Prediction of the Aggressive Clinical Course of Papillary Thyroid Carcinoma Based on Fine Needle Aspiration Biopsy Molecular Testing
Abstract
1. Introduction
2. Results
- The TPO (thyroid peroxidase) gene: the decline in its expression is associated with resistance to radioactive iodine therapy [29]. We detected that TPO expression depends on tumor size while being weakly associated with extrathyroidal invasion (p = 0.01) and a high/low recurrence risk (p = 0.02). Taking into account the fact that radioactive iodine-resistant tumors are more likely to be large-sized, it appears that thyroid peroxidase activity is its consequence rather than a cause.
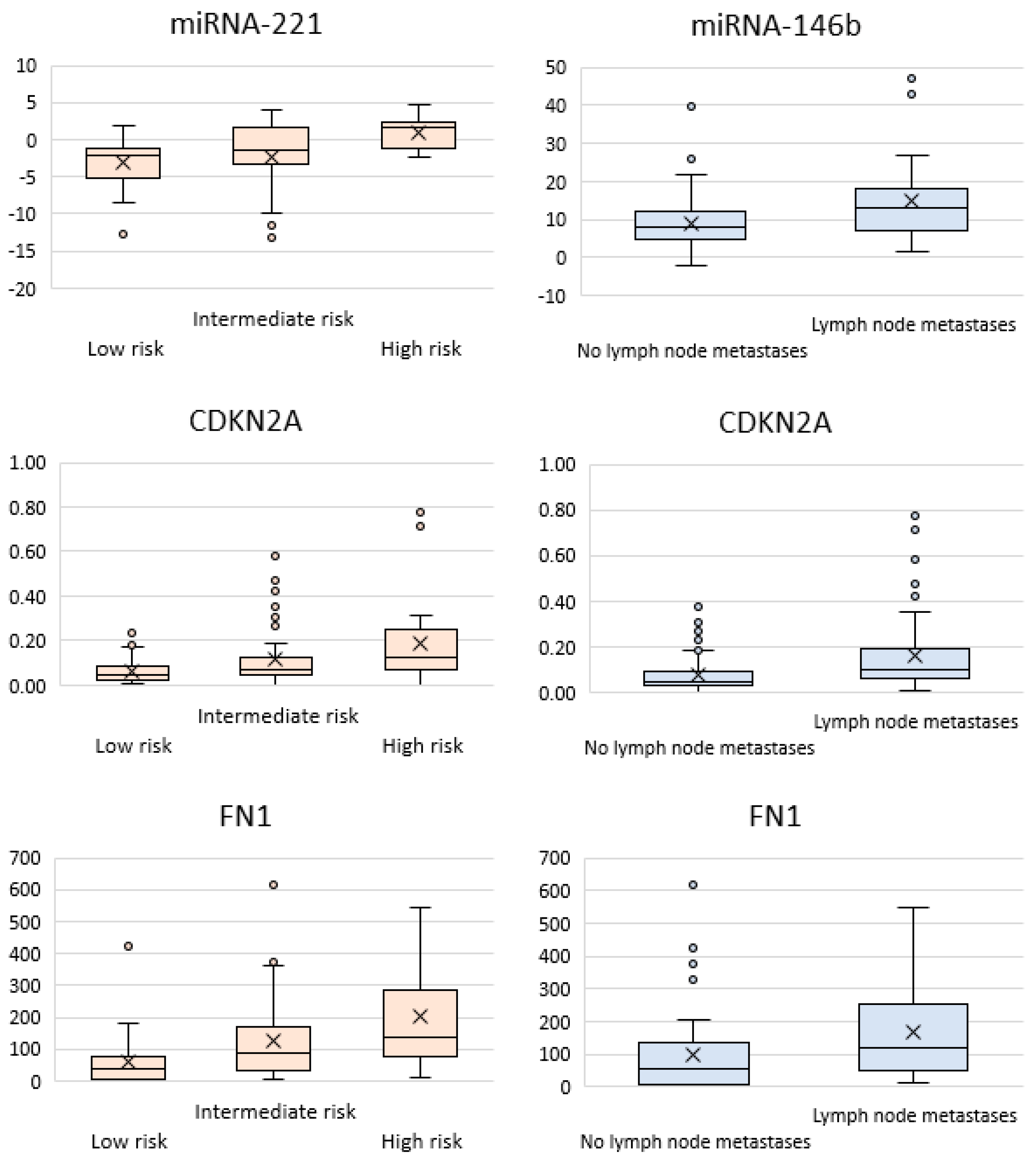
- The CITED1 gene, which is associated with the development of follicular cancer [28]. Differences were observed for such parameters as cervical lymph node metastases (p = 0.01), extrathyroidal extension (p = 0.02), and high/low recurrence risk (p = 0.004).
