Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review
Abstract
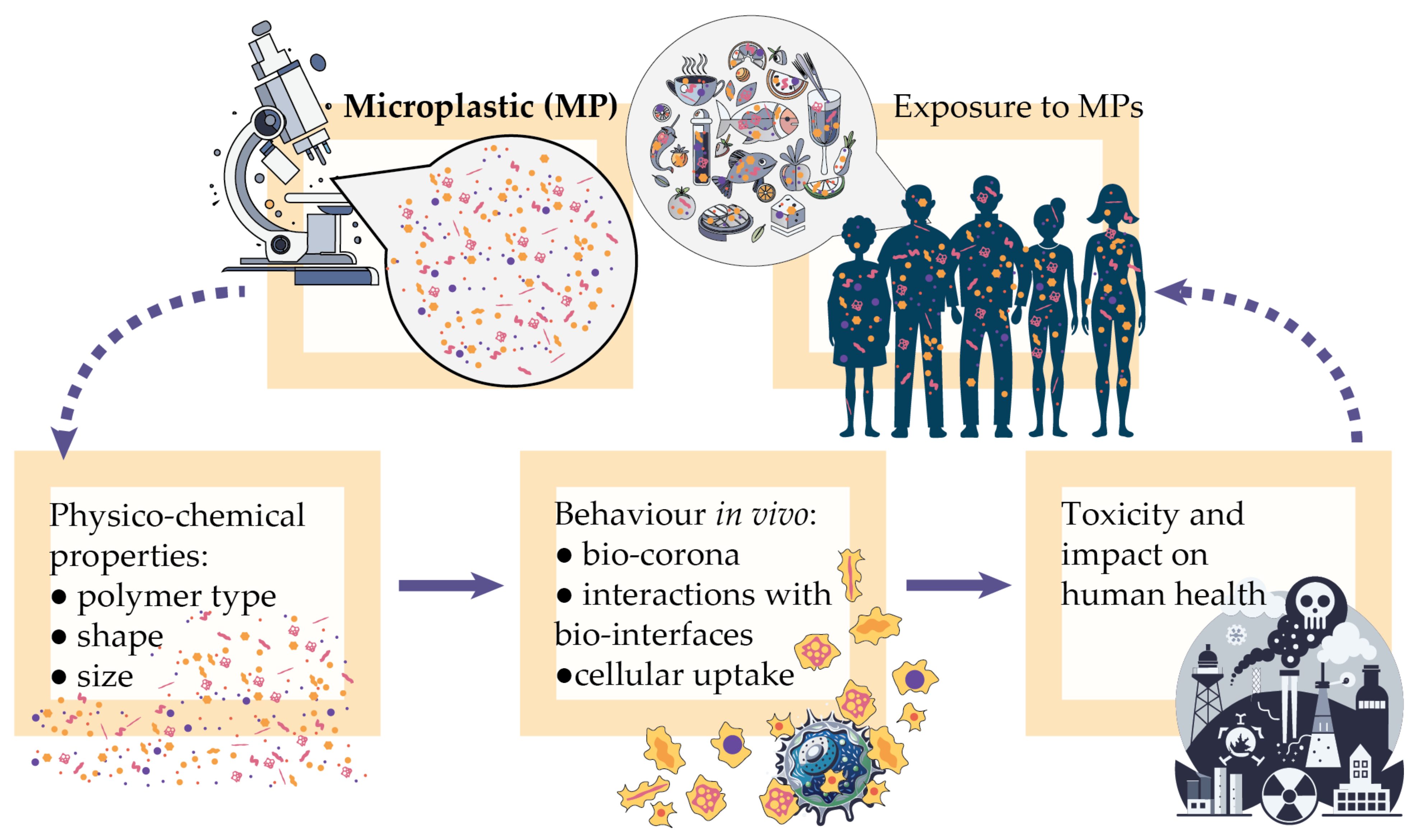
1. Introduction
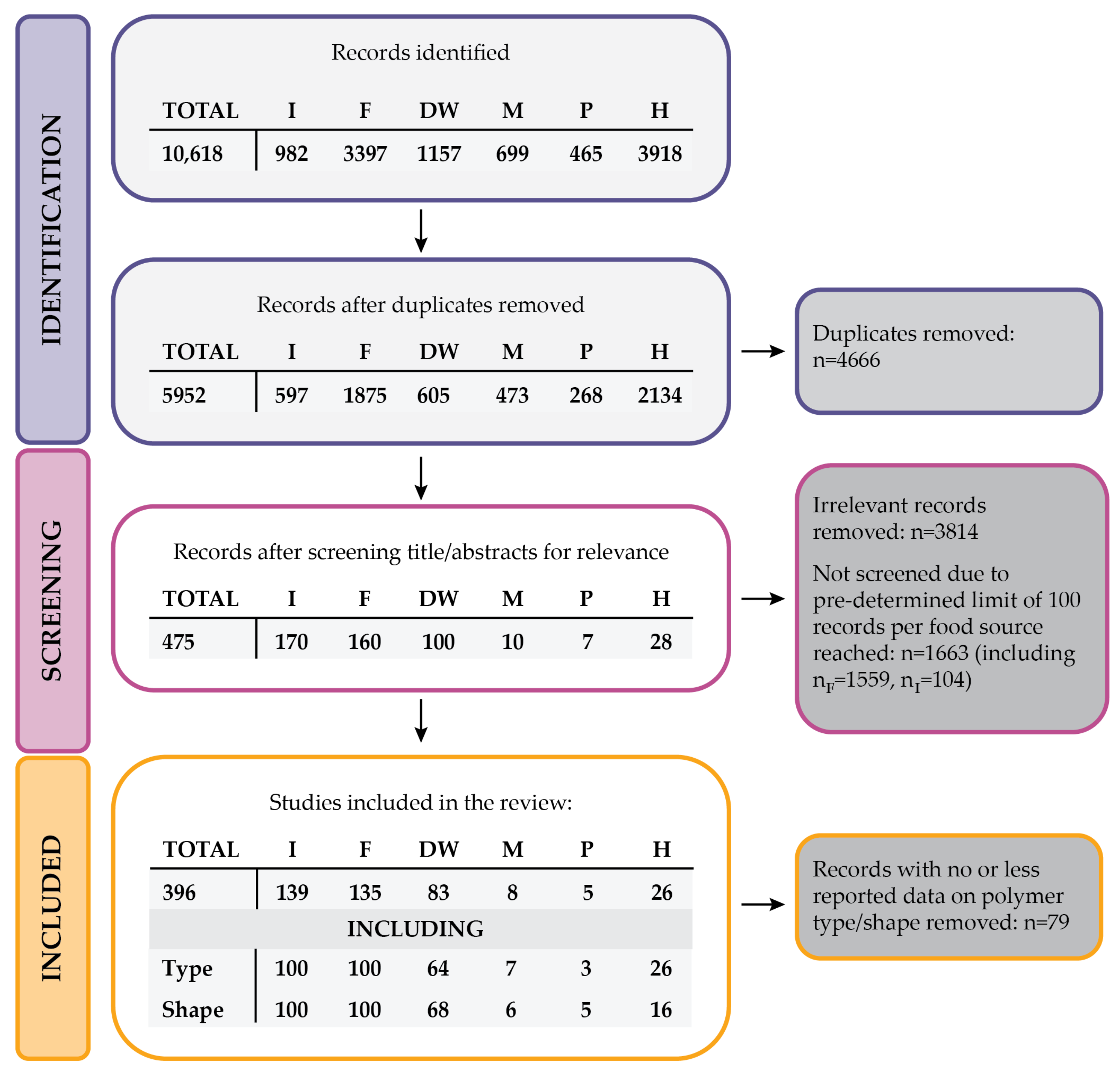
2. Methods
3. Results and Discussion
3.1. Critical Analysis of Human Exposure to MPs through Food and Drinking Water
3.1.1. Description of Data Sources
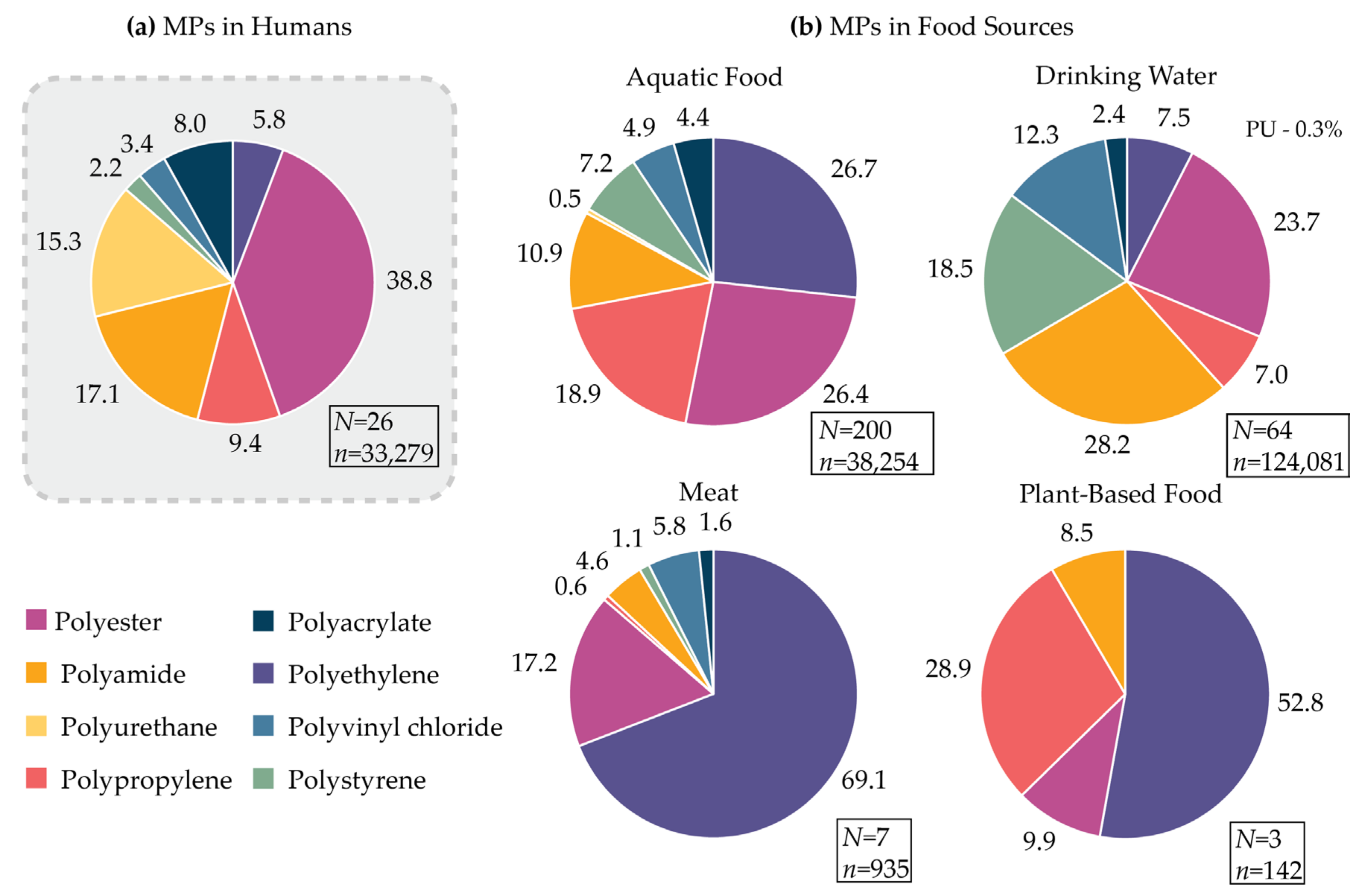
3.1.2. Human Exposure to MPs: Evaluation of Polymer Chemistry
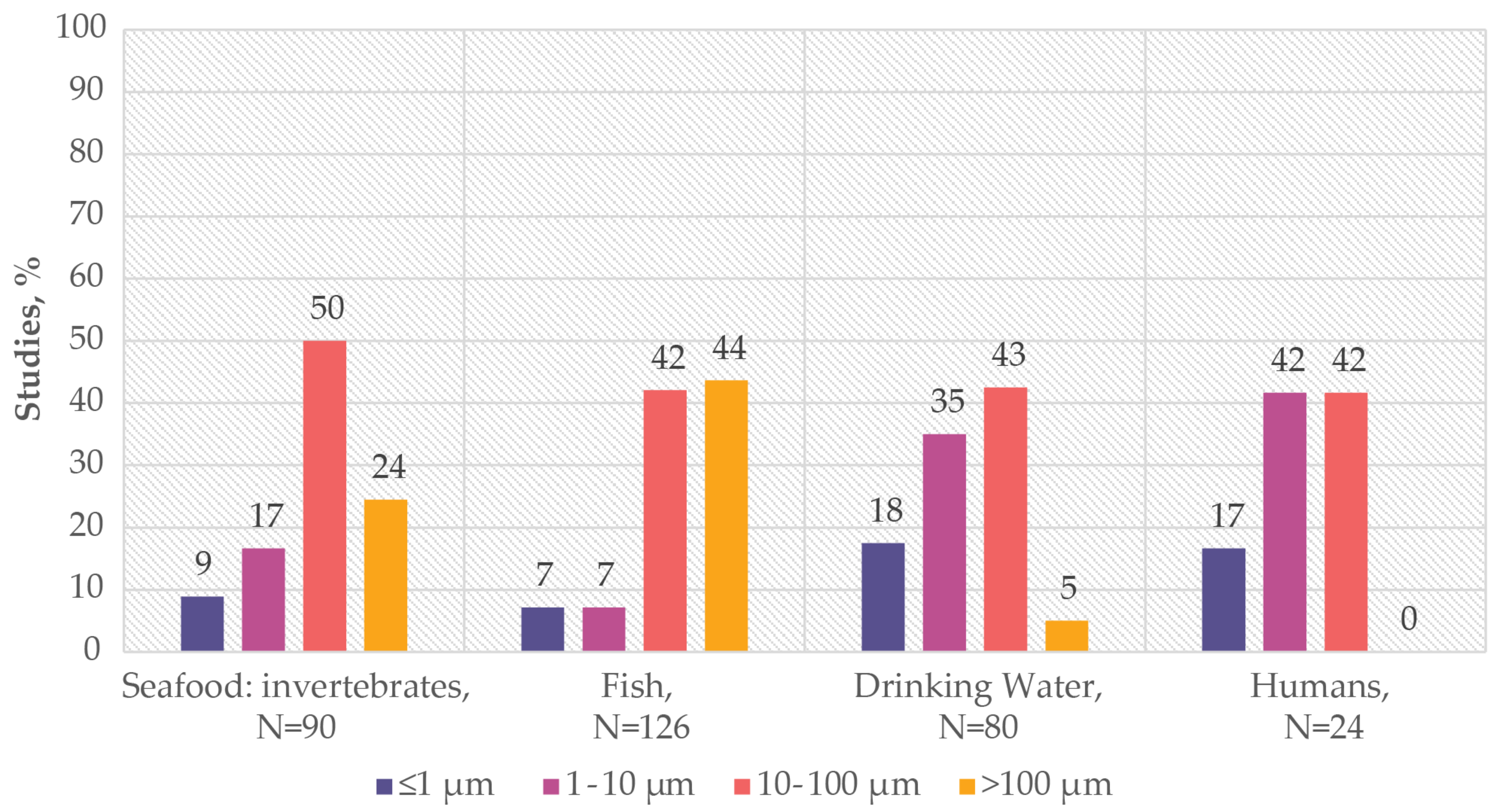
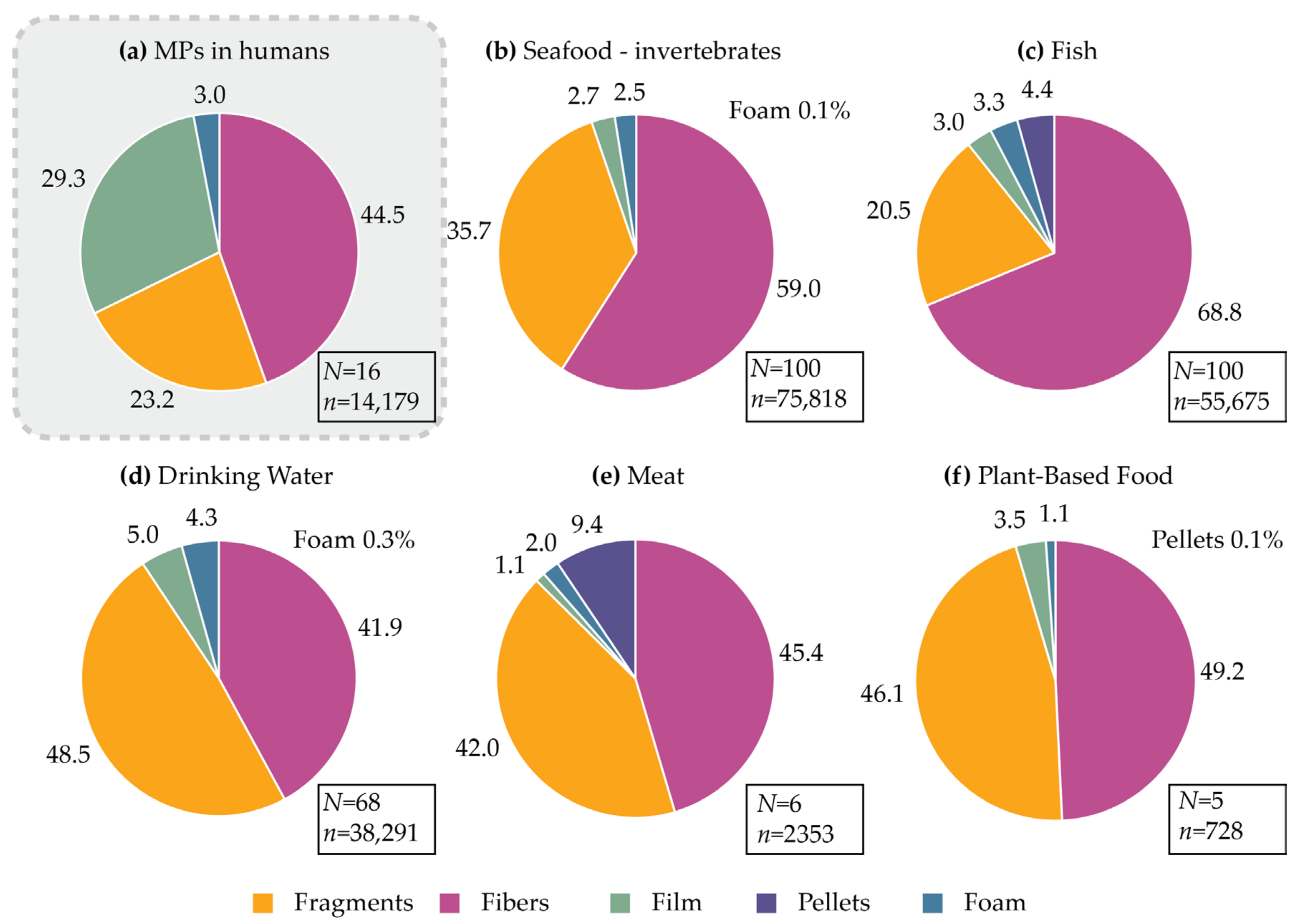
3.1.3. Human Exposure to MPs: Particle Shape
3.2. Outlook on the Impact of MPs’ Physico-Chemical Properties on Toxicology
3.2.1. Toxicology of MPs: Effect of Polymer Chemistry
3.2.2. Toxicology of MPs: Effect of MP Shape
3.2.3. Toxicology of MPs: Effect of MPs’ Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Network, T.B.P. Plastic a Call To Action; The Birmingham Plastic Network: Birmingham, UK, 2023. [Google Scholar] [CrossRef]
- European Chemicals Agency. General Report 2018; European Chemicals Agency: Helsinki, Finland, 2018. [Google Scholar]
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2023, 20, 789. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Bahrani, F.; Dobaradaran, S.; De-La-Torre, G.E.; Ramavandi, B.; Saeedi, R.; Tekle-Röttering, A. Occurrence of microplastics in edible tissues of livestock (cow and sheep). Environ. Sci. Pollut. Res. 2023, 31, 22145–22157. [Google Scholar] [CrossRef]
- Bilal, M.; Taj, M.; Ul Hassan, H.; Yaqub, A.; Shah, M.I.A.; Sohail, M.; Rafiq, N.; Atique, U.; Abbas, M.; Sultana, S.; et al. First Report on Microplastics Quantification in Poultry Chicken and Potential Human Health Risks in Pakistan. Toxics 2023, 11, 612. [Google Scholar] [CrossRef]
- Milne, M.H.; De Frond, H.; Rochman, C.M.; Mallos, N.J.; Leonard, G.H.; Baechler, B.R. Exposure of U.S. adults to microplastics from commonly-consumed proteins. Environ. Pollut. 2023, 343, 123233. [Google Scholar] [CrossRef]
- Sultan, M.H.; Al-Ahmady, K.K.; Mhemid, R.K.S. Microplastics Evaluation in Tap Water in Left Side Districts of Mosul City, Iraq. J. Ecol. Eng. 2023, 24, 353–362. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Habib, R.Z.; Kindi, R.A.; Salem, F.A.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. [Google Scholar] [CrossRef]
- Habib, R.Z.; Poulose, V.; Alsaidi, R.; al Kendi, R.; Iftikhar, S.H.; Mourad, A.H.I.; Kittaneh, W.F.; Thiemann, T. Plastic cutting boards as a source of microplastics in meat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 609–619. [Google Scholar] [CrossRef]
