Plasma miRNA-146b-3p, -222-3p, -221-5p, -21a-3p Expression Levels and TSHR Methylation: Diagnostic Potential and Association with Clinical and Pathological Features in Papillary Thyroid Cancer
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Expression of miRNA-146b-3p, -222-3p, -21a-3p, and -221-5p and Methylation of TSHR in Plasma Samples of Patients with PTC and HCs
2.3. Plasma miRNA Expression and TSHR Methylation Levels in Patients with PTC before and after Thyroidectomy
2.4. Association of TSHR Methylation Level and miRNA-221-5p, miRNA-21a-3p, miRNA-222-3p, and miRNA-146b-3p Expression with Clinicopathological Features of PTC
2.5. Correlation of miRNA Expression with TSHR Methylation Levels
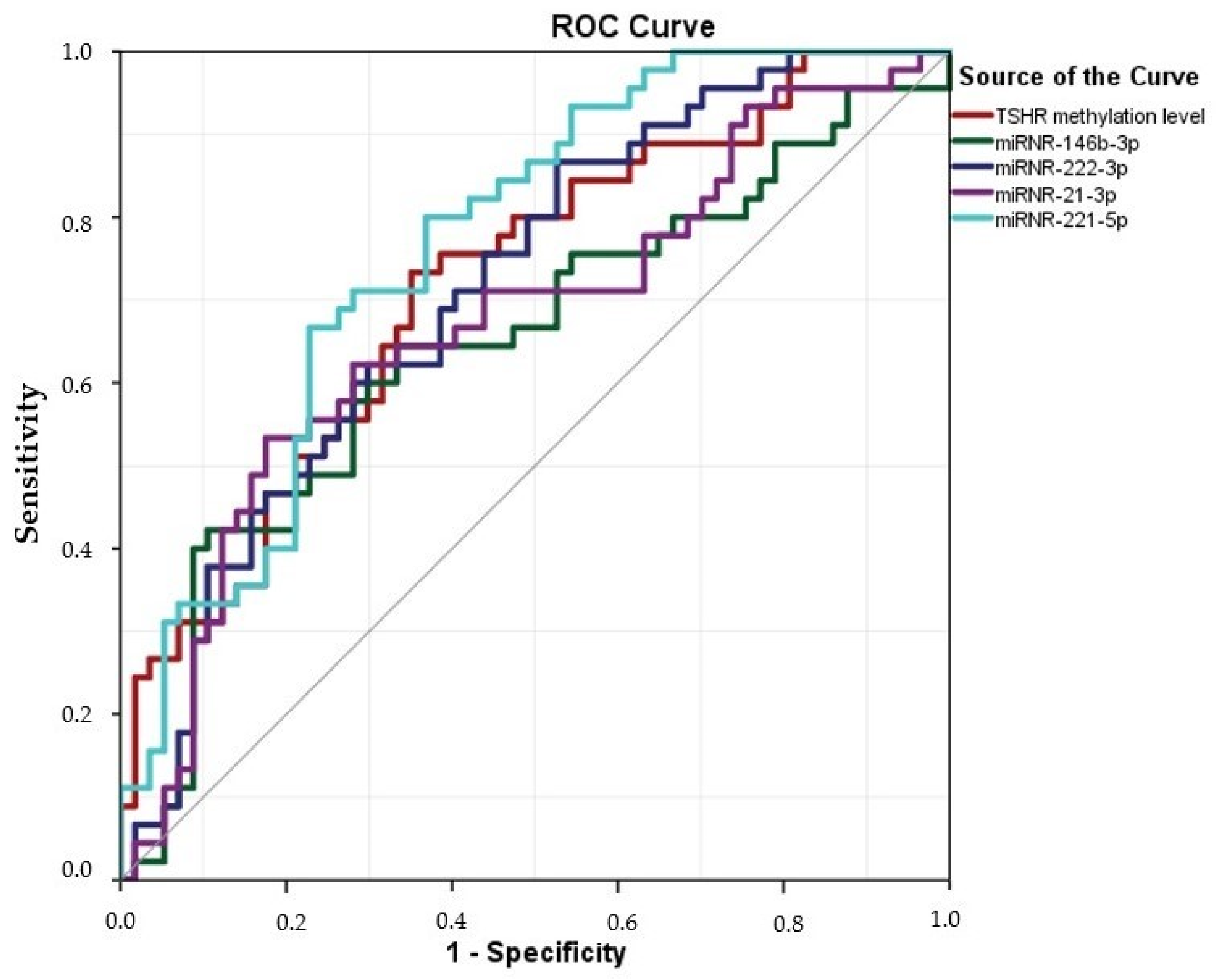
2.6. The Value of miRNA-146b-3p, -222-3p, -21a-3p, -221-5p, and TSHR Methylation Assays in PTC Plasma Samples for Diagnosis of PTC
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. DNA Samples
4.3. DNA and RNA Extraction
4.4. Bisulfite Conversion and Quantitative Methylation-Specific PCR
4.5. Quantitative Reverse Transcription–Polymerase Chain Reaction
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limaiem, F.; Rehman, A.; Anastasopoulou, C.; Mazzoni, T. Papillary thyroid carcinoma. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Fukushima, M.; Higashiyama, T.; Miya, A. Overall survival of papillary thyroid carcinoma patients: A single-institution long-term follow-up of 5897 patients. World J. Surg. 2018, 42, 615–622. [Google Scholar] [CrossRef]
- Medas, F.; Canu, G.L.; Boi, F.; Lai, M.L.; Erdas, E.; Calò, P.G. Predictive factors of recurrence in patients with differentiated thyroid carcinoma: A retrospective analysis on 579 patients. Cancers 2019, 11, 1230. [Google Scholar] [CrossRef]
- Lewinski, A.; Adamczewski, Z. Papillary thyroid carcinoma: A cancer with an extremely diverse genetic background and prognosis. Pol. Arch. Intern. Med. 2017, 127, 388–389. [Google Scholar] [CrossRef]
- Ward, L.S.; Kloos, R.T. Marcadores moleculares no diagnóstico de nódulos tireoidianos. Arq. Bras. Endocrinol. Metabol. 2013, 57, 89–97. [Google Scholar] [CrossRef]
- Mon, S.Y.; Riedlinger, G.; Abbott, C.E.; Seethala, R.; Ohori, N.P.; Nikiforova, M.N.; Nikiforov, Y.E.; Hodak, S.P. Cancer risk and clinicopathological characteristics of thyroid nodules harboring thyroid-stimulating hormone receptor gene mutations. Diagn. Cytopathol. 2018, 46, 369–377. [Google Scholar] [CrossRef]
- Farid, N.R.; Kascur, V.; Balazs, C. The human thyrotropin receptor is highly mutable: A review of gain-of-function mutations. Eur. J. Endocrinol. 2000, 143, 25–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alsina, J.; Alsina, R.; Gulec, S. A concise atlas of thyroid cancer next-generation sequencing panel ThyroSeq v. 2. Mol. Imaging Radionucl. Ther. 2017, 26 (Suppl. S1), 102–117. [Google Scholar] [CrossRef]