- The HMGA2 gene: expression of this gene is believed to be associated with lymphogenic metastasis and vascular invasion [30]. According to our data, weak differences were observed for the groups of patients with/without metastases (p = 0.02), with/without extrathyroidal extension (p = 0.01), with/without vascular invasion (p = 0.05), and with moderate/high recurrence risk (p = 0.01).
- The NIS (sodium/iodine symporter) gene, whose expression level is reduced in most thyroid carcinomas [31]. Differences were observed for the groups of patients with a low/high (p = 0.05) and moderate/high (p = 0.0049) recurrence risk.
- The CLU gene (clusterin alpha chain, an extracellular chaperone preventing the aggregation of non-native proteins) whose upregulated expression is associated with better survival prognosis [24]. Differences were observed in groups of patients with/without metastases (p = 0.005) and multifocal/unifocal cancer (p = 0.01).
- The SERPINA1 (serine protease inhibitor) gene: its association with the stage and the multifocal nature of thyroid cancer has been reported [32]. Differences were observed for the groups of patients with/without metastases (p = 0.004).
- The TFF3 gene: its downregulated expression was observed in patients with follicular thyroid cancer [21]. Differences were detected in the groups with/without metastases (p = 0.02), with/without extrathyroidal extension (p = 0.002), and with a high/low (p = 0.01) and moderate/high risk (p = 0.003).
- The TMPRSS4 (transmembrane serine protease) gene is characterized by increased expression in patients with PTC [27]. Differences in groups of patients with/without metastases (p = 0.04), and with a low/intermediate (p = 0.05) and low/high (p = 0.01) recurrence risk.
3. Discussion
4. Materials and Methods
4.1. Clinical Material
4.2. Choosing the Set of Molecular Markers
4.3. Total Nucleic Acid Extraction
4.4. Semi-Quantification of Messenger RNA Level
4.5. MicroRNA Detection
4.6. Quantification of the Ratio between the Mitochondrial and Nuclear DNA Copy Number (the mtDNA/nDNA Ratio)
4.7. Detection of Somatic BRAF Mutation
4.8. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Thyroid cancer survival in the United States: Observational data from 1973 to 2005. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 440–444. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef]
- Sun, J.H.; Li, Y.R.; Chang, K.H.; Liou, M.J.; Lin, S.F.; Tsai, S.S.; Yu, M.C.; Hsueh, C.; Chen, S.T. Evaluation of recurrence risk in patients with papillary thyroid cancer through tumor-node-metastasis staging: A single-center observational study in Taiwan. Biomed. J. 2022, 45, 923–930. [Google Scholar] [CrossRef]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef]