- Logvina, Y.; Matas, I.M.; Ribeiro, H.; Pinto da Silva, L.; Rodrigues, P.; Leitão, J.; da Silva, J.E. Micro- and Nanoplastics in the Atmosphere: Methodology for Microplastics Size-Fractionation Sampling. Microplastics 2024, 3, 82–97. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Winkler, A.; Fumagalli, F.; Cella, C.; Gilliland, D.; Tremolada, P.; Valsesia, A. Detection and formation mechanisms of secondary nanoplastic released from drinking water bottles. Water Res. 2022, 222, 118848. [Google Scholar] [CrossRef]
- Joseph, A.; Parveen, N.; Ranjan, V.P.; Goel, S. Drinking hot beverages from paper cups: Lifetime intake of microplastics. Chemosphere 2023, 317, 137844. [Google Scholar] [CrossRef]
- Hussain, K.A.; Romanova, S.; Okur, I.; Zhang, D.; Kuebler, J.; Huang, X.; Wang, B.; Fernandez-Ballester, L.; Lu, Y.; Schubert, M.; et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health. Environ. Sci. Technol. 2023, 57, 9782–9792. [Google Scholar] [CrossRef]
- Brouwer, H.; Porbahaie, M.; Boeren, S.; Busch, M.; Bouwmeester, H. The in vitro gastrointestinal digestion-associated protein corona of polystyrene nano- and microplastics increases their uptake by human THP-1-derived macrophages. Part. Fibre Toxicol. 2024, 21, 4. [Google Scholar] [CrossRef]
- Krishna de Guzman, M.; Stanic-Vucinic, D.; Gligorijevic, N.; Wimmer, L.; Gasparyan, M.; Lujic, T.; Vasovic, T.; Dailey, L.A.; Van Haute, S.; Cirkovic Velickovic, T. Small polystyrene microplastics interfere with the breakdown of milk proteins during static in vitro simulated human gastric digestion. Environ. Pollut. 2023, 335, 122282. [Google Scholar] [CrossRef]
- Ma, L.; Wu, Z.; Lu, Z.; Yan, L.; Dong, X.; Dai, Z.; Sun, R.; Hong, P.; Zhou, C.; Li, C. Differences in toxicity induced by the various polymer types of nanoplastics on HepG2 cells. Sci. Total Environ. 2024, 918, 170664. [Google Scholar] [CrossRef]
- Yang, Z.S.; Bai, Y.L.; Jin, C.H.; Na, J.; Zhang, R.; Gao, Y.; Pan, G.W.; Yan, L.J.; Sun, W. Evidence on Invasion of Blood, Adipose Tissues, Nervous System and Reproductive System of Mice After a Single Oral Exposure: Nanoplastics versus Microplastics. Biomed. Environ. Sci. 2022, 35, 1025–1037. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in human urine: Characterisation using μFTIR and sampling challenges using healthy donors and endometriosis participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Shi, C.; Lv, J.; Xu, Y.; Yang, J.; Chua, S.L.; Jia, L.; Chen, H.; Liu, Q.; et al. Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation. Nat. Nanotechnol. 2023, 18, 403–411. [Google Scholar] [CrossRef]
- Fournier, E.; Ratel, J.; Denis, S.; Leveque, M.; Ruiz, P.; Mazal, C.; Amiard, F.; Edely, M.; Bezirard, V.; Gaultier, E.; et al. Exposure to polyethylene microplastics alters immature gut microbiome in an infant in vitro gut model. J. Hazard. Mater. 2023, 443, 130383. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. Microplastics: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Zhou, X.; Su, L.; Wang, M.; Wang, T.; Zhang, H. Nanoplastic-induced vascular endothelial injury and coagulation dysfunction in mice. Sci. Total Environ. 2023, 865, 161271. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef]