- Chu, Y.; Yeh, C. The molecular function and clinical role of thyroid stimulating hormone receptor in cancer cells. Cells 2020, 9, 1730. [Google Scholar] [CrossRef]
- Kartal, K.; Onder, S.; Kosemehmetoglu, K.; Kilickap, S.; Tezel, Y.G.; Kaynaroglu, V. Methylation status of TSHr in well-differentiated thyroid cancer by using cytologic material. BMC Cancer 2015, 15, 824. [Google Scholar] [CrossRef]
- Qu, M.; Wan, S.; Ren, B.; Wu, H.; Liu, L.; Shen, H. Association between TSHR gene methylation and papillary thyroid cancer: A meta-analysis. Endocrine 2020, 69, 508–515. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Yu, X.; Tao, Y.; Bode, A.M.; Dong, Z.; Cao, Y. Regulation of microRNAs by epigenetics and their interplay involved in cancer. J. Exp. Clin. Cancer Res. 2013, 32, 96. [Google Scholar] [CrossRef][Green Version]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Tan, W.; Liu, B.; Qu, S.; Liang, G.; Luo, W.; Gong, C. MicroRNAs and cancer: Key paradigms in molecular therapy. Oncol. Lett. 2018, 15, 2735–2742. [Google Scholar] [CrossRef]
- Occhipinti, G.; Giulietti, M.; Principato, G.; Piva, F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumour Biol. 2016, 37, 11657–11665. [Google Scholar] [CrossRef]
- Pardini, B.; Calin, G.A. MicroRNAs and long non-coding RNAs and their hormone-like activities in cancer. Cancers 2019, 11, 378. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Junit, S.M.; Ng, K.L.; Jayapalan, J.J.; Karikalan, B.; Hashim, O.H. Papillary thyroid cancer: Genetic alterations and molecular biomarker investigations. Int. J. Med. Sci. 2019, 16, 450–460. [Google Scholar] [CrossRef]
- Sondermann, A.; Andreghetto, F.M.; Moulatlet, A.C.B.; Victor, E.d.S.; de Castro, M.G.; Nunes, F.D.; Brandão, L.G.; Severino, P. MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin. Exp. Metastasis 2015, 32, 521–530. [Google Scholar] [CrossRef]
- Thomaidou, A.C.; Batsaki, P.; Adamaki, M.; Goulielmaki, M.; Baxevanis, C.N.; Zoumpourlis, V.; Fortis, S.P. Promising biomarkers in head and neck cancer: The most clinically important miRNAs. Int. J. Mol. Sci. 2022, 23, 8257. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Cui, S. Matrine inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/akt pathway. Oncol. Lett. 2018, 16, 2965–2970. [Google Scholar] [CrossRef]
