Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs
Abstract
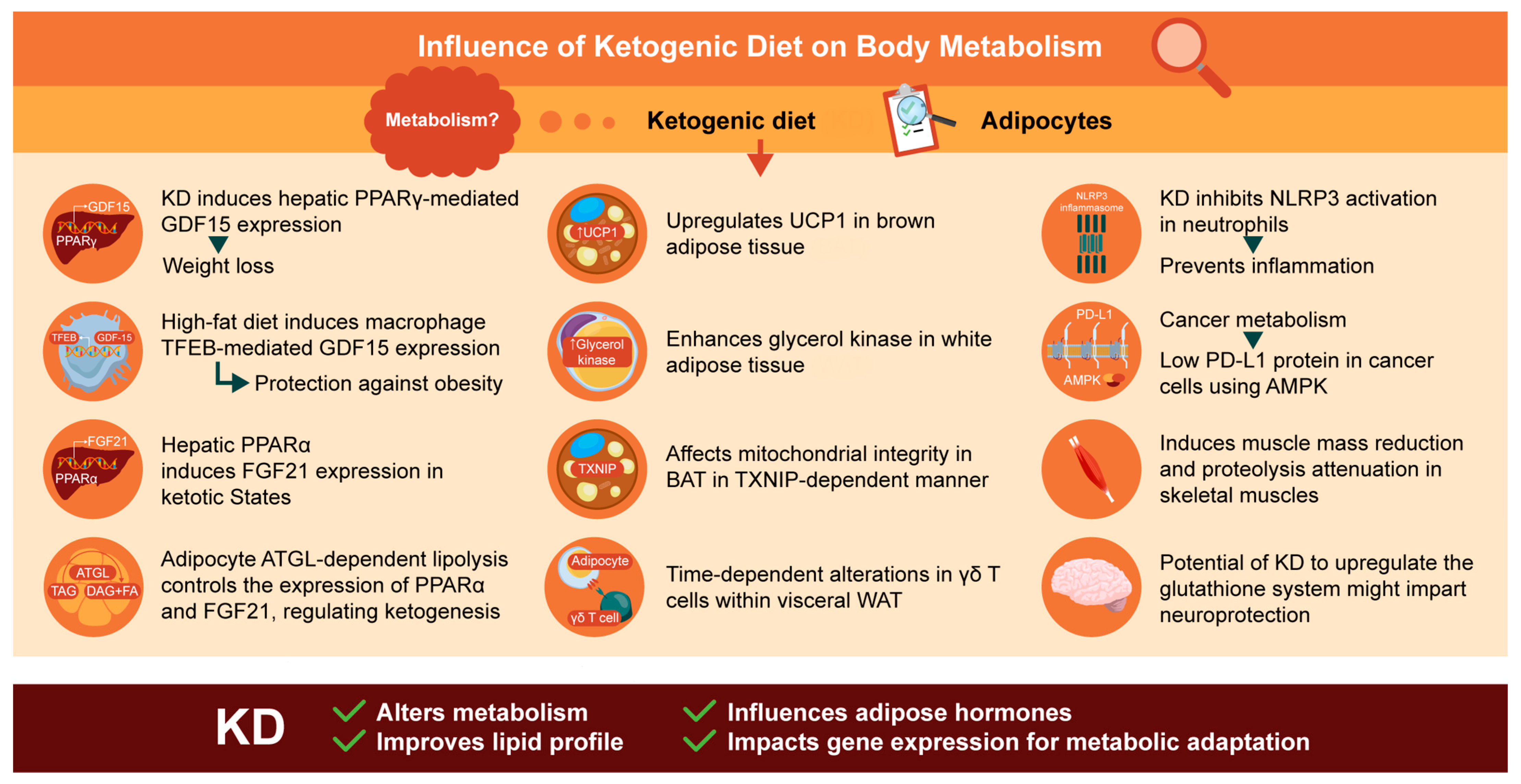
:1. Introduction
2. Glucose and Lipid Metabolism under the KD
3. Insights and Mechanisms of Brown Adipose Tissue (BAT) in the Context of the KD
4. Metabolic Regulation of WAT by the KD
5. Gene Expression Profiles of Metabolic Enzymes under the KD
6. Metabolic Signaling by Fibroblast Growth Factor 21 (FGF21) and Growth Differentiation Factor 15 (GDF15) under the KD
6.1. FGF21
6.2. GDF15
7. The KD: Interconnections between Adipose Tissue and Other Organs
7.1. Liver
7.2. Cardiac and Skeletal Muscles
7.3. Neurological Disorders
7.4. Inflammation
7.5. Cancer Cachexia
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Masood, W.; Annamaraju, P.; Khan Suheb, M.Z.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearlsl: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weber, A.; Medak, K.D.; Townsend, L.K.; Wright, D.C. Ketogenic diet-induced weight loss occurs independent of housing temperature and is followed by hyperphagia and weight regain after cessation in mice. J. Physiol. 2022, 600, 4677–4693. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; Sole, T.; Vega, N.; Thomas, T.; Balcerczyk, A.; Strigini, M.; Pirola, L. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metab. 2022, 65, 101578. [Google Scholar] [CrossRef]
- Arsyad, A.; Idris, I.; Rasyid, A.A.; Usman, R.A.; Faradillah, K.R.; Latif, W.O.U.; Lubis, Z.I.; Aminuddin, A.; Yustisia, I.; Djabir, Y.Y. Long-term ketogenic diet induces metabolic acidosis, anemia, and oxidative stress in healthy wistar rats. J. Nutr. Metab. 2020, 2020, 3642035. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Coopmans, E.C.; Berk, K.A.C.; El-Sayed, N.; Neggers, S.; van der Lely, A.J. Eucaloric Very-Low-Carbohydrate Ketogenic Diet in Acromegaly Treatment. N. Engl. J. Med. 2020, 382, 2161–2162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, Y.; Meireles, C.; Sharma, K.; Qi, L.; Castillo, A.; Wang, J. Adherence to ketogenic diet in lifestyle interventions in adults with overweight or obesity and type 2 diabetes: A scoping review. Nutr. Diabetes 2023, 13, 16. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Bastias-Perez, M.; Serra, D.; Herrero, L. Dietary Options for Rodents in the Study of Obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef]
- Schugar, R.C.; Huang, X.; Moll, A.R.; Brunt, E.M.; Crawford, P.A. Role of choline deficiency in the Fatty liver phenotype of mice fed a low protein, very low carbohydrate ketogenic diet. PLoS ONE 2013, 8, e74806. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Purello, C.; Booth, S.L.; Bennett, B.; Wiley, C.D.; Korstanje, R. Chow diet in mouse aging studies: Nothing regular about it. Geroscience 2023, 45, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Ulamek-Koziol, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.R.; Kim, H.D.; Kang, H.C. Lower fat and better quality diet therapy for children with pharmacoresistant epilepsy. Korean J. Pediatr. 2013, 56, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Desli, E.; Spilioti, M.; Evangeliou, A.; Styllas, F.; Magkos, F.; Dalamaga, M. The Efficacy and Safety of Ketogenic Diets in Drug-Resistant Epilepsy in Children and Adolescents: A Systematic Review of Randomized Controlled Trials. Curr. Nutr. Rep. 2022, 11, 102–116. [Google Scholar] [CrossRef]
- Baylie, T.; Ayelgn, T.; Tiruneh, M.; Tesfa, K.H. Effect of Ketogenic Diet on Obesity and Other Metabolic Disorders: Narrative Review. Diabetes Metab. Syndr. Obes. 2024, 17, 1391–1401. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef]
- Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C.; Gnessi, L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes. Rev. 2020, 21, e13053. [Google Scholar] [CrossRef]
