Reducing FKBP51 Expression in the Ventral Hippocampus Decreases Auditory Fear Conditioning in Male Rats
Abstract
1. Introduction
2. Results
2.1. Decreasing FKBP51 Expression in the VH Reduces Fear Acquisition and Recall
2.2. Effects of Decreasing VH FKBP51 Expression on Fear Extintion
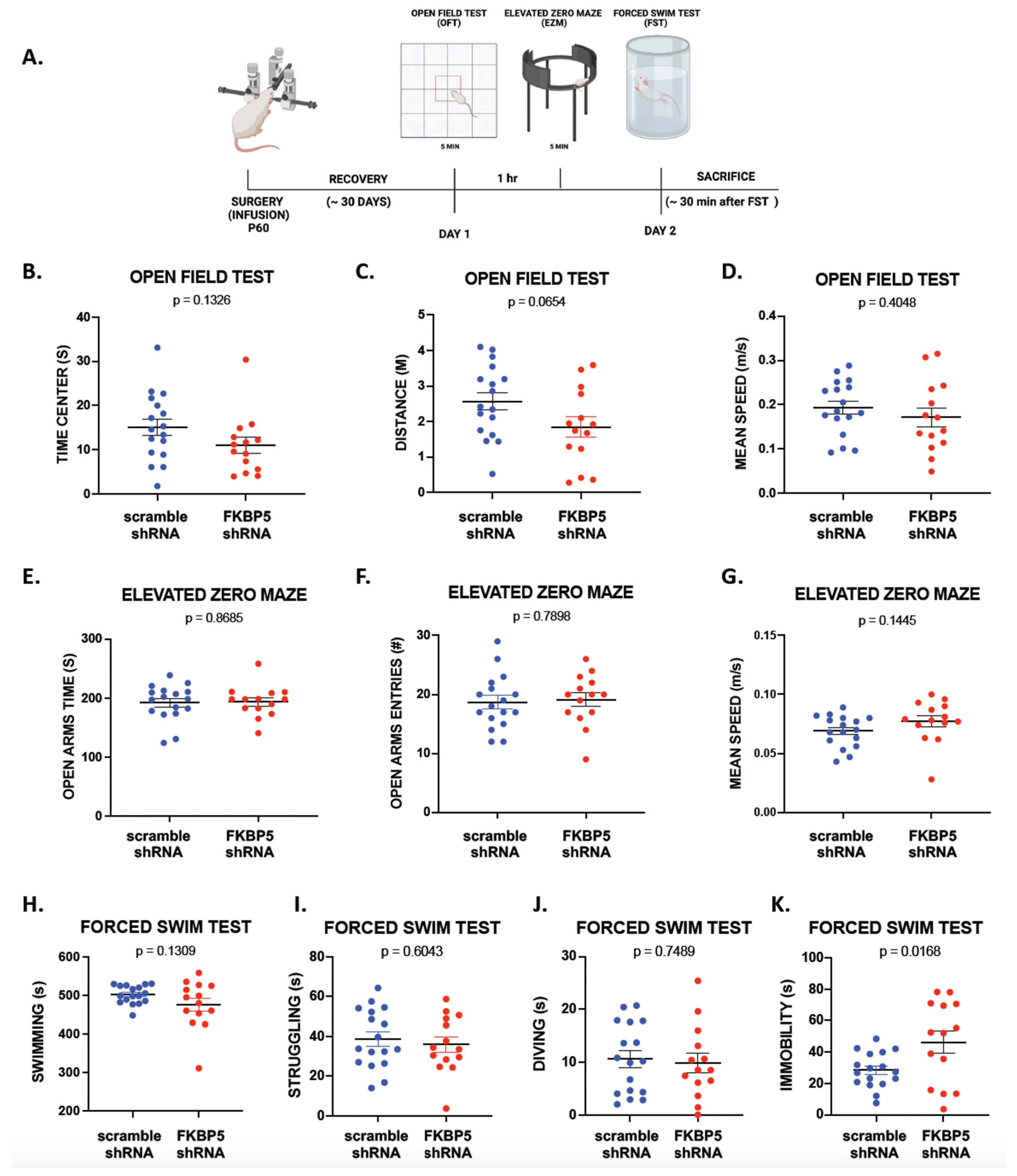
2.3. Reducing FKBP51 in VH Does Not Affect Exploritory Behavior or Locomotion but Induces Passive Behavior during the Forced Swim Test
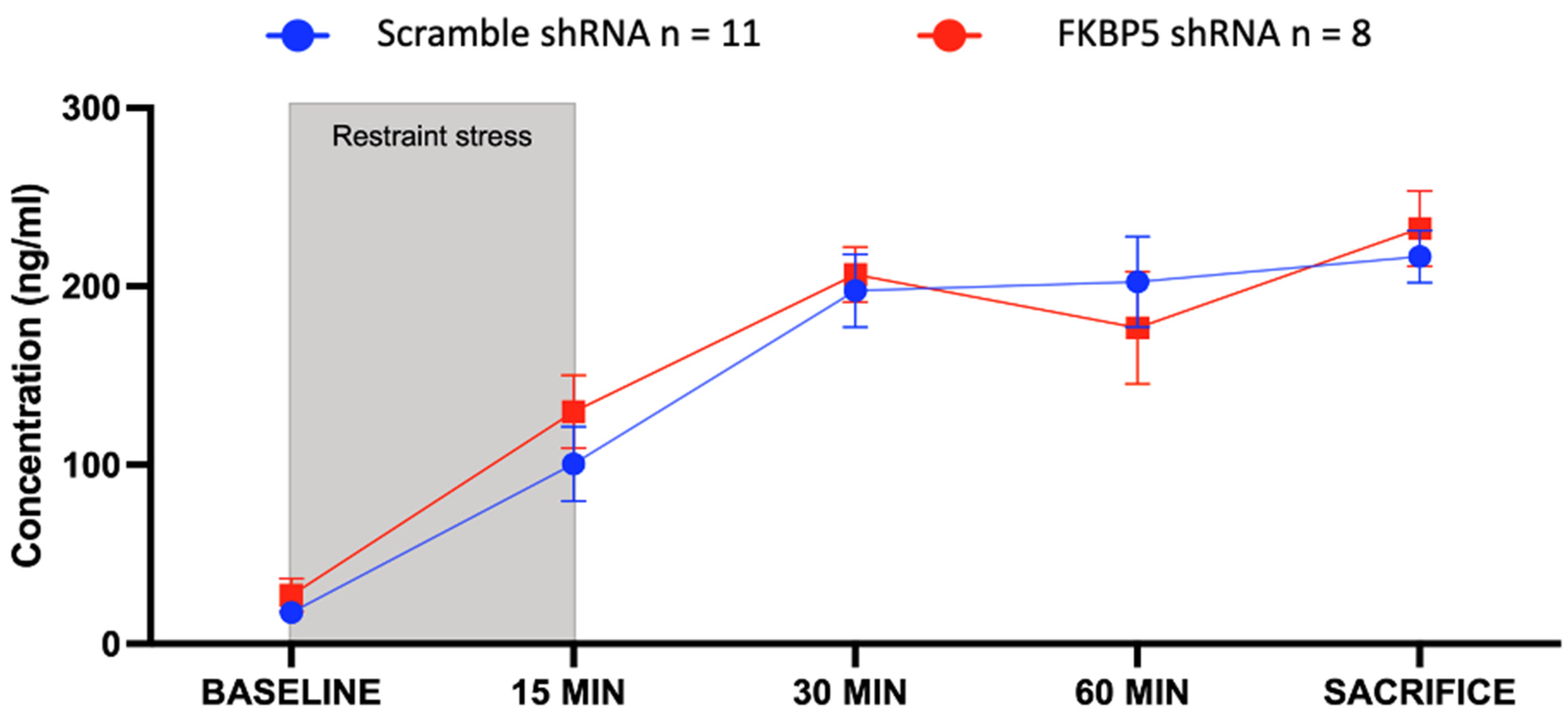
2.4. Reducing FKBP51 in the VH Does Not Disrupt the HPA Axis Response to Acute Restraint Stress
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Short Hairpin RNA Construct
4.3. Stereotaxic Surgery
4.4. Auditory Fear Conditioning and Extinction Training
4.5. Forced Swim Test (FST)
4.6. Open Field Test (OFT)
4.7. Elevated Zero Maze Test (EZM)
4.8. ELISA Assay of Corticosterone
4.9. Immunofluorescence
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Finsterwald, C.; Alberini, C.M. Stress and Glucocorticoid Receptor-Dependent Mechanisms in Long-Term Memory: From Adaptive Responses to Psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef]
- Sarabdjitsingh, R.A.; Meijer, O.C.; de Kloet, E.R. Specificity of Glucocorticoid Receptor Primary Antibodies for Analysis of Receptor Localization Patterns in Cultured Cells and Rat Hippocampus. Brain Res. 2010, 1331, 1–11. [Google Scholar] [CrossRef]