- Visone, R.; Russo, L.; Pallante, P.; De Martino, I.; Ferraro, A.; Leone, V.; Borbone, E.; Petrocca, F.; Alder, H.; Croce, C.M.; et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr. Relat. Cancer 2007, 14, 791–798. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, L.; Luo, D.; Yan, F.; Liu, J.; Shao, S.; Zhao, L.; Jin, T.; Zhao, J.; Gao, L. MicroRNA-146b-3p promotes cell metastasis by directly targeting NF2 in human papillary thyroid cancer. Thyroid. 2018, 28, 1627–1641. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Claret, F.X. Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics 2017, 12, 187–197. [Google Scholar] [CrossRef]
- Saviana, M.; Le, P.; Micalo, L.; Del Valle-Morales, D.; Romano, G.; Acunzo, M.; Li, H.; Nana-Sinkam, P. Crosstalk between miRNAs and DNA methylation in cancer. Genes 2023, 14, 1075. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory mechanisms of epigenetic miRNA relationships in human cancer and potential as therapeutic targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Suzuki, H.; Maruyama, R.; Yamamoto, E.; Kai, M. DNA methylation and microRNA dysregulation in cancer. Mol. Oncol. 2012, 6, 567–578. [Google Scholar] [CrossRef]
- Woeller, C.F.; Roztocil, E.; Hammond, C.; Feldon, S.E. TSHR signaling stimulates proliferation through PI3K/akt and induction of miR-146a and miR-155 in thyroid eye disease orbital fibroblasts. Invest. Ophthalmol. Vis. Sci. 2019, 60, 4336–4345. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) as new tools for cancer therapy: First steps from bench to bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Abdul Manap, A.S.; Wisham, A.A.; Wong, F.W.; Ahmad Najmi, H.R.; Ng, Z.F.; Diba, R.S. Mapping the function of MicroRNAs as a critical regulator of tumor-immune cell communication in breast cancer and potential treatment strategies. Front. Cell Dev. Biol. 2024, 12, 1390704. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as biomarkers and therapeutic targets for cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Cheng, G. Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy. Adv. Drug Deliv. Rev. 2015, 81, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Asl, E.R.; Sarabandi, S.; Shademan, B.; Dalvandi, K.; Sheikhansari, G.; Nourazarian, A. MicroRNA targeting: A novel therapeutic intervention for ovarian cancer. Biochem. Biophys. Rep. 2023, 35, 101519. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Dolcimascolo, A.; Torrisi, F.; Zappala, A.; Gulino, R.; Parenti, R. MiR-19a overexpression in FTC-133 cell line induces a more de-differentiated and aggressive phenotype. Int. J. Mol. Sci. 2018, 19, 3944. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.; Chen Wongworawat, Y.; Khan, S. Differential MicroRNA-signatures in thyroid cancer subtypes. J. Oncol. 2020, 2020, 2052396. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, S.; Zarif-Yeganeh, M.Z.; Farashi, S.; Azizi, F.; Kia, S.K.; Teimoori-Toolabi, L.; Hedayati, M. Promoter methylation of tumor suppressors in thyroid carcinoma: A systematic review. Iran. J. Public Health 2021, 50, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European thyroid association clinical practice guidelines for thyroid nodule management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef] [PubMed]