- Valderrabano, P.; Leon, M.E.; Centeno, B.A.; Otto, K.J.; Khazai, L.; McCaffrey, J.C.; Russell, J.S.; McIver, B. Institutional prevalence of malignancy of indeterminate thyroid cytology is necessary but insufficient to accurately interpret molecular marker tests. Eur. J. Endocrinol. 2016, 174, 621–629. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., 3rd; et al. The American Association of Endocrine Surgeons Guidelines for the definitive surgical management of thyroid disease in adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef]
- Panebianco, F.; Nikitski, A.V.; Nikiforova, M.N.; Nikiforov, Y.E. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019, 8, 5831–5839. [Google Scholar] [CrossRef]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef]
- Patel, S.G.; Carty, S.E.; McCoy, K.L.; Ohori, N.P.; LeBeau, S.O.; Seethala, R.R.; Nikiforova, M.N.; Nikiforov, Y.E.; Yip, L. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery 2017, 161, 168–175. [Google Scholar] [CrossRef]
- Song, Y.S.; Lim, J.A.; Choi, H.; Won, J.K.; Moon, J.H.; Cho, S.W.; Lee, K.E.; Park, Y.J.; Yi, K.H.; Park, D.J.; et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016, 122, 1370–1379. [Google Scholar] [CrossRef]
- Yip, L.; Nikiforova, M.N.; Yoo, J.Y.; McCoy, K.L.; Stang, M.T.; Armstrong, M.J.; Nicholson, K.J.; Ohori, N.P.; Coyne, C.; Hodak, S.P.; et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients. Ann. Surg. 2015, 262, 519–525. [Google Scholar] [CrossRef]
- Schumm, M.A.; Shu, M.L.; Hughes, E.G.; Nikiforov, Y.E.; Nikiforova, M.N.; Wald, A.I.; Lechner, M.G.; Tseng, C.H.; Sajed, D.P.; Wu, J.X.; et al. Prognostic Value of Preoperative Molecular Testing and Implications for Initial Surgical Management in Thyroid Nodules Harboring Suspected (Bethesda V) or Known (Bethesda VI) Papillary Thyroid Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 735–742. [Google Scholar] [CrossRef]
- Yip, L.; Gooding, W.E.; Nikitski, A.; Wald, A.I.; Carty, E.; Karslioglu-French, E.; Seethala, R.R.; Zandberg, D.P.; Ferris, R.L.; Nikiforova, M.N.; et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: A matched case-control study. Cancer 2021, 127, 1779–1787. [Google Scholar] [CrossRef]
- Liu, J.B.; Baugh, K.A.; Ramonell, K.M.; McCoy, K.L.; Karslioglu-French, E.; Morariu, E.M.; Ohori, N.P.; Nikiforova, M.N.; Nikiforov, Y.E.; Carty, S.E.; et al. Molecular Testing Predicts Incomplete Response to Initial Therapy in Differentiated Thyroid Carcinoma Without Lateral Neck or Distant Metastasis at Presentation: Retrospective Cohort Study. Thyroid 2023, 33, 705–714. [Google Scholar] [CrossRef]
- Zafon, C.; Gil, J.; Pérez-González, B.; Jordà, M. DNA methylation in thyroid cancer. Endocr. Relat. Cancer 2019, 26, R415–R439. [Google Scholar] [CrossRef]
- Rogucki, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 2021, 10, 4738. [Google Scholar] [CrossRef]
- Nieto, H.R.; Thornton, C.E.M.; Brookes, K.; Nobre de Menezes, A.; Fletcher, A.; Alshahrani, M.; Kocbiyik, M.; Sharma, N.; Boelaert, K.; Cazier, J.B.; et al. Recurrence of Papillary Thyroid Cancer: A Systematic Appraisal of Risk Factors. J. Clin. Endocrinol. Metab. 2022, 107, 1392–1406. [Google Scholar] [CrossRef]