- Talib, W.H.; Al-Dalaeen, A.; Mahmod, A.I. Ketogenic diet in cancer management. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Drabinska, N.; Juskiewicz, J.; Wiczkowski, W. The Effect of the Restrictive Ketogenic Diet on the Body Composition, Haematological and Biochemical Parameters, Oxidative Stress and Advanced Glycation End-Products in Young Wistar Rats with Diet-Induced Obesity. Nutrients 2022, 14, 4805. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, Y.; Zhang, J.; Ma, J. The Role of a Ketogenic Diet in the Treatment of Dementia in Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1971. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Prins, P.J.; Volek, J.S.; D‘Agostino, D.P.; Koutnik, A.P. Low carbohydrate high fat ketogenic diets on the exercise crossover point and glucose homeostasis. Front. Physiol. 2023, 14, 1150265. [Google Scholar] [CrossRef] [PubMed]
- Skytte, M.J.; Samkani, A.; Astrup, A.; Frystyk, J.; Rehfeld, J.F.; Holst, J.J.; Madsbad, S.; Burling, K.; Fenger, M.; Thomsen, M.N.; et al. Effects of carbohydrate restriction on postprandial glucose metabolism, beta-cell function, gut hormone secretion, and satiety in patients with Type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E7–E18. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Hanson, P.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations. Nutrients 2021, 13, 1187. [Google Scholar] [CrossRef]
- Hall, K.D.; Chung, S.T. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 308–312. [Google Scholar] [CrossRef]
- Arbour, M.W.; Stec, M.; Walker, K.C.; Wika, J.C. Clinical Implications for Women of a Low-Carbohydrate or Ketogenic Diet With Intermittent Fasting. Nurs. Womens Health 2021, 25, 139–151. [Google Scholar] [CrossRef]
- Paoli, A.; Bianco, A.; Moro, T.; Mota, J.F.; Coelho-Ravagnani, C.F. The Effects of Ketogenic Diet on Insulin Sensitivity and Weight Loss, Which Came First: The Chicken or the Egg? Nutrients 2023, 15, 3120. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimaki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Jarvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Jani, S.; Da Eira, D.; Stefanovic, M.; Ceddia, R.B. The ketogenic diet prevents steatosis and insulin resistance by reducing lipogenesis, diacylglycerol accumulation and protein kinase C activity in male rat liver. J. Physiol. 2022, 600, 4137–4151. [Google Scholar] [CrossRef] [PubMed]
- Lejk, A.; Chrzanowski, J.; Cieslak, A.; Fendler, W.; Mysliwiec, M. Reduced Carbohydrate Diet Influence on Postprandial Glycemia-Results of a Short, CGM-Based, Interventional Study in Adolescents with Type 1 Diabetes. Nutrients 2022, 14, 4689. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.V.; Abild, C.B.; Bangshaab, M.; Gormsen, L.C.; Sondergaard, E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients 2022, 14, 1322. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, J.; Jiang, T.; Zhang, Z.; Wang, Z.; Gong, M.; Zhao, L.; Zhang, C. Ketogenic Diets Induced Glucose Intolerance and Lipid Accumulation in Mice with Alterations in Gut Microbiota and Metabolites. mBio 2021, 12. [Google Scholar] [CrossRef]
- Valsdottir, T.D.; Henriksen, C.; Odden, N.; Nellemann, B.; Jeppesen, P.B.; Hisdal, J.; Westerberg, A.C.; Jensen, J. Effect of a Low-Carbohydrate High-Fat Diet and a Single Bout of Exercise on Glucose Tolerance, Lipid Profile and Endothelial Function in Normal Weight Young Healthy Females. Front. Physiol. 2019, 10, 1499. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Altinoz, S.; Micili, S.C.; Soy, S.; Engur, D.; Baysal, B.; Kumral, A. Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain. Nutrients 2023, 15, 1994. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Brown adipose tissue thermogenesis and obesity. Prog. Lipid Res. 1989, 28, 67–115. [Google Scholar] [CrossRef]