- Geropoulos, G.; Psarras, K.; Papaioannou, M.; Giannis, D.; Meitanidou, M.; Kapriniotis, K.; Symeonidis n Pavlidis, E.T.; Pavlidis, T.E.; Sapalidis, K.; Ahmed, N.M.; et al. Circulating microRNAs and Clinicopathological Findings of Papillary Thyroid Cancer: A Systematic Review. In Vivo 2022, 36, 1551–1569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Zambrano, A.K. Differential microRNA expression for diagnosis and prognosis of papillary thyroid cancer. Front. Med. 2023, 10, 1139362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, A.; Shostrom, V.; Treude, K.; Lydiatt, W.; Smith, R.; Goldner, W. Serum Thyroglobulin: Preoperative Levels and Factors Affecting Postoperative Optimal Timing following Total Thyroidectomy. Int. J. Endocrinol. 2019, 2019, 1384651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torlontano, M.; Crocetti, U.; D’Aloiso, L.; Bonfitto, N.; Di Giorgio, A.; Modoni, S.; Valle, G.; Frusciante, V.; Bisceglia, M.; Filetti, S.; et al. Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2003, 148, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahraki, K.; Pak, V.I.; Najafi, A.; Shahraki, K.; Boroumand, P.G.; Sheervalilou, R. Non-coding RNA-mediated epigenetic alterations in grave’s ophthalmopathy: A scoping systematic review. Non-Coding RNA Res. 2023, 8, 426–450. [Google Scholar] [CrossRef] [PubMed]
- Nylén, C.; Mechera, R.; Maréchal-Ross, I.; Tsang, V.; Chou, A.; Gill, A.J.; Clifton-Bligh, R.J.; Robinson, B.G.; Sywak, M.S.; Sidhu, S.B.; et al. Molecular markers guiding thyroid cancer management. Cancers 2020, 12, 2164. [Google Scholar] [CrossRef] [PubMed]
- Spirina, L.V.; Kovaleva, I.V.; Chizhevskaya, S.Y.; Chebodaeva, A.V.; Tarasenko, N.V. Autophagy-related MicroRNA: Tumor miR-125b and thyroid cancers. Genes 2023, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yuan, Z.; Fan, Y.; Deng, X.; Zheng, Q. Integrated analyses of microRNA and mRNA expression profiles in aggressive papillary thyroid carcinoma. Mol. Med. Rep. 2013, 8, 1353–1358. [Google Scholar] [CrossRef]
- Lee, J.C.; Zhao, J.T.; Clifton-Bligh, R.J.; Gill, A.; Gundara, J.S.; Ip, J.C.; Glover, A.; Sywak, M.S.; Delbridge, L.W.; Robinson, B.G.; et al. MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer 2013, 119, 4358–4365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, J.; Xu, D.; Yang, Z.; Sun, J.; Sun, L.; Wu, Y.; Qiao, H. Combination of serum microRNAs and ultrasound profile as predictive biomarkers of diagnosis and prognosis for papillary thyroid microcarcinoma. Oncol. Rep. 2018, 40, 3611–3624. [Google Scholar] [CrossRef] [PubMed]