- Titov, S.E.; Ivanov, M.K.; Demenkov, P.S.; Katanyan, G.A.; Kozorezova, E.S.; Malek, A.V.; Veryaskina, Y.A.; Zhimulev, I.F. Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears. BMC Cancer 2019, 19, 1010. [Google Scholar] [CrossRef]
- Ravi, N.; Yang, M.; Mylona, N.; Wennerberg, J.; Paulsson, K. Global RNA Expression and DNA Methylation Patterns in Primary Anaplastic Thyroid Cancer. Cancers 2020, 12, 680. [Google Scholar] [CrossRef]
- Wojtas, B.; Pfeifer, A.; Oczko-Wojciechowska, M.; Krajewska, J.; Czarniecka, A.; Kukulska, A.; Eszlinger, M.; Musholt, T.; Stokowy, T.; Swierniak, M.; et al. Gene Expression (mRNA) Markers for Differentiating between Malignant and Benign Follicular Thyroid Tumours. Int. J. Mol. Sci. 2017, 18, 1184. [Google Scholar] [CrossRef]
- Poma, A.M.; Giannini, R.; Piaggi, P.; Ugolini, C.; Materazzi, G.; Miccoli, P.; Vitti, P.; Basolo, F. A six-gene panel to label follicular adenoma, low- and high-risk follicular thyroid carcinoma. Endocr. Connect. 2018, 7, 124–132. [Google Scholar] [CrossRef]
- Mussazhanova, Z.; Shimamura, M.; Kurashige, T.; Ito, M.; Nakashima, M.; Nagayama, Y. Causative role for defective expression of mitochondria-eating protein in accumulation of mitochondria in thyroid oncocytic cell tumors. Cancer Sci. 2020, 111, 2814–2823. [Google Scholar] [CrossRef]
- Nan, B.Y.; Xiong, G.F.; Zhao, Z.R.; Gu, X.; Huang, X.S. Comprehensive Identification of Potential Crucial Genes and miRNA-mRNA Regulatory Networks in Papillary Thyroid Cancer. Biomed Res. Int. 2021, 2021, 6752141. [Google Scholar] [CrossRef]
- Ma, J.; Han, W.; Lu, K. Comprehensive Pan-Cancer Analysis and the Regulatory Mechanism of ASF1B, a Gene Associated With Thyroid Cancer Prognosis in the Tumor Micro-Environment. Front. Oncol. 2021, 11, 711756. [Google Scholar] [CrossRef]
- Zafereo, M.; McIver, B.; Vargas-Salas, S.; Domínguez, J.M.; Steward, D.L.; Holsinger, F.C.; Kandil, E.; Williams, M.; Cruz, F.; Loyola, S.; et al. A Thyroid Genetic Classifier Correctly Predicts Benign Nodules with Indeterminate Cytology: Two Independent, Multicenter, Prospective Validation Trials. Thyroid 2020, 30, 704–712. [Google Scholar] [CrossRef]
- Kebebew, E.; Peng, M.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann. Surg. 2005, 242, 353–361. [Google Scholar] [CrossRef]
- Fryknäs, M.; Wickenberg-Bolin, U.; Göransson, H.; Gustafsson, M.G.; Foukakis, T.; Lee, J.J.; Landegren, U.; Höög, A.; Larsson, C.; Grimelius, L.; et al. Molecular markers for discrimination of benign and malignant follicular thyroid tumors. Tumour Biol. 2006, 27, 211–220. [Google Scholar]
- Colombo, C.; Minna, E.; Gargiuli, C.; Muzza, M.; Dugo, M.; De Cecco, L.; Pogliaghi, G.; Tosi, D.; Bulfamante, G.; Greco, A.; et al. The molecular and gene/miRNA expression profiles of radioiodine resistant papillary thyroid cancer. J. Exp. Clin. Cancer Res. 2020, 39, 245. [Google Scholar] [CrossRef]