- Kalafut, K.C.; Mitchell, S.J.; MacArthur, M.R.; Mitchell, J.R. Short-Term Ketogenic Diet Induces a Molecular Response That Is Distinct From Dietary Protein Restriction. Front. Nutr. 2022, 9, 839341. [Google Scholar] [CrossRef]
- Da Eira, D.; Jani, S.; Stefanovic, M.; Ceddia, R.B. Obesogenic versus ketogenic diets in the regulation of the renin-angiotensin system in rat white and brown adipose tissues. Nutrition 2023, 105, 111862. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, L.; Li, M.; Liu, Y.; Shi, Y.; Zhang, J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules 2024, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Waldhart, A.N.; Muhire, B.; Johnson, B.; Pettinga, D.; Madaj, Z.B.; Wolfrum, E.; Dykstra, H.; Wegert, V.; Pospisilik, J.A.; Han, X.; et al. Excess dietary carbohydrate affects mitochondrial integrity as observed in brown adipose tissue. Cell Rep. 2021, 36, 109488. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Van Thi-Tuong, N.; Van Vu, V.; Van Pham, P. Brown adipocyte and browning thermogenesis: Metabolic crosstalk beyond mitochondrial limits and physiological impacts. Adipocyte 2023, 12, 2237164. [Google Scholar] [CrossRef]
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 2013, 65, 58–66. [Google Scholar] [CrossRef]
- Sebaa, R.; Johnson, J.; Pileggi, C.; Norgren, M.; Xuan, J.; Sai, Y.; Tong, Q.; Krystkowiak, I.; Bondy-Chorney, E.; Davey, N.E.; et al. SIRT3 controls brown fat thermogenesis by deacetylation regulation of pathways upstream of UCP1. Mol. Metab. 2019, 25, 35–49. [Google Scholar] [CrossRef]
- Okamatsu-Ogura, Y.; Kuroda, M.; Tsutsumi, R.; Tsubota, A.; Saito, M.; Kimura, K.; Sakaue, H. UCP1-dependent and UCP1-independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism 2020, 113, 154396. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, T.; Chen, J.; Li, J.; You, J. Fibroblast growth factor 21: An emerging pleiotropic regulator of lipid metabolism and the metabolic network. Genes. Dis. 2024, 11, 101064. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Hasegawa, S.; Ozaki, S.; Imai, M.; Saito, D.; Takahashi, N. High-Fat-Diet Suppressed Ketone Body Utilization for Lipogenic Pathway in Brown Adipose Tissues. Metabolites 2023, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Douris, N.; Desai, B.N.; Cisu, T.; Fowler, A.J.; Zarebidaki, E.; Nguyen, N.L.T.; Morgan, D.A.; Bartness, T.J.; Rahmouni, K.; Flier, J.S. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Mol. Metab. 2017, 6, 854–862. [Google Scholar] [CrossRef]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Shen, Q.; Wang, B.; Tang, X.; Jiang, J.; Zheng, Y.; Huang, H.; Zhuo, W.; Wang, W.; Gao, Y.; et al. Spexin ameliorated obesity-related metabolic disorders through promoting white adipose browning mediated by JAK2-STAT3 pathway. Nutr. Metab. 2024, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.J.; Li, W.; Dinh, C.; Zhang, Y.; Hu, J.K.; Daniele, S.G.; Hou, X.; Yang, Z.; Asara, J.M.; Hu, G.F.; et al. CDK6 inhibits de novo lipogenesis in white adipose tissues but not in the liver. Nat. Commun. 2024, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Kennedy, A.R.; Adams, A.C.; Pissios, P.; Maratos-Flier, E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1197–E1204. [Google Scholar] [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Nunno, N.D.; et al. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. Int. J. Mol. Sci. 2020, 21, 3228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Sun, X. The molecular mechanism of macrophage-adipocyte crosstalk in maintaining energy homeostasis. Front. Immunol. 