- Rogucki, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 2021, 10, 4738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panico, A.; Tumolo, M.R.; Leo, C.G.; Donno, A.; Grassi, T.; Bagordo, F.; Serio, F.; Idolo, A.; Masi, R.; Mincarone, P.; et al. The influence of lifestyle factors on miRNA expression and signal pathways: A review. Epigenomics 2021, 13, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, D.; Katare, R. Exosomal microRNAs in diabetic heart disease. Cardiovasc. Diabetol. 2022, 21, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 2021, 11, e468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keller, A.; Rounge, T.; Backes, C.; Ludwig, N.; Gislefoss, R.; Leidinger, P.; Langseth, H.; Meese, E. Sources to variability in circulating human miRNA signatures. RNA Biol. 2017, 14, 1791–1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garofalo, M.; Quintavalle, C.; Romano, G.; Croce, C.M.; Condorelli, G. miR221/222 in cancer: Their role in tumor progression and response to therapy. Curr. Mol. Med. 2012, 12, 27–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Said, M.N.; Muawia, S.; Helal, A.; Fawzy, A.; Allam, R.M.; Shafik, N.F. Regulation of CDK inhibitor p27 by microRNA 222 in breast cancer patients. Exp. Mol. Pathol. 2021, 123, 104718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Wang, F.; Xiao, J.J.; Song, Y.; Zhao, Y.Y.; Cao, Y.; Bei, Y.H.; Yang, C.Q. MiR-222 overexpression promotes proliferation of human hepatocellular carcinoma HepG2 cells by downregulating p27. Int. J. Clin. Exp. Med. 2014, 7, 893–902. [Google Scholar] [PubMed] [PubMed Central]
- Zhang, C.; Chang, Q.; Hu, Y.; Chang, W.; Guo, X.; Fu, L.; Tang, G.; Chen, C. MiR-222-3p promotes the proliferation, migration and invasion of papillary thyroid carcinoma cells through targeting SLC4A4. Histol. Histopathol. 2021, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiang, D.; Li, W.; Zheng, X.; Wang, L.; Li, Z.; Chen, T. BRAFV600E Mutation-Responsive miRNA-222-3p Promotes Metastasis of Papillary Thyroid Cancer Cells via Snail-Induced EMT. Front. Endocrinol. 2022, 13, 843334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weissenbacher, T.; Hirte, E.; Kuhn, C.; Janni, W.; Mayr, D.; Karsten, U.; Rack, B.; Friese, K.; Jeschke, U.; Heublein, S.; et al. Multicentric and multifocal versus unifocal breast cancer: Differences in the expression of E-cadherin suggest differences in tumor biology. BMC Cancer 2013, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Rebouissou, S.; de Koning, L.; Masmoudi, A.; Hérault, A.; Dubois, T.; Maille, P.; Soyeux, P.; Sibony, M.; de la Taille, A.; et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int. J. Cancer 2014, 134, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Govender, P.; Ghai, M.; Okpeku, M. Sex-specific DNA methylation: Impact on human health and development. Mol. Genet. Genom. 