxylin and eosin staining (HES) IHC | Carboplatin and paclitaxel | Average 4 cycles | 52 months |
| Kim et al. 2018 [27] | Retrospective | 266 | 76 | HGSC | Omental biopsies | HES, IHC (Ventana XT automated stainer) | Taxane/platinum combinations | A median of 3 cycles (range, 2–6 cycles) | Median: 30 months |
| Chen et al. 2020 [28] | Retrospective | 189 | 15 | HGSC, CC, EC, MC | NA | HES, IHC (Aperio ScanScope AT Turbo scanner) | Platinum-based treatment | N/A | 37 months |
| Leary et al. 2020 [29] | Retrospective | 150 | 53 | HGSC, EC, CC, other high-grade, LGSC, MC | NA | HES, IHC | Platinum and paclitaxel | Mean number of cycles: 4 | Median: 80 months |
| Félix Blanc-Durand et al. 2020 [30] | Retrospective | 98 | 63 | HGSC, LGSC, mixed, poorly differentiated, EC, CC, MC | NA | HES, IHC (Ventana XT automated stainer) | Platinum-based treatment | N/A | N/A |
| Lee et al. 2022 [31] | Retrospective | 147 | 147 | HGSC | NA | HES, IHC (Ventana XT automated stainer), whole-transcriptome sequencing (WTS) | Platinum-based chemotherapy | Median of 3 cycles (range, 3–4 cycles) | Median: 28.2 months |
| Stromal | Prognostic Association Pre-NACT | Prognostic Association Post-NACT | Study |
|---|---|---|---|
| TILs | PFS increase with ↑ | PFS increase with ↑ | Mesnage et al. [26] |
| None | None | Kim et al. [27] | |
|
PFS increase with
↑
OS increase with ↑ | None | Lee et al. [31] | |
| CD8+ | None | None | Leary et al. [29] |
|
PFS increase with
↑
OS increase with ↑ | None | Lee et al. [31] | |
| CD3+ | None | None | Leary et al. [29] |
| CD4+ | None | None | Leary et al. [29] |
| None | None | Bohm et al. [24] | |
| Foxp3+ | None |
PFS increase with
↑
OS none | Leary et al. [29] |
|
PFS increase with
↑
OS increase with ↑ | None | Lee et al. [31] |
| Tumor | Prognostic Association Pre-NACT | Prognostic Association Post-NACT | Study |
|---|---|---|---|
| TILs | None | None | Mesnage et al. [26] |
| CD8+ | None | None | Polcher et al. [23] |
| None | None | Lo et al. [25] | |
| None | None | Leary et al. [29] | |
| CD3+ | None | None | Lo et al. [25] |
| None | None | Leary et al. [29] | |
| CD4+ | None | None | Polcher et al. [23] |
| None | None | Leary et al. [29] | |
| Foxp3+ | None | PFS increase with ↓ OS increase with ↓ | Polcher et al. [23] |
| None | None | Lo et al. [25] | |
| None | None | Leary et al. [29] | |
| CD20+ | None | PFS none OS increase with ↑ | Lo et al. [25] |
| CD8+/Foxp3+ | None | None | Polcher et al. [23] |
| CD8+/CD4+ | None | PFS none OS increase with ↑ | Polcher et al. [23] |
| Prognostic Association Pre-NACT | Prognostic Association Post-NACT | Study | |
|---|---|---|---|
| Granzime B+ | None | PFS increase with ↑ OS none | Polcher et al. [23] |
| GranzymeB/Foxp3 | None | PFS increase with ↑ OS none | Polcher et al. [23] |
| PD-L1 | None | None | Mesnage et al. [26] |
| None | None | Kim et al. [27] | |
| None | None | Bohm et al. [24] | |
| None | None | Lo et al. [25] | |
| None | PFS increase with ↑ OS increase with ↑ | Chen et al. [28] | |
| None | None | Félix Blanc-Durand et al. [30] | |
| PFS increase with ↑ OS increase with ↑ | None | Lee et al. [31] | |
| PD-1 | None | None | Lo et al. [25] |
| IDO-1 | None | None | Félix Blanc-Durand et al. [30] |
| TIM3+ | None | None | Félix Blanc-Durand et al. [30] |
| LAG3+ | None | None | Félix Blanc-Durand et al. [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnol, G.; Ghisoni, E.; Morotti, M.; De Tommasi, O.; Marchetti, M.; Bigardi, S.; Tuninetti, V.; Tasca, G.; Noventa, M.; Saccardi, C.; et al. The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 7070. https://doi.org/10.3390/ijms25137070
Spagnol G, Ghisoni E, Morotti M, De Tommasi O, Marchetti M, Bigardi S, Tuninetti V, Tasca G, Noventa M, Saccardi C, et al. The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature. International Journal of Molecular Sciences. 2024; 25(13):7070. https://doi.org/10.3390/ijms25137070
Chicago/Turabian StyleSpagnol, Giulia, Eleonora Ghisoni, Matteo Morotti, Orazio De Tommasi, Matteo Marchetti, Sofia Bigardi, Valentina Tuninetti, Giulia Tasca, Marco Noventa, Carlo Saccardi, and et al. 2024. "The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature" International Journal of Molecular Sciences 25, no. 13: 7070. https://doi.org/10.3390/ijms25137070