2024, 15, 1378202. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Neri, I.G.; Trentani, C.; Ferraris, C.; De Amicis, R.; Battezzati, A.; Veggiotti, P.; De Giorgis, V.; Tagliabue, A. Short-term effects of ketogenic diet on anthropometric parameters, body fat distribution, and inflammatory cytokine production in GLUT1 deficiency syndrome. Nutrition 2015, 31, 981–987. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Roberson, R.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.F.; Kahn, B.B.; et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, N.C.; Riezu-Boj, J.I.; Martinez, J.A.; Garcia-Calzon, S.; Milagro, F.I. A Predictive Tool Based on DNA Methylation Data for Personalized Weight Loss through Different Dietary Strategies: A Pilot Study. Nutrients 2023, 15, 5023. [Google Scholar] [CrossRef]
- Ungaro, P.; Nettore, I.C.; Franchini, F.; Palatucci, G.; Muscogiuri, G.; Colao, A.; Macchia, P.E. Epigenome modulation induced by ketogenic diets. Nutrients 2022, 14, 3245. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bu, X.; Gao, Y.; Guo, J.; Hu, J.; Jiang, C.; Zhang, Z.; Xu, K.; Duan, J.; He, S.; et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 2021, 81, 2317–2331.e2316. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Izquierdo, A.G.; Primo, D.; Milagro, F.I.; Sajoux, I.; Jacome, A.; Fernandez-Quintela, A.; Portillo, M.P.; Martinez, J.A.; Martinez-Olmos, M.A.; et al. Epigenetic landscape in blood leukocytes following ketosis and weight loss induced by a very low calorie ketogenic diet (VLCKD) in patients with obesity. Clin. Nutr. 2021, 40, 3959–3972. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, D.; Gimenez-Cassina, A. Ketone Bodies in the Brain Beyond Fuel Metabolism: From Excitability to Gene Expression and Cell Signaling. Front. Mol. Neurosci. 2021, 14, 732120. [Google Scholar] [CrossRef]
- Tozzi, R.; Cipriani, F.; Masi, D.; Basciani, S.; Watanabe, M.; Lubrano, C.; Gnessi, L.; Mariani, S. Ketone bodies and SIRT1, synergic epigenetic regulators for metabolic health: A narrative review. Nutrients 2022, 14, 3145. [Google Scholar] [CrossRef]
- Bandera-Merchan, B.; Boughanem, H.; Crujeiras, A.B.; Macias-Gonzalez, M.; Tinahones, F.J. Ketotherapy as an epigenetic modifier in cancer. Rev. Endocr. Metab. Disord. 2020, 21, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Murano, C.; Binda, A.; Palestini, P.; Baruscotti, M.; DiFrancesco, J.C.; Rivolta, I. Effect of the ketogenic diet in excitable tissues. Am. J. Physiol. Cell Physiol. 2021, 320, C547–C553. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Choe, W.; Yoon, K.-S.; Ha, J.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular mechanisms for ketone body metabolism, signaling functions, and therapeutic potential in cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Cowart, K. Oral Semaglutide: First-in-Class Oral GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes Mellitus. Ann. Pharmacother. 2020, 54, 478–485. [Google Scholar] [CrossRef]
- Keipert, S.; Ost, M. Stress-induced FGF21 and GDF15 in obesity and obesity resistance. Trends Endocrinol. Metab. 2021, 32, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Mangelsdorf, D.J. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cao, H.; Shen, Y.; Li, W.; Wang, W.; Cheng, L.; Cai, M.; Xu, F. Role of liver FGF21-KLB signaling in ketogenic diet-induced amelioration of hepatic steatosis. Nutr. Diabetes 2024, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Altirriba, J.; Rohner-Jeanrenaud, F.; Jornayvaz, F.R. Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue. PLoS ONE 2015, 10, e0126364. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef]