- Binabaj, M.M.; Soleimani, A.; Rahmani, F.; Avan, A.; Khazaei, M.; Fiuji, H.; Soleimanpour, S.; Ryzhikov, M.; Ferns, G.A.; Bahrami, A.; et al. Prognostic value of high mobility group protein A2 (HMGA2) over-expression in cancer progression. Gene 2019, 706, 131–139. [Google Scholar] [CrossRef]
- Tavares, C.; Coelho, M.J.; Eloy, C.; Melo, M.; Gaspar da Rocha, A.; Pestana, A.; Batista, R.; Bueno Ferreira, L.; Rios, E.; Selmi-Ruby, S.; et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocr. Connect. 2018, 7, 78–90. [Google Scholar] [CrossRef]
- Chai, L.; Han, D.; Li, J.; Lv, Z. The construction and analysis of gene co-expression network of differentially expressed genes identifies potential biomarkers in thyroid cancer. Transl. Cancer Res. 2018, 7, 1235–1243. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Feng, J.; Shen, F.; Cai, W.; Gan, X.; Deng, X.; Xu, B. Survival of aggressive variants of papillary thyroid carcinoma in patients under 55 years old: A SEER population-based retrospective analysis. Endocrine 2018, 61, 499–505. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Jung, C.K.; Jung, S.H.; Jeon, S.; Jeong, Y.M.; Kim, Y.; Lee, S.; Bae, J.S.; Chung, Y.J. Risk Stratifcation Using a Novel Genetic Classifer Including PLEKHS1 Promoter Mutations for Diferentiated Thyroid Cancer with Distant Metastasis. Thyroid 2020, 30, 1589–1600. [Google Scholar] [CrossRef]
- Santiago, K.; Chen Wongworawat, Y.; Khan, S. Differential MicroRNA-Signatures in Thyroid Cancer Subtypes. J. Oncol. 2020, 2020, 2052396. [Google Scholar] [CrossRef]
- Lukyanov, S.A.; Sergiyko, S.V.; Titov, S.E.; Reshetov, I.V.; Veryaskina, Y.A.; Vazhenin, A.V.; Gostimsky, A.V.; Ippolitov, L.I.; Rogova, M.O. Stratification of papillary thyroid cancer relapse risk based on the results of molecular genetic studies. Head Neck Tumors 2020, 10, 93–100. (In Russian) [Google Scholar] [CrossRef]
- Titov, S.E.; Demenkov, P.S.; Lukyanov, S.A.; Sergiyko, S.V.; Katanyan, G.A.; Veryaskina, Y.A.; Ivanov, M.K. Preoperative detection of malignancy in fine-needle aspiration cytology (FNAC) smears with indeterminate cytology (Bethesda III, IV) by a combined molecular classifier. J. Clin. Pathol. 2020, 73, 722–727. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]

| Characteristic | N (%) |
|---|---|
| Median age (Q1–Q3) | 47.5 (37–60.3) |
| Sex ratio (male/female) | 22/86 |
| Metastases in central lymph nodes | 28 (26%) |
| Metastases in lateral lymph nodes | 25 (23.1%) |
| Multifocal nature | 61 (56.5%) |
| Extrathyroidal extension (macroscopic invasion) | 25 (23.1%) |
| Vascular invasion | 58 (53.4%) |
| Variants of PTC | |
| Classical | 35 (32.4%) |
| Oncocytic | 30 (27.8%) |
| Tall cell | 19 (17.6%) |
| Follicular | 15 (13.9%) |
| Warthin-like | 5 (4.6%) |
| Solid | 4 (3.7%) |