2022, 297, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age-Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, J.; Maitra, A. Maternal DNA Methylation During Pregnancy: A Review. Reprod. Sci. 2021, 28, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Simpkin, A.J.; Victora, C.G.; Relton, C.L.; Caramaschi, D. Association between Breastfeeding and DNA Methylation over the Life Course: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Nutrients 2020, 12, 3309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, R.; Sun, W.; Huang, J.; Shao, L.; Zhang, H. Sex-biased DNA methylation in papillary thyroid cancer. Biomark. Med. 2021, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
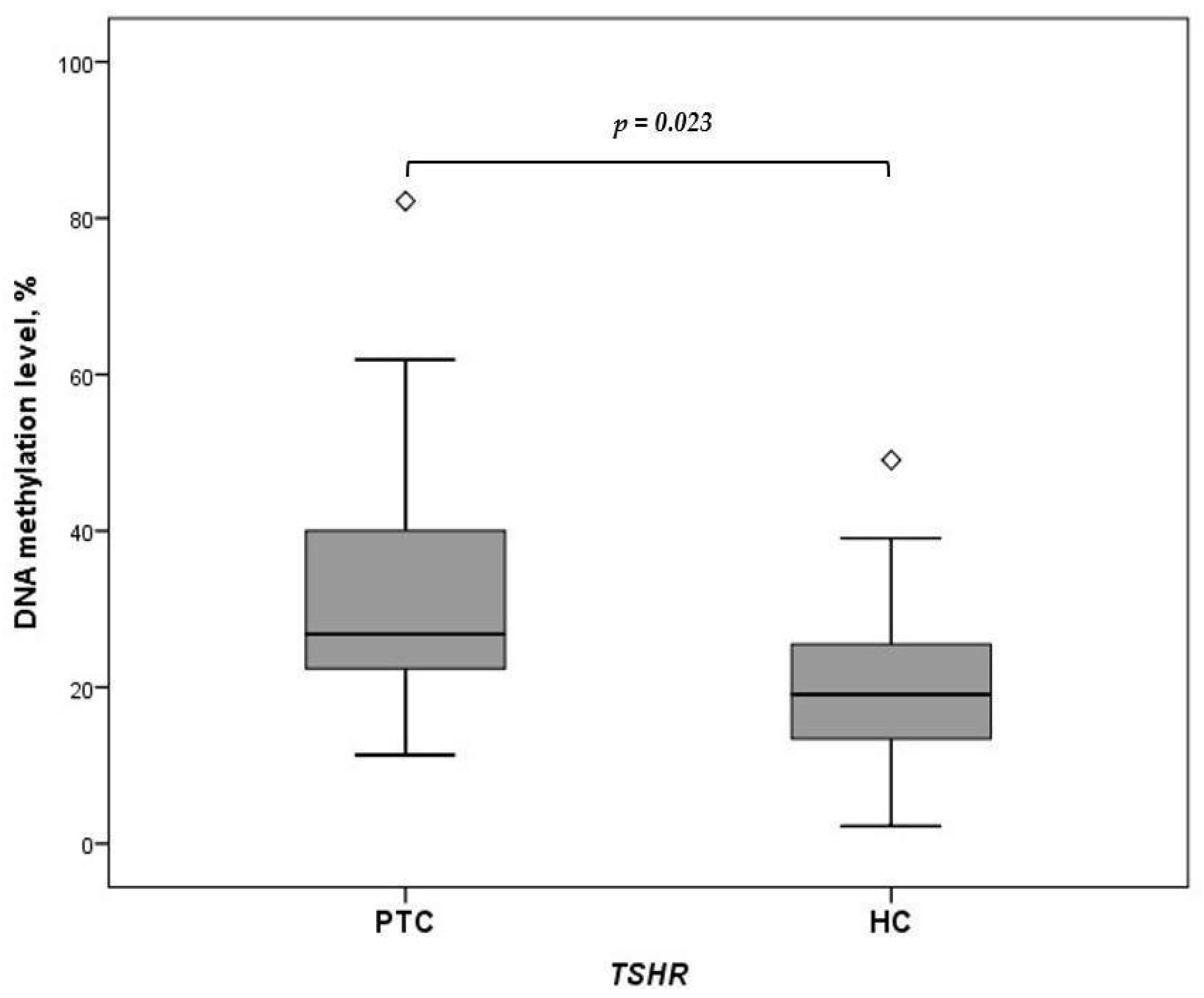
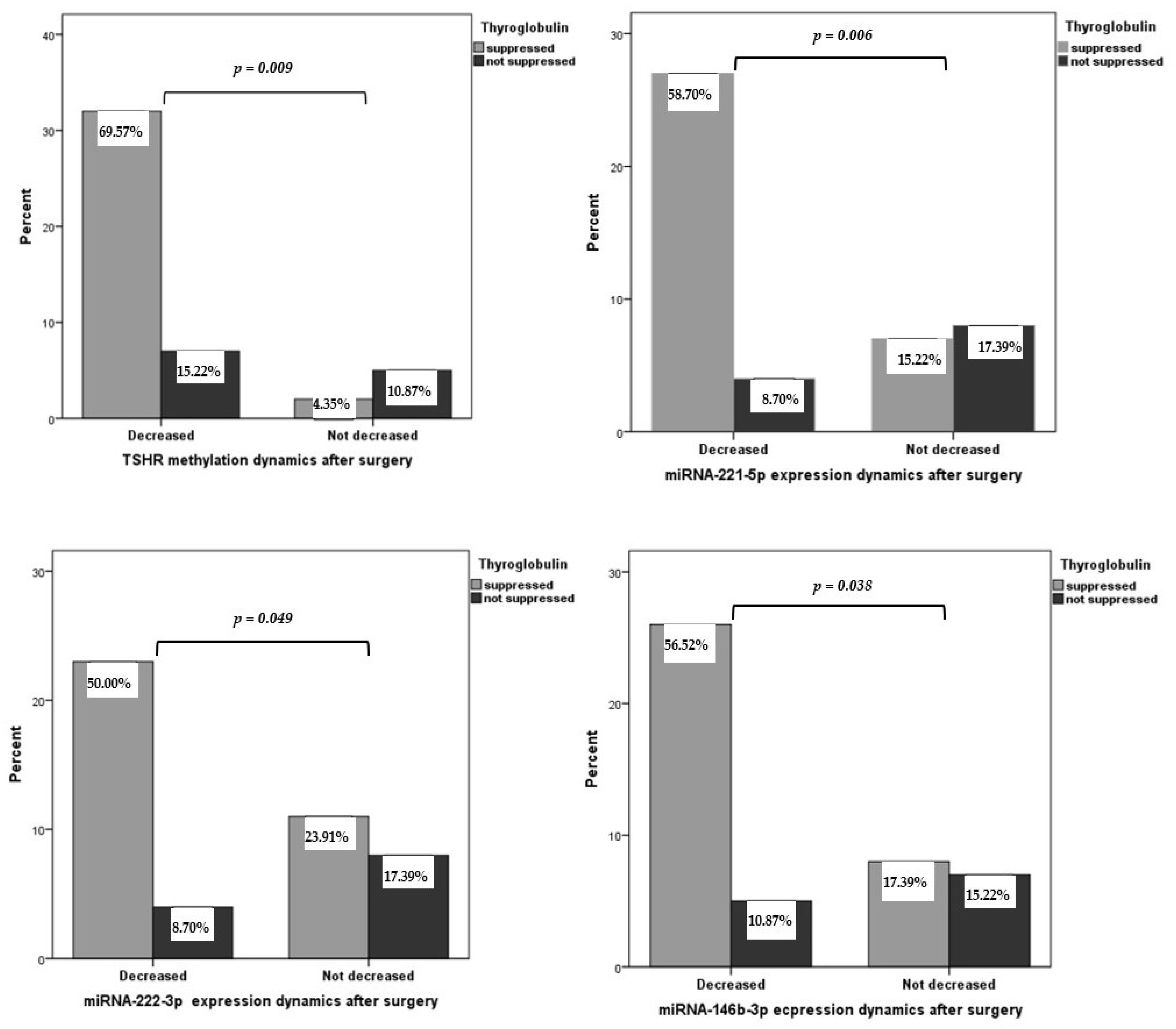
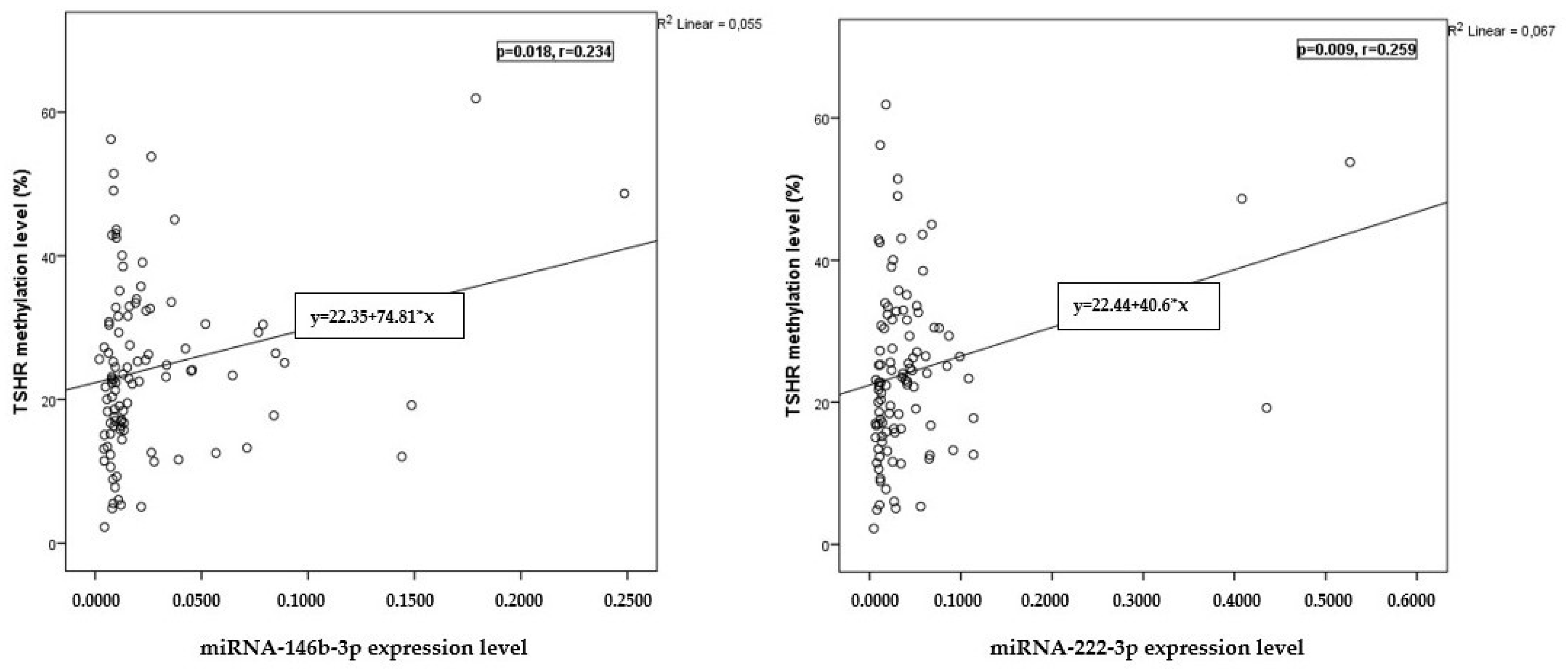
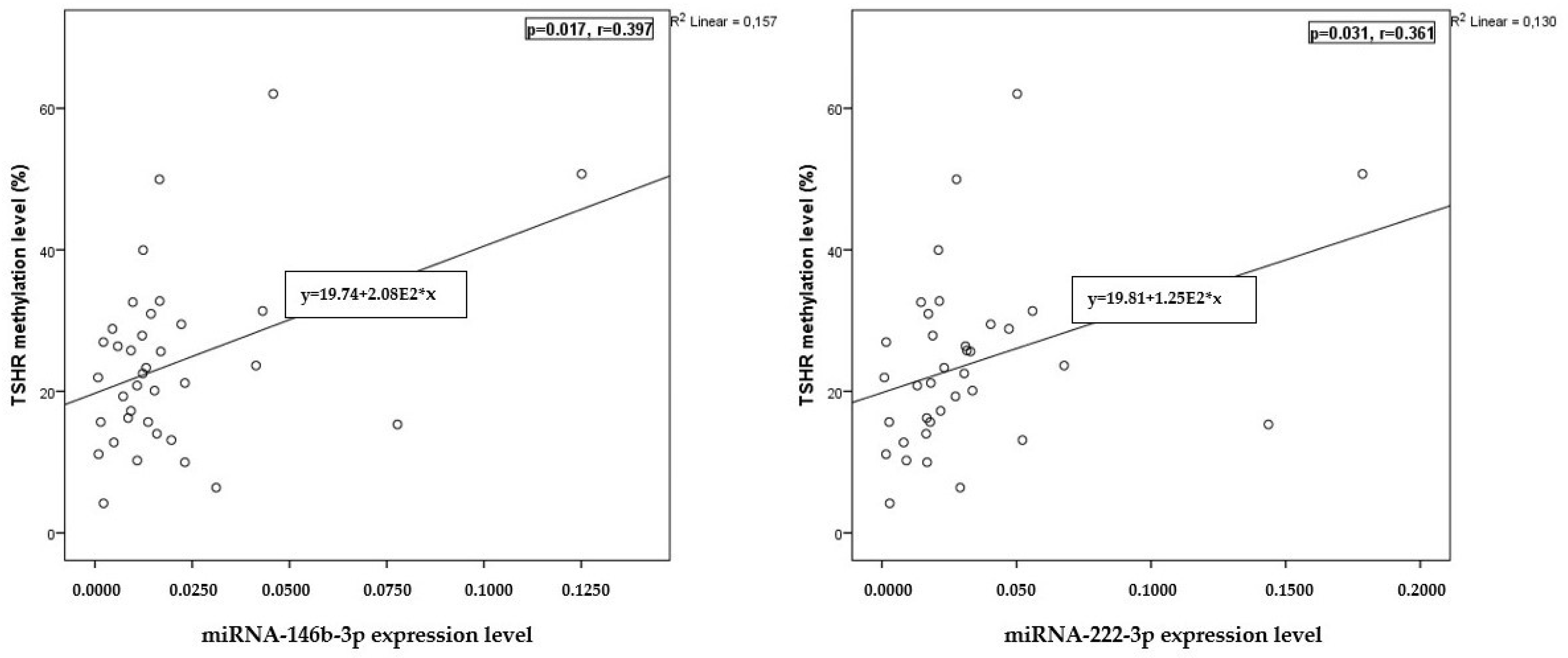
| Characteristic | PTC n = 46 | HC n = 57 | p-Value |
|---|---|---|---|
| Gender | |||
| Male | 8 (17.4%) | 12 (21.1%) | |
| Female | 38 (82.6%) | 45 (78.9%) | |
| Age at initial surgery (years) | 48.6 (14.6) | 45.21 (12.9) | p = 0.236 |
| T (TNM), n (%) | - | - | |
| pT1a | 15 (32.6) | ||
| pT1b | 4 (8.7) | ||
| pT2 | 3 (6.5) | ||
| pT3a | 15 (32.6) | ||
| pT3b | 9 (19.6) | ||
| Tumor size (cm) | - | - | |
| ≤2 | 32 (69.6) | ||
| >2 | 14 (30.4) | ||
| Lymph node metastases at initial surgery | - | - | |
| Yes | 12 (26.1) | ||
| No | 34 (73.9) | ||
| Variant of PTC, n (%) | - | - | |
| Classical variant | 18 (39.1) | ||
| Follicular variant | 10 (21.7) | ||
| Diffuse sclerosing variant | 15 (32.6) | ||
| Tall cell carcinoma | 3 (6.5) | ||
| Extrathyroidal extension | - | - | |
| Yes | 24 (52.2) | ||
| No | 22 (47.8) | ||
| Lymphovascular invasion | - | - | |
| Yes | 25 (54.3) | ||
| No | 21 (45.7) | ||
| Multifocality | - | - | |
| Yes | 13 (28.3) | ||
| No | 33 (71.7) |
| miRNA | Relative Expression 2−ΔΔCt: Median (IQR) | p | |
|---|---|---|---|
| Healthy Controls (n = 57) | Preoperative PTC (n = 46) | ||
| 146b-3p | 0.011 (0.009) | 0.016 ( 0.027) | 0.009 |
| 222-3p | 0.018 (0.026) | 0.038 (0.04) | <0.001 |
| 21a-3p | 0.072 (0.063) | 0.13 (0.21) | <0.001 |
| 221-5p | 0.007 (0.008) | 0.015 (0.013) | 0.003 |
| miRNA | Relative Expression 2−ΔΔCt: Median (IQR) TSHR Methylation Levels: Median (IQR) | p | |
|---|---|---|---|
| Preoperative PTC (n = 46) | Postoperative PTC (n = 46) | ||