- Song, P.; Zechner, C.; Hernandez, G.; Canovas, J.; Xie, Y.; Sondhi, V.; Wagner, M.; Stadlbauer, V.; Horvath, A.; Leber, B.; et al. The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol. Cell Metab. 2018, 27, 1338–1347.e1334. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Heblinski, M.; Wahl, D.; McMahon, A.C.; Warren, A.; Durrant-Whyte, J.; Walters, K.A.; Krycer, J.R.; et al. Defining the Nutritional and Metabolic Context of FGF21 Using the Geometric Framework. Cell Metab. 2016, 24, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Nakamura, H.; Konishi, M.; Murata, Y.; Takagi, H.; Matsumura, S.; Inoue, K.; Fushiki, T.; Itoh, N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 2009, 150, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an update of the physiological and pathological roles it plays: A review. Pflug. Arch. 2020, 472, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Zhu, M.Q.; Xia, B.; Zhang, N.N.; Liu, X.P.; Liu, H.; Zhang, R.X.; Xiao, J.Y.; Yang, H.; Zhang, Y.Q.; et al. GDF15 is a major determinant of ketogenic diet-induced weight loss. Cell Metab. 2023, 35, 2165–2182.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jorgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Patel, S.; Haider, A.; Alvarez-Guaita, A.; Bidault, G.; El-Sayed Moustafa, J.S.; Guiu-Jurado, E.; Tadross, J.A.; Warner, J.; Harrison, J.; Virtue, S.; et al. Combined genetic deletion of GDF15 and FGF21 has modest effects on body weight, hepatic steatosis and insulin resistance in high fat fed mice. Mol. Metab. 2022, 65, 101589. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Recarte, D.; Barroso, E.; Palomer, X.; Wahli, W.; Vazquez-Carrera, M. Knocking on GDF15’s door for the treatment of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2022, 33, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.H.; Kang, H.; Lee, S.; Park, S.Y.; Cho, Y.; Lim, Y.M.; Ahn, J.W.; Kim, Y.H.; Chung, S.; et al. TFEB-GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat. Metab. 2021, 3, 410–427. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, D.Y. Research Progress on the Role and Mechanism of GDF15 in Body Weight Regulation. Obes. Facts 2024, 17, 1–11. [Google Scholar] [CrossRef]
- Sierawska, O.; Sawczuk, M. Interaction between Selected Adipokines and Musculoskeletal and Cardiovascular Systems: A Review of Current Knowledge. Int. J. Mol. Sci. 2023, 24, 17287. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Szerenos, E.; Lewandowski, D.; Toczylowski, K.; Sulik, A. The Role of Adipokines in the Pathologies of the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 14684. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; De Nucci, S.; Castellana, F.; Di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The Effects of Eight Weeks’ Very Low-Calorie Ketogenic Diet (VLCKD) on Liver Health in Subjects Affected by Overweight and Obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Sun, S.; Zhang, S.; Yuan, L.; Xu, Y.; Li, X.; Chen, G.; Wei, X.; Liu, C. Ketogenic diet alleviates beta-cell dedifferentiation but aggravates hepatic lipid accumulation in db/db mice. Nutrition 2024, 119, 112284. [Google Scholar] [CrossRef] [PubMed]
- Battaglioni, S.; Benjamin, D.; Walchli, M.; Maier, T.; Hall, M.N. mTOR substrate phosphorylation in growth control. Cell 2022, 185, 1814–1836. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Nishio, K.; Mochiyama, T.; Konishi, M.; Shimada, M.; Ohta, H.; Itoh, N. Fgf21 impairs adipocyte insulin sensitivity in mice fed a low-carbohydrate, high-fat ketogenic diet. PLoS ONE 2013, 8, e69330. [Google Scholar] [CrossRef] [PubMed]