- Du, T.; Yu, X.; Shao, S.; Li, T.; Xu, S.; Wu, L. Aging of Nanoplastics Significantly Affects Protein Corona Composition Thus Enhancing Macrophage Uptake. Environ. Sci. Technol. 2023, 57, 3206–3217. [Google Scholar] [CrossRef]
- Wen, J.; Sun, H.; Liu, Z.; Zhu, X.; Qin, Z.; Song, E.; Song, Y. Aging Processes Dramatically Alter the Protein Corona Constitution, Cellular Internalization, and Cytotoxicity of Polystyrene Nanoplastics. Environ. Sci. Technol. Lett. 2022, 9, 962–968. [Google Scholar] [CrossRef]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of Shape, Size, and Aspect Ratio on Nanoparticle Penetration and Distribution inside Solid Tissues Using 3D Spheroid Models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailänder, V. How shape influences uptake: Interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 2012, 8, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Rahman, M.M.; Akbor, M.A.; Siddique, M.A.B.; Uddin, M.K.; Malafaia, G. Is there tea complemented with the appealing flavor of microplastics? A pioneering study on plastic pollution in commercially available tea bags in Bangladesh. Sci. Total Environ. 2022, 837, 155833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, L.; Jiang, Y.; Zhang, Y.; Fan, Y.; Rao, W.; Qian, X. Microplastics in infant milk powder. Environ. Pollut. 2023, 323, 121225. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Rahman, M.M.; Hossain, M.N.; Uddin, M.K.; Malafaia, G. Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci. Total Environ. 2022, 837, 155849. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in honey, beer, milk and refreshments in Ecuador as emerging contaminants. Sustainbility 2020, 12, 5514. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Ho, Y.W.; Lim, J.Y.; Yeoh, Y.K.; Chiou, J.C.; Zhu, Y.; Lai, K.P.; Li, L.; Chan, P.K.S.; Fang, J.K.H. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Massardo, S.; Verzola, D.; Alberti, S.; Caboni, C.; Santostefano, M.; Eugenio Verrina, E.; Angeletti, A.; Lugani, F.; Ghiggeri, G.M.; Bruschi, M.; et al. MicroRaman spectroscopy detects the presence of microplastics in human urine and kidney tissue. Environ. Int. 2024, 184, 108444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Demirkaya Miloglu, F.; Kilic Baygutalp, N.; Ceylan, O.; Yildirim, S.; Eser, G.; Gul, H.İ. Higher number of microplastics in tumoral colon tissues from patients with colorectal adenocarcinoma. Environ. Chem. Lett. 2023, 21, 639–646. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue accumulation of microplastics and potential health risks in human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Wei, H.; Xia, Y.; Luo, Y. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Sadofsky, L.; Palmer, T.M. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS ONE 2023, 18, e0280594. [Google Scholar] [CrossRef]
- Guan, Q.; Jiang, J.; Huang, Y.; Wang, Q.; Liu, Z.; Ma, X.; Yang, X.; Li, Y.; Wang, S.; Cui, W.; et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mater. 2023, 442, 130138. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Gao, X.; Du, J.; Sui, H.; Wu, J.; Zhong, Y.; Liang, B.; Huang, Y.; Ye, R.; et al. Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy. Toxics 2023, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy evidence of microplastics in human semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association Between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2023, 57, 17774–17785. [Google Scholar] [CrossRef] [PubMed]
- Weingrill, R.B.; Lee, M.J.; Benny, P.; Riel, J.; Saiki, K.; Garcia, J.; Oliveira, L.F.A.d.M.; Fonseca, E.J.d.S.; Souza, S.T.d.; D’Amato, F.d.O.S.; et al. Temporal trends in microplastic accumulation in placentas from pregnancies in Hawaiʻi. Environ. Int. 2023, 180, 108220. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xu, Z.; Hu, X.; Lu, Y.; Zhao, Y.; Zhang, H. Microplastics in maternal amniotic fluid and their associations with gestational age. Sci. Total Environ. 2024, 920, 171044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Y.; Maimaiti, Z.; Fu, J.; Yang, F.; Li, Z.Y.; Shi, Y.; Hao, L.B.; Chen, J.Y.; Xu, C. Identification and analysis of microplastics in human lower limb joints. J. Hazard. Mater. 2024, 461, 132640. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Zhang, L.; Ma, D.; Wen, K.; Cai, J.; Cai, Z.; Wang, C.; Chai, X.; Zhong, J.; et al. Revealing new insights: Two-center evidence of microplastics in human vitreous humor and their implications for ocular health. Sci. Total Environ. 2024, 921, 171109. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Ellis-Hutchings, R.; Thornton Hampton, L.M.; Lemieux, C.L.; Wright, S.L. Screening and prioritization of nano- and microplastic particle toxicity studies for evaluating human health risks—Development and application of a toxicity study assessment tool. Microplastics Nanoplastics 2022, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Thornton Hampton, L.M.; Bouwmeester, H.; Brander, S.M.; Coffin, S.; Cole, M.; Hermabessiere, L.; Mehinto, A.C.; Miller, E.; Rochman, C.M.; Weisberg, S.B. Research recommendations to better understand the potential health impacts of microplastics to humans and aquatic ecosystems. Microplastics Nanoplastics 2022, 2, 18. [Google Scholar] [CrossRef]