| 146b-3p | 0.016 (0.027) | 0.012 (0.011) | 0.009 |
| 222-3p | 0.038 (0.04) | 0.022 (0.016) | <0.001 |
| 21a-3p | 0.13 (0.21) | 0.069 (0.08) | 0.003 |
| 221-5p | 0.015 (0.013) | 0.009 (0.007) | <0.001 |
| TSHR methylation | 26.041 (18.740) | 21.382 (16.220) | <0.001 |
| PTC Clinicopathological Feature | Relative Expression 2−ΔΔCt: Median (IQR) | DNA Methylation Level: Median (IQR) | |||
|---|---|---|---|---|---|
| miRNA-146b-3p | miRNA-222-3p | miRNA-21a-3p | miRNA-221-5p | TSHR | |
| Age | |||||
| <55 years (n = 30; 68.2%) | 0.017 (0.026) | 0.038 (0.045) | 0.13 (0.17) | 0.014 (0.013) | 30.41(18.78) |
| ≥55 years (n = 16; 33.8%) | 0.018 (0.034) | 0.038 (0.021) | 0.10 (0.24) | 0.016 (0.015) | 24.09 (10.99) |
| p | 0.908 | 0.963 | 0.729 | 0.344 | 0.219 |
| Gender | |||||
| Male (n = 8; 16.3%) | 0.021 (0.036) | 0.035 (0.033) | 0.079 (0.256) | 0.017 (0.015) | 24.09 (12.01) |
| Female (n = 38; 83.7%) | 0.017 (0.246) | 0.04 (0.041) | 0.134 (0.184) | 0.013 (0.014) | 30.88 (21.32) |
| p | 0.701 | 0.831 | 0.467 | 0.201 | 0.038 * |
| Multifocality | |||||
| Single (n = 13; 28.3%) | 0.015 (0.018) | 0.032 (0.035) | 0.103 (0.152) | 0.013 (0.013) | 30.39 (20.13) |
| Multiple (≥2) (n = 33; 71.7%) | 0.033 (0.051) | 0.061 (0.046) | 0.232 (0.383) | 0.018 (0.013) | 25.04 (13.01) |
| p | 0.154 | 0.016 * | 0.147 | 0.329 | 0.259 |
| Extrathyroidal extension | |||||
| Yes (n = 24; 71.4%) | 0.025 (0.027) | 0.028 (0.04) | 0.079 (0.112) | 0.013 (0.009) | 26.04 (11.80) |
| No (n = 22; 28.6%) | 0.013 (0.02) | 0.045 (0.04) | 0.199 (0.169) | 0.0162 (0.013) | 27.07 (23.00) |
| p | 0.09 | 0.132 | 0.008 * | 0.132 | 0.856 |
| Lymphovascular invasion | |||||
| Yes (n = 24; 52.2%) | 0.011± 0.017 | 0.031 (0.04) | 0.085 (0.119) | 0.012 (0.013) | 30.41 (19.40) |
| No (n = 22; 48.8%) | 0.03 (0.028) | 0.049 (0.042) | 0.207 (0.131) | 0.016 (0.013) | 23.99 (14.78) |
| p | 0.005 * | 0.087 | 0.007 * | 0.147 | 0.039 * |
| T (TNM) | |||||
| T1a, T1b (n = 19; 20.6%) | 0.018 ( 0.027) | 0.046 (0.042) | 0.192 (0.21) | 0.016 (0.013) | 29.34 (23.00) |
| T2, T3 (n = 27; 79.4%) | 0.016 (0.26) | 0.034 (0.035) | 0.088 (0.1840 | 0.014 (0.011) | 26.04 (11.80) |
| p | 0.289 | 0.220 | 0.051 | 0.326 | 0.816 |
| Tumor size (cm) | |||||
| ≤2 (n = 32; 69.6%) | 0.017 (0.029) | 0.038 (0.046) | 0.134 (0.164) | 0.014 (0.013) | 24.23 (14.18) |
| >2 (n = 14; 30.6%) | 0.018 (0.019) | 0.036 (0.030) | 0.087 (0.12) | 0.015 (0.013) | 32.64 (27.58) |
| p | 0.402 | 0.272 | 0.733 | 0.412 | 0.002 * |
| Lymph node metastases (preoperative PTC plasma samples) | |||||
| Yes (n = 12; 25%) | 0.026 (0.056) | 0.034 ( 0.039) | 0.136 (0.372) | 0.013 (0.016) | 26.04 (17.86) |
| No (n = 34; 75%) | 0.015 (0.027) | 0.04 ( 0.056) | 0.14 (0.208) | 0.013 (0.014) | 27.07 (19.92) |