| ATA risk stratification | |
| Low risk | 23 (21.3%) |
| Intermediate risk | 60 (55.6%) |
| High risk | 25 (23.1%) |
| Parameter | Total Number | BRAF Mutations | Odds Ratio (95% CI) | p | |
|---|---|---|---|---|---|
| yes | no | ||||
| Sex | |||||
| females | 86 | 64 | 22 | 0.84 (0.26–2.76) | 0.78 |
| males | 22 | 18 | 4 | ||
| Multifocal nature | |||||
| unifocal | 47 | 35 | 12 | 0.86 (0.35–2.1) | 0.75 |
| multifocal | 61 | 47 | 14 | ||
| Extrathyroidal extension | |||||
| no | 25 | 21 | 4 | 0.52 (0.16–1.71) | 0.28 |
| yes | 83 | 61 | 22 | ||
| Metastases to the cervical lymph nodes | |||||
| no | 55 | 39 | 16 | 0.56 (0.23–1.39) | 0.21 |
| yes | 53 | 43 | 10 | ||
| Vascular invasion | |||||
| no | 50 | 34 | 16 | 0.44 (0.17–1.09) | 0.07 |
| yes | 58 | 48 | 10 | ||
| ATA recurrence risk | |||||
| low | 23 | 13 | 10 | low/intermediate 0.32 (0.11–0.91) | 0.03 |
| intermediate | 60 | 48 | 12 | intermediate/high 0.76 (0.22–2.6) | 0.66 |
| high | 25 | 21 | 4 | low/high 0.24 (0.06–0.95) | 0.04 |
| Group | miR-146b | miR-199b | miR-221 | miR-223 | miR-31 | miR-375 |
| Metastases to cervical lymph nodes | 0.0003 | 0.84 | 0.01 | 0.05 | 0.04 | 0.14 |
| Extrathyroidal extension | 0.15 | 0.23 | 0.00006 | 0.38 | 0.01 | 0.02 |
| Vascular invasion | 0.38 | 0.24 | 0.58 | 0.98 | 0.28 | 0.09 |
| Multifocal nature | 0.12 | 0.39 | 0.84 | 0.04 | 0.18 | 0.95 |
| Low/intermediate | 0.04 | 0.60 | 0.06 | 0.16 | 0.87 | 0.1 |
| Low/high | 0.02 | 0.59 | 0.00001 | 0.10 | 0.09 | 0.007 |
| Intermediate/high | 0.4 | 0.18 | 0.001 | 0.6 | 0.01 | 0.08 |
| Group | miR-451a | miR-551b | miR-148b | miR-21 | miR-125b | mtDNA |
| Metastases to cervical lymph nodes | 0.20 | 0.07 | 0.04 | 0.41 | 0.34 | 0.01 |
| Extrathyroidal extension | 0.55 | 0.01 | 0.90 | 0.95 | 0.03 | 0.58 |
| Vascular invasion | 0.93 | 0.22 | 0.70 | 0.26 | 0.63 | 0.05 |
| Multifocal nature | 0.26 | 0.21 | 0.005 | 0.48 | 0.89 | 0.01 |
| Low/intermediate | 0.18 | 0.20 | 0.07 | 0.17 | 0.98 | 0.004 |
| Low/high | 0.15 | 0.01 | 0.18 | 0.50 | 0.11 | 0.01 |
| Intermediate/high | 0.91 | 0.02 | 0.65 | 0.70 | 0.03 | 0.72 |
| Group | FN1 | GMNN | CDKN2A | TIMP1 | CITED1 | TPO |
| Metastases to cervical lymph nodes | 0.0004 | 0.25 | 0.00015 | 0.05 | 0.01 | 0.09 |
| Extrathyroidal extension | 0.002 | 0.85 | 0.003 | 0.27 | 0.02 | 0.02 |
| Vascular invasion | 0.33 | 0.26 | 0.24 | 0.83 | 0.63 | 0.28 |
| Multifocal nature | 0.11 | 0.74 | 0.05 | 0.12 | 0.09 | 0.57 |
| Low/intermediate | 0.001 | 0.71 | 0.03 | 0.38 | 0.09 | 0.12 |
| Low/high | 0.00006 | 0.88 | 0.0012 | 0.20 | 0.004 | 0.02 |
| Intermediate/high | 0.03 | 0.74 | 0.01 | 0.40 | 0.08 | 0.06 |
| Group | SLC26A7 | HMGA2 | CPQ | RXRG | SPATA18 | APOE |
| Metastases to cervical lymph nodes | 0.53 | 0.02 | 0.59 | 0.46 | 0.05 | 0.54 |
| Extrathyroidal extension | 0.06 | 0.01 | 0.81 | 0.65 | 0.82 | 0.12 |
| Vascular invasion | 0.64 | 0.05 | 0.35 | 0.7 | 0.10 | 0.53 |