- Abdurrachim, D.; Teo, X.Q.; Woo, C.C.; Ong, S.Y.; Salleh, N.F.; Lalic, J.; Tan, R.S.; Lee, P.T.H. Cardiac metabolic modulation upon low-carbohydrate low-protein ketogenic diet in diabetic rats studied in vivo using hyperpolarized (13) C pyruvate, butyrate and acetoacetate probes. Diabetes Obes. Metab. 2019, 21, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes. Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef]
- Konwerski, M.; Gasecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. [Google Scholar] [CrossRef]
- Antonio Paoli, A.; Mancin, L.; Caprio, M.; Monti, E.; Narici, M.V.; Cenci, L.; Piccini, F.; Pincella, M.; Grigoletto, D.; Marcolin, G. Effects of 30 days of ketogenic diet on body composition, muscle strength, muscle area, metabolism, and performance in semi-professional soccer players. J. Int. Soc. Sports Nutr. 2021, 18, 62. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Sci. Rep. 2019, 9, 19652. [Google Scholar] [CrossRef] [PubMed]
- Yakupova, E.I.; Bocharnikov, A.D.; Plotnikov, E.Y. Effects of Ketogenic Diet on Muscle Metabolism in Health and Disease. Nutrients 2022, 14, 3842. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Lopez-Soriano, J.; Almendro, V.; Busquets, S.; Lopez-Soriano, F.J. Cross-talk between skeletal muscle and adipose tissue: A link with obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Liu, X.; Zhang, J.; Liu, G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediat. Inflamm. 2019, 2019, 8373060. [Google Scholar] [CrossRef]
- Murakami, M.; Tognini, P. Molecular Mechanisms Underlying the Bioactive Properties of a Ketogenic Diet. Nutrients 2022, 14, 782. [Google Scholar] [CrossRef]
- Simeone, T.A.; Matthews, S.A.; Simeone, K.A. Synergistic protection against acute flurothyl-induced seizures by adjuvant treatment of the ketogenic diet with the type 2 diabetes drug pioglitazone. Epilepsia 2017, 58, 1440–1450. [Google Scholar] [CrossRef]
- Li, R.J.; Liu, Y.; Liu, H.Q.; Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar] [CrossRef] [PubMed]
- Parimisetty, A.; Dorsemans, A.C.; Awada, R.; Ravanan, P.; Diotel, N.; Lefebvre d‘Hellencourt, C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflamm. 2016, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Daas, S.I.; Rizeq, B.R.; Nasrallah, G.K. Adipose tissue dysfunction in cancer cachexia. J. Cell Physiol. 2018, 234, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.Z.C.; Arabaci, D.H.; Kir, S. Metabolic Reprogramming in Adipose Tissue During Cancer Cachexia. Front. Oncol. 2022, 12, 848394. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, K.L.; Ke, J.Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 2015, 16, 886–897. [Google Scholar] [CrossRef]
- Sripongpun, P.; Churuangsuk, C.; Bunchorntavakul, C. Current Evidence Concerning Effects of Ketogenic Diet and Intermittent Fasting in Patients with Nonalcoholic Fatty Liver. J. Clin. Transl. Hepatol. 2022, 10, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Correa de Mello, L.L.; Hasenstab, K.A.; Spina, L.; Bussade, I.; Prata Mesiano, J.M.; Coutinho, W.; Guzman, G.; Sajoux, I. MRI estimated changes in visceral adipose tissue and liver fat fraction in patients with obesity during a very low-calorie-ketogenic diet compared to a standard low-calorie diet. Clin. Radiol. 2020, 75, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Zhao, Y.; Shi, J.; Lan, R.; Gan, Y.; Ren, H.; Zhu, B.; Qian, M.; Du, B. Long-term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice. Nutr. Res. 2016, 36, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ravaut, G.; Carneiro, A.; Mounier, C. Exploring the impacts of ketogenic diet on reversible hepatic steatosis: Initial analysis in male mice. Front. Nutr. 2024, 11, 1290540. [Google Scholar] [CrossRef] [PubMed]