| p | 0.317 | 0.591 | 0.764 | 0.548 | 0.490 |
| miRNA | AUC | Asymptotic 95% CI | p | miRNA’s Expression 2−ΔΔCt, TSHR Methylation Level Cut-Offs | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| 146b-3p | 0.651 | 0.541 | 0.761 | 0.007 * | 0.0126 | 65.2% | 33.33% |
| 222-3p | 0.708 | 0.609 | 0.807 | <0.0001 * | 0.0143 | 87.9% | 52.6% |
| 21a-3p | 0.673 | 0.566 | 0.779 | 0.001 * | 0.1300 | 52.2% | 17.5% |
| 221-5p | 0.765 | 0.674 | 0.855 | <0.0001 * | 0.0103 | 66.0% | 71.7% |
| TSHR methylation levels | 0.719 | 0.620 | 0.818 | <0.0001 * | 16.4960 | 86.7% | 63.2% |
| Gene Symbol | DNA Strand | Primer ID | Primer Sequence (5′→3′) | Amplicon Size, bp |
|---|---|---|---|---|
| TSHR | Sense | Fwd | GGTGTAGAGTTGAGAATGAGGTGATTTC | 122 |
| Rev | GCCCAAATCCCTAAACAAATCG | |||
| Probe | FAM-ACAACACCAACTACAACAAATCCGCCGA-BHQ1 | |||
| ACTB | Antisense | Fwd | TGGTGATGGAGGAGGTTTAGTAAGT | 133 |
| Rev | AACCAATAAAACCTACTCCTCCCTTAA | |||
| Probe | FAM-ACCACCACCCAACACACAATAACAAACACA-BHQ- 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazlauskiene, M.; Klimaite, R.; Kondrotiene, A.; Dauksa, A.; Dauksiene, D.; Verkauskiene, R.; Zilaitiene, B. Plasma miRNA-146b-3p, -222-3p, -221-5p, -21a-3p Expression Levels and TSHR Methylation: Diagnostic Potential and Association with Clinical and Pathological Features in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 8412. https://doi.org/10.3390/ijms25158412
Kazlauskiene M, Klimaite R, Kondrotiene A, Dauksa A, Dauksiene D, Verkauskiene R, Zilaitiene B. Plasma miRNA-146b-3p, -222-3p, -221-5p, -21a-3p Expression Levels and TSHR Methylation: Diagnostic Potential and Association with Clinical and Pathological Features in Papillary Thyroid Cancer. International Journal of Molecular Sciences. 2024; 25(15):8412. https://doi.org/10.3390/ijms25158412
Chicago/Turabian StyleKazlauskiene, Mintaute, Raimonda Klimaite, Aiste Kondrotiene, Albertas Dauksa, Dalia Dauksiene, Rasa Verkauskiene, and Birute Zilaitiene. 2024. "Plasma miRNA-146b-3p, -222-3p, -221-5p, -21a-3p Expression Levels and TSHR Methylation: Diagnostic Potential and Association with Clinical and Pathological Features in Papillary Thyroid Cancer" International Journal of Molecular Sciences 25, no. 15: 8412. https://doi.org/10.3390/ijms25158412
APA StyleKazlauskiene, M., Klimaite, R., Kondrotiene, A., Dauksa, A., Dauksiene, D., Verkauskiene, R., & Zilaitiene, B. (2024). Plasma miRNA-146b-3p, -222-3p, -221-5p, -21a-3p Expression Levels and TSHR Methylation: Diagnostic Potential and Association with Clinical and Pathological Features in Papillary Thyroid Cancer. International Journal of Molecular Sciences, 25(15), 8412. https://doi.org/10.3390/ijms25158412