- Materials Market Report; Textile Exchange: Lamesa, TX, USA, 2023; p. 75.
- Jones, J.I.; Vdovchenko, A.; Cooling, D.; Murphy, J.F.; Arnold, A.; Pretty, J.L.; Spencer, K.L.; Markus, A.A.; Vethaak, A.D.; Resmini, M. Systematic analysis of the relative abundance of polymers occurring as microplastics in freshwaters and estuaries. Int. J. Environ. Res. Public Health 2020, 17, 9304. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2017, 120, 2017. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, H.; Ghayebzadeh, M.; Ganji, F.; Mousavi, S.; Azizi, N. Tracking microplastics contamination in drinking water in Zahedan, Iran: From source to consumption taps. Sci. Total Environ. 2023, 872, 162121. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Hensel, F.; Gomiero, A.; Iordachescu, L.; Vianello, A.; Wittgren, H.B.; Vollertsen, J. Drinking plastics?—Quantification and qualification of microplastics in drinking water distribution systems by µFTIR and Py-GCMS. Water Res. 2021, 188, 116519. [Google Scholar] [CrossRef] [PubMed]
- Montarsolo, A.; Mossotti, R.; Patrucco, A.; Caringella, R.; Zoccola, M.; Pozzo, P.D.; Tonin, C. Study on the microplastics release from fishing nets. Eur. Phys. J. Plus 2018, 133, 494. [Google Scholar] [CrossRef]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential microplastic release from beached fishing gear in Great Britain’s region of highest fishing litter density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef]
- Battaglini, E.; Miralles, P.; Lotti, N.; Soccio, M.; Fiorini, M.; Coscollà, C. Analysis of microplastics in commercial vegetable edible oils from Italy and Spain. Food Chem. 2024, 443, 138567. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Blanca, V.; Edo, C.; González-Pleiter, M.; Albentosa, M.; Bayo, J.; Beiras, R.; Fernández-Piñas, F.; Gago, J.; Gómez, M.; Gonzalez-Cascon, R.; et al. Occurrence and size distribution study of microplastics in household water from different cities in continental Spain and the Canary Islands. Water Res. 2023, 238, 120044. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from synthetic textiles as a major source of microplastics in the environment: A review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Dronjak, L.; Exposito, N.; Rovira, J.; Florencio, K.; Emiliano, P.; Corzo, B.; Schuhmacher, M.; Valero, F.; Sierra, J. Screening of microplastics in water and sludge lines of a drinking water treatment plant in Catalonia, Spain. Water Res. 2022, 225, 119185. [Google Scholar] [CrossRef] [PubMed]