- Liskiewicz, D.; Liskiewicz, A.; Grabowski, M.; Nowacka-Chmielewska, M.M.; Jablonska, K.; Wojakowska, A.; Marczak, L.; Barski, J.J.; Malecki, A. Upregulation of hepatic autophagy under nutritional ketosis. J. Nutr. Biochem. 2021, 93, 108620. [Google Scholar] [CrossRef] [PubMed]
- Bence, K.K.; Birnbaum, M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101143. [Google Scholar] [CrossRef]
- Jump, D.B. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr. Opin. Lipidol. 2002, 13, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Gonzalez-Rodriguez, A.; Garcia-Monzon, C.; Valverde, A.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Bhatti, M.R.; Kellenberger, A.; Sun, W.; Modica, S.; Horing, M.; Liebisch, G.; Krieger, J.P.; Wolfrum, C.; Challa, T.D. A low-carbohydrate diet induces hepatic insulin resistance and metabolic associated fatty liver disease in mice. Mol. Metab. 2023, 69, 101675. [Google Scholar] [CrossRef]
- Martinez-Una, M.; Lopez-Mancheno, Y.; Dieguez, C.; Fernandez-Rojo, M.A.; Novelle, M.G. Unraveling the Role of Leptin in Liver Function and Its Relationship with Liver Diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Zhao, M.F.; Zhang, X.G.; Tang, Y.P.; Zhu, Y.X.; Nie, H.Y.; Bu, D.D.; Fang, L.; Li, C.J. Ketone bodies promote epididymal white adipose expansion to alleviate liver steatosis in response to a ketogenic diet. J. Biol. Chem. 2024, 300, 105617. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Heather, L.C.; Luiken, J. CD36 as a gatekeeper of myocardial lipid metabolism and therapeutic target for metabolic disease. Physiol. Rev. 2024, 104, 727–764. [Google Scholar] [CrossRef]
- Balteau, M.; Van Steenbergen, A.; Timmermans, A.D.; Dessy, C.; Behets-Wydemans, G.; Tajeddine, N.; Castanares-Zapatero, D.; Gilon, P.; Vanoverschelde, J.L.; Horman, S.; et al. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1120–H1133. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Ericsson, M.; Joibari, M.M.; Anderson, F.; Carlsson, L.; Nilsson, S.K.; Sjodin, A.; Buren, J. A low-carbohydrate high-fat diet decreases lean mass and impairs cardiac function in pair-fed female C57BL/6J mice. Nutr. Metab. 2016, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzym. Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Smith, D.; Burke, L.M.; Angus, D.J.; Tunstall, R.J.; Cox, G.R.; Bonen, A.; Hawley, J.A.; Hargreaves, M. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am. J. Clin. Nutr. 2003, 77, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Khanal, S. Leptin in Atherosclerosis: Focus on Macrophages, Endothelial and Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 22, 5446. [Google Scholar] [CrossRef]
- Sweeney, G. Cardiovascular effects of leptin. Nat. Rev. Cardiol. 2010, 7, 22–29. [Google Scholar] [CrossRef]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef]
- Liskiewicz, D.; Liskiewicz, A.; Nowacka-Chmielewska, M.M.; Grabowski, M.; Pondel, N.; Grabowska, K.; Student, S.; Barski, J.J.; Malecki, A. Differential Response of Hippocampal and Cerebrocortical Autophagy and Ketone Body Metabolism to the Ketogenic Diet. Front. Cell Neurosci. 2021, 15, 733607. [Google Scholar] [CrossRef]
- Badoer, E.; Kosari, S.; Stebbing, M.J. Resistin, an Adipokine with Non-Generalized Actions on Sympathetic Nerve Activity. Front. Physiol. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Dobri, A.M.; Dudau, M.; Enciu, A.M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chyra, M.; Swietochowska, E.; Gorska-Flak, K.; Dudzinska, M.; Oswiecimska, J. The effect of the ketogenic diet on leptin, chemerin and resistin levels in children with epilepsy. Neuro Endocrinol. Lett. 2021, 42, 489–499. [Google Scholar] [PubMed]