| Multifocal nature | 0.69 | 0.61 | 0.47 | 0.84 | 0.14 | 0.80 |
| Low/intermediate | 0.96 | 0.71 | 0.05 | 0.34 | 0.35 | 0.13 |
| Low/high | 0.28 | 0.18 | 0.22 | 0.81 | 0.50 | 0.05 |
| Intermediate/high | 0.05 | 0.01 | 0.38 | 0.48 | 0.99 | 0.25 |
| Group | ASF1B | AFAP1L2 | CLU | ECM1 | DIO1 | NIS |
| Metastases to cervical lymph nodes | 0.40 | 0.82 | 0.005 | 0.91 | 0.61 | 0.07 |
| Extrathyroidal extension | 0.27 | 0.73 | 0.80 | 0.54 | 0.43 | 0.005 |
| Vascular invasion | 0.15 | 0.17 | 0.47 | 0.88 | 0.61 | 0.81 |
| Multifocal nature | 0.94 | 0.99 | 0.01 | 0.17 | 0.98 | 0.06 |
| Low/intermediate | 0.05 | 0.91 | 0.28 | 0.48 | 0.49 | 0.82 |
| Low/high | 0.74 | 0.81 | 0.56 | 0.86 | 0.38 | 0.05 |
| Intermediate/high | 0.11 | 0.73 | 0.55 | 0.47 | 0.53 | 0.0049 |
| Group | SERPINA1 | TFF3 | TMPRSS4 | TSHR | ||
| Metastases to cervical lymph nodes | 0.004 | 0.02 | 0.04 | 0.39 | ||
| Extrathyroidal extension | 0.47 | 0.002 | 0.12 | 0.48 | ||
| Vascular invasion | 0.28 | 0.84 | 0.07 | 0.81 | ||
| Multifocal nature | 0.19 | 0.18 | 0.78 | 0.05 | ||
| Low/intermediate | 0.04 | 0.34 | 0.05 | 0.31 | ||
| Low/high | 0.09 | 0.01 | 0.01 | 0.21 | ||
| Intermediate/high | 0.8 | 0.003 | 0.36 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukyanov, S.A.; Titov, S.E.; Kozorezova, E.S.; Demenkov, P.S.; Veryaskina, Y.A.; Korotovskii, D.V.; Ilyina, T.E.; Vorobyev, S.L.; Zhivotov, V.A.; Bondarev, N.S.; et al. Prediction of the Aggressive Clinical Course of Papillary Thyroid Carcinoma Based on Fine Needle Aspiration Biopsy Molecular Testing. Int. J. Mol. Sci. 2024, 25, 7090. https://doi.org/10.3390/ijms25137090
Lukyanov SA, Titov SE, Kozorezova ES, Demenkov PS, Veryaskina YA, Korotovskii DV, Ilyina TE, Vorobyev SL, Zhivotov VA, Bondarev NS, et al. Prediction of the Aggressive Clinical Course of Papillary Thyroid Carcinoma Based on Fine Needle Aspiration Biopsy Molecular Testing. International Journal of Molecular Sciences. 2024; 25(13):7090. https://doi.org/10.3390/ijms25137090
Chicago/Turabian StyleLukyanov, Sergei A., Sergei E. Titov, Evgeniya S. Kozorezova, Pavel S. Demenkov, Yulia A. Veryaskina, Denis V. Korotovskii, Tatyana E. Ilyina, Sergey L. Vorobyev, Vladimir A. Zhivotov, Nikita S. Bondarev, and et al. 2024. "Prediction of the Aggressive Clinical Course of Papillary Thyroid Carcinoma Based on Fine Needle Aspiration Biopsy Molecular Testing" International Journal of Molecular Sciences 25, no. 13: 7090. https://doi.org/10.3390/ijms25137090
APA StyleLukyanov, S. A., Titov, S. E., Kozorezova, E. S., Demenkov, P. S., Veryaskina, Y. A., Korotovskii, D. V., Ilyina, T. E., Vorobyev, S. L., Zhivotov, V. A., Bondarev, N. S., Sleptsov, I. V., & Sergiyko, S. V. (2024). Prediction of the Aggressive Clinical Course of Papillary Thyroid Carcinoma Based on Fine Needle Aspiration Biopsy Molecular Testing. International Journal of Molecular Sciences, 25(13), 7090. https://doi.org/10.3390/ijms25137090