- Negrete Velasco, A.; Ramseier Gentile, S.; Zimmermann, S.; Le Coustumer, P.; Stoll, S. Contamination and removal efficiency of microplastics and synthetic fibres in a conventional drinking water treatment plant in Geneva, Switzerland. Sci. Total Environ. 2023, 880, 163270. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, Z.; Jamal, A.H.M.S.I.M.; Momtaz, N.; Beauty, S.A. Removal efficiencies of microplastics of the three largest drinking water treatment plants in Bangladesh. Sci. Total Environ. 2023, 895, 165155. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kauts, S.; Shabir, S.; Yousuf, S.; Mishra, Y.; Bhardwaj, R.; Milibari, A.A.; Singh, S.K.; Singh, M.P. The evidence of in-vivo and in-vitro studies on microplastic and nano plastic toxicity in mammals: A possible threat for an upcoming generation? Phys. Chem. Earth 2023, 132, 103511. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in Mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol. In Vitro 2021, 70, 105021. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brito, W.A.; Singer, D.; Miebach, L.; Saadati, F.; Wende, K.; Schmidt, A.; Bekeschus, S. Comprehensive in vitro polymer type, concentration, and size correlation analysis to microplastic toxicity and inflammation. Sci. Total Environ. 2023, 854, 158731. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, A.F.R.M.; Jasinski, J.; Völkl, M.; Witzmann, T.; Meinhart, M.; Jérôme, V.; Kretschmer, W.P.; Freitag, R.; Senker, J.; Fery, A.; et al. Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses. J. Hazard. Mater. 2022, 425, 127961. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.B. The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Olga, V.; Xue, Y.; Lv, S.; Diao, X.; Zhang, Y.; Han, Q.; Zhou, H. The potential effects of microplastic pollution on human digestive tract cells. Chemosphere 2022, 291, 132714. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Shi, X.; Wang, Y.; Xu, S. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ. Pollut. 2023, 324, 121388. [Google Scholar] [CrossRef] [PubMed]
| Group | Abbreviation | Search Term 1 | Search Terms 2–3 |
|---|---|---|---|
| Seafood: invertebrates | I | microplastic * | 2) digestive OR gut OR ingestion OR uptake 3) Mussel OR oyster OR clam OR lobster OR shrimp OR sea urchin OR snail OR sea cucumbers OR crab |
| Fish | F | 2) digestive OR gut OR ingestion OR uptake 3) fish | |
| Drinking Water | DW | 2) drinking water | |
| Meat | M | 2) meat OR pork OR beef OR chicken OR goat OR turkey OR duck OR lamb OR buffalo | |
| Plant-Based food | P | 2) edible plant OR vegetable OR fruit OR herb OR plant based | |
| Humans | H | 2) digestive OR gut OR ingestion OR uptake OR internalization OR tissue OR organ OR bioaccumulation 3) human * |
| Shape Group | Terms Included |
|---|---|