- Liu, Y.; Wang, D.; Li, T.; Yang, F.; Li, Z.; Bai, X.; Wang, Y. The role of NLRP3 inflammasome in inflammation-related skeletal muscle atrophy. Front. Immunol. 2022, 13, 1035709. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Kim, S.R.; Cho, W.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Kim, J.H.; Yu, J.W.; Lee, B.W.; et al. Short Term Isocaloric Ketogenic Diet Modulates NLRP3 Inflammasome Via B-hydroxybutyrate and Fibroblast Growth Factor 21. Front. Immunol. 2022, 13, 843520. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Newman, J.C. Ketogenic diet and adipose tissue inflammation-a simple story? Fat chance! Nat. Metab. 2020, 2, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Jayashankar, S.S.; Tajul Arifin, K.; Nasaruddin, M.L. beta-Hydroxybutyrate Regulates Activated Microglia to Alleviate Neurodegenerative Processes in Neurological Diseases: A Scoping Review. Nutrients 2023, 15, 524. [Google Scholar] [CrossRef]
- Tavener, S.K.; Jackson, M.I.; Panickar, K.S. Immune-Modulating Effects of Low-Carbohydrate Ketogenic Foods in Healthy Canines. Curr. Dev. Nutr. 2024, 8, 102128. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Molony, R.D.; Kudo, E.; Sidorov, S.; Kong, Y.; Dixit, V.D.; Iwasaki, A. Ketogenic diet activates protective gammadelta T cell responses against influenza virus infection. Sci. Immunol. 2019, 4, eaav2026. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Lopez-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Yan, L.; Liao, Z. Abnormal lipid metabolism in cancer-associated cachexia and potential therapy strategy. Front. Oncol. 2023, 13, 1123567. [Google Scholar] [CrossRef]
- Alves, M.J.; Figueredo, R.G.; Azevedo, F.F.; Cavallaro, D.A.; Neto, N.I.; Lima, J.D.; Matos-Neto, E.; Radloff, K.; Riccardi, D.M.; Camargo, R.G.; et al. Adipose tissue fibrosis in human cancer cachexia: The role of TGFbeta pathway. BMC Cancer 2017, 17, 190. [Google Scholar] [CrossRef]
| Diet of Humans and Rodents | Types of Diets | Carbohydrates (%) | Fats (%) | Proteins (%) | References/ Product No. |
|---|---|---|---|---|---|
| Standard American diet | regular diet | 50–60 | 25–35 | 10–20 | [14] |
| Human ketogenic diet therapy | classic long-chain triglyceride ketogenic diet | 5–10 | 80–90 | 25–35 | [10,15] |
| medium-chain triglyceride ketogenic diet | 10 | 70 | 20 | [10,16] | |
| modified Atkins ketogenic diet | 5–10 | 70–80 | 15–25 | [10,17] | |
| low glycemic index treatment | 10 | 60 | 30 | [10,18] | |
| Representative rodent diets for animal research (Research Diets Inc.) | normal chow diet (standard regular diet) | 65–70 | 10–15 | 20 | D12450 |
| high fat diet (HFD) | 20–35 | 45–65 | 20 | D12492, D12451 | |
| ketogenic diet | 0–5 | 80–90 | 10–20 | D10070801, D05052004 | |
| Control diet for ketogenic diet (high carbohydrate diet) | 80 | 10 | 10 | D10070802, D19082304 |
| Organ-Specific Diseases | Effects of Ketogenic Diet | References |
|---|---|---|
| Non-alcoholic fatty liver disease |
| [30] |
| [94] | |
| [95] | |
| [96] | |
| [97] | |
| [98] | |
| Cardiovascular diseases |
| [37] |
| [99] | |
| [100] | |
| [101] | |
| [102] | |
| Sarcopenic obesity |
| [103] |
| [104] | |
| [105] | |
| [106] | |
| [107] | |
| [108] | |
| Neuroinflammation |
| [109] |
| [110] | |
| [111] | |
| [112] | |
| [113] | |
| Cancer cachexia |
| [114] |
| [115] | |
| [116] | |
| [117] | |
| [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. https://doi.org/10.3390/ijms25137076
Ahmad Y, Seo DS, Jang Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. International Journal of Molecular Sciences. 2024; 25(13):7076. https://doi.org/10.3390/ijms25137076
Chicago/Turabian StyleAhmad, Yusra, Dong Soo Seo, and Younghoon Jang. 2024. "Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs" International Journal of Molecular Sciences 25, no. 13: 7076. https://doi.org/10.3390/ijms25137076