| Fibres | Fibre, filament, thread, line, fibrous MP |
| Fragments | Fragment, angular, irregularly shaped |
| Films | Film, sheet, flat MP |
| Foams | Foam, sponge-like |
| Pellets | Pellet, bead, sphere |
| Food Group | Food Sources Included in the Database | N Studies |
|---|---|---|
| Seafood Invertebrates | Mussels, oysters, clams, lobsters, shrimps, sea urchins, snails, sea cucumbers, crabs | 139 |
| Fish | Freshwater and seawater fish, wild (only edible species) or farmed, canned fish, fishmeal | 135 |
| Drinking Water | Tap water, bottled water, borehole drinking water, water after purification in drinking water treatment plants | 83 |
| Meat | chicken, beef, pork, chicken eggs, duck (edible species) | 8 |
| Plant-Based food | Edible fruits and vegetables, lettuce, vegetable oil, processed plant-based food | 5 |
| Health Status | Sample Analysed | N Patients | n MPs | Sizemin | Ref. |
|---|---|---|---|---|---|
| Gastrointestinal Tract | |||||
| healthy | stool | 8 | 727 | 50 μm | [39] |
| healthy and inflammatory bowel disease | stool | 102 | 8529 | 5 μm | [40] |
| healthy and liver cirrhosis | liver tissue | 6 | 102 | 4 μm | [41] |
| colorectal cancer and normal colon | colon tissue | 11 | 3638 | NM | [42] |
| healthy | stool | 8 | 129 | 30 μm | [43] |
| healthy | urine and kidney tissue | 10 | 26 | 1 μm | [44] |
| healthy | stool | 26 | 213 | 20 μm | [45] |
| healthy, colorectal adenocarcinoma | colon tissues | 32 | 3 | 1 μm | [46] |
| various conditions | lung, intestine, tonsil tissues | 41 | 37 | 20 μm | [47] |
| Vascular system/body fluids | |||||
| thrombosis | thrombi | 26 | 1 | 2.1 μm | [48] |
| patients undergoing surgery | saphenous vein tissue | 5 | 102 | 5 μm | [49] |
| NM | blood, cerebrospinal fluid, effusions and cyst fluids | 104 | 17 | 2 μm | [50] |
| patients undergoing cardiac surgery | blood, vascular tissues | 15 | 17,961 | 20 μm | [51] |
| healthy | blood | 22 | 27 | 0.7 μm | [52] |
| atherosclerosis | coronary artery, carotid artery, carotid aortic samples | 17 | 628 | NM | [53] |
| Reproductive system and new-borns health | |||||
| healthy | breastmilk | 34 | 41 | 2 μm | [54] |
| healthy infants | meconium | 37 | 0 | 10 μm | [55] |
| preterm birth | amniotic fluid and placenta | 20 | 21 | 10 μm | [56] |
| healthy | semen | 10 | 16 | 2 μm | [57] |
| healthy | placentas and meconium | 36 | 941 | 20 μm | [58] |
| healthy | placentas | 30 | 142 | 1 μm | [59] |
| healthy, endometriosis | urine | 38 | 355 | 5 μm | [21] |
| acute caesarean sections | maternal amniotic fluid | 40 | 776 | 20 μm | [60] |
| healthy | testis, semen | 30 | 56 | 20 μm | [61] |
| Other systems | |||||
| hip or knee arthroplasty | lower limb joints | 45 | 343 | 11 μm | [62] |
| ocular diseases | vitreous samples | 49 | 1745 | 20 μm | [63] |
| Polymer | Global Production, Mt [66,67] | Chemical Structure | Examples of Uses [67] |
|---|---|---|---|
| PES (textiles) | 63.3 |  | Apparel, home textiles, water filtration systems, conveyor belts, safety belts, geotextiles, non-woven fabrics, medical masks, etc. |
| PES (PET) | 27.6 | Bottles for water, soft drinks, juices, cleaners, etc., food jars/pots, clothes, plastic films, microwavable packaging, etc. | |
| PA | 6.1 |  | Fibres, bristles for toothbrushes, tubing, clothing, fishing lines and nets, conveyor belts, food packaging, medical devices, etc. |
| PU | 28.4 | 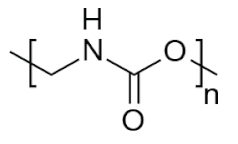 | Building insulation, pillows and mattresses, insulating foams, surface coatings, rollers for printing used in cars, carpet, flexible foam in furniture, elastic fibre, etc. |
| PP | 69.3 |  | Food packaging, sweets and snacks, wrappers, hinged caps, microwave containers, pipes, automotive parts, bank notes, etc. |
| PAC | - | 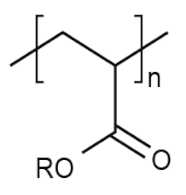 | diapers, sanitary napkins, textile finishing, nail polishes, and skincare products, contact lenses, touch screens, paper coatings, etc. |
| PE | 106.6 | 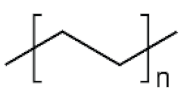 | Reusable bags, trays and containers, agricultural film, food packaging film, toys, milk bottles, shampoo bottles, pipes, houseware, etc. |
| PVC | 35.9 |  | Window frames, pipes, plumbing and guttering, shower curtains, synthetic leather, cosmetic containers, commercial cling wrap, etc. |
| PS | 23.0 | 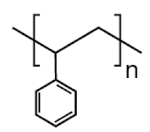 | Food packaging (dairy, fishery), electrical and electronic equipment, inner liner for fridges, eyeglasses frames, food containers, disposable cups, plates and cutlery, boxes for compact discs and cassettes, etc. |
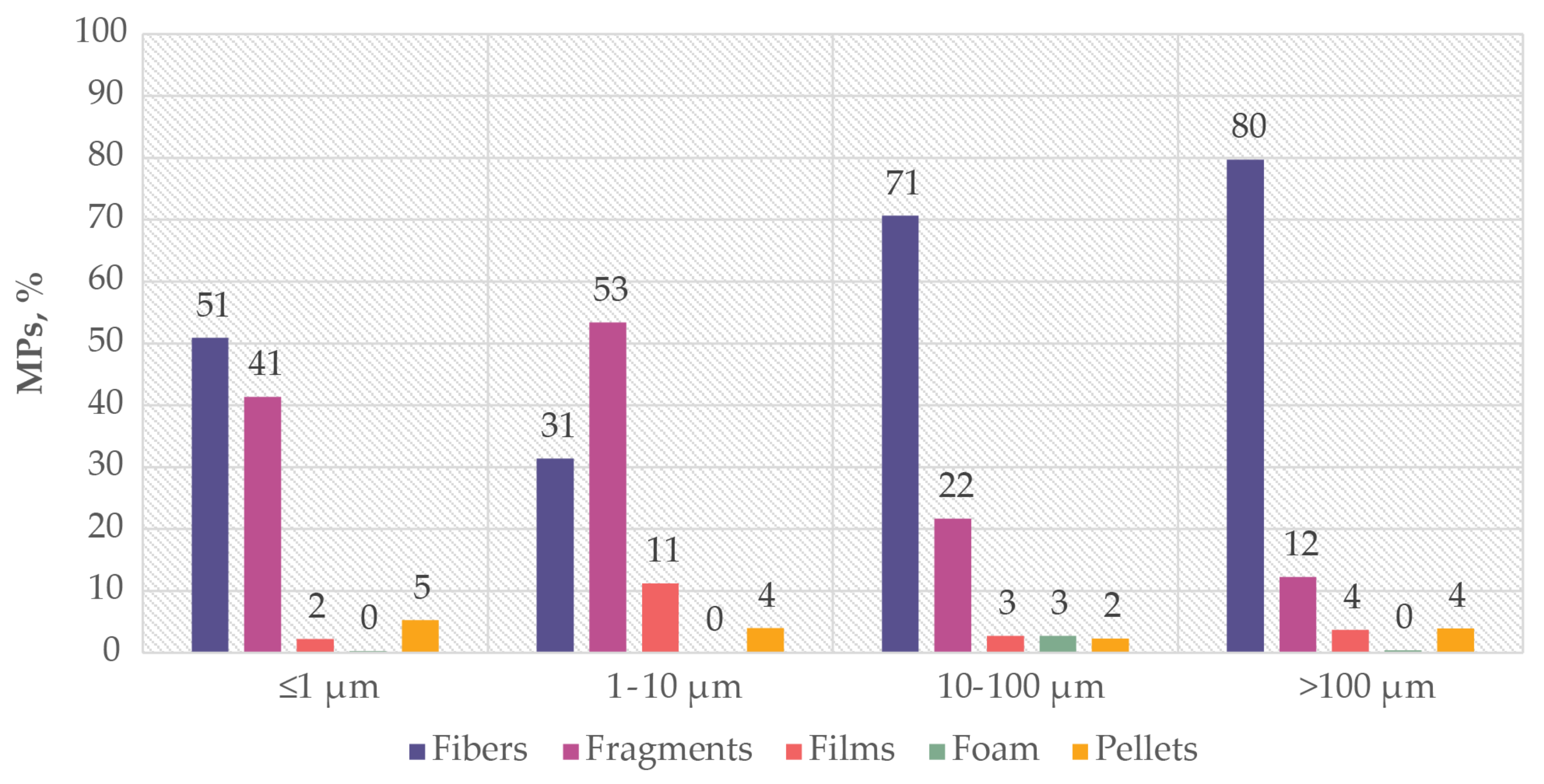
| Size Group | N Studies | n MPs | MPs/Study |
|---|---|---|---|
| ≤1 μm | 38 | 115,183 | 3031 |
| 1–10 μm | 63 | 59,038 | 937 |
| 10–100 μm | 147 | 106,660 | 726 |
| >100 μm | 84 | 17,696 | 211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. https://doi.org/10.3390/ijms25137074
Vdovchenko A, Resmini M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. International Journal of Molecular Sciences. 2024; 25(13):7074. https://doi.org/10.3390/ijms25137074
Chicago/Turabian StyleVdovchenko, Alena, and Marina Resmini. 2024. "Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review" International Journal of Molecular Sciences 25, no. 13: 7074. https://doi.org/10.3390/ijms25137074
APA StyleVdovchenko, A., & Resmini, M. (2024). Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. International Journal of Molecular Sciences, 25(13), 7074. https://doi.org/10.3390/ijms25137074