- Sala, M. Stress and Hippocampal Abnormalities in Psychiatric Disorders. Eur. Neuropsychopharmacol. 2004, 14, 393–405. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.J. Neurocognitive Effects of Stress: A Metaparadigm Perspective. Mol. Psychiatry 2023, 28, 2750–2763. [Google Scholar] [CrossRef]
- Roux, C.M.; Leger, M.; Freret, T. Memory Disorders Related to Hippocampal Function: The Interest of 5-HT4Rs Targeting. Int. J. Mol. Sci. 2021, 22, 12082. [Google Scholar] [CrossRef]
- Eachus, H.; Ryu, S. Glucocorticoid Effects on the Brain: From Adaptive Developmental Plasticity to Allostatic Overload. J. Exp. Biol. 2024, 227 (Suppl. 1), jeb246128. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Mennesson, M.; Revest, J.-M. Glucocorticoid-Responsive Tissue Plasminogen Activator (TPA) and Its Inhibitor Plasminogen Activator Inhibitor-1 (PAI-1): Relevance in Stress-Related Psychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 4496. [Google Scholar] [CrossRef]
- Szabó, C.; Kelemen, O.; Kéri, S. Changes in FKBP5 Expression and Memory Functions during Cognitive–Behavioral Therapy in Posttraumatic Stress Disorder: A Preliminary Study. Neurosci. Lett. 2014, 569, 116–120. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of Glucocorticoid Negative Feedback in the Regulation of HPA Axis Pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Watkins, L.E.; Han, S.; Harpaz-Rotem, I.; Mota, N.P.; Southwick, S.M.; Krystal, J.H.; Gelernter, J.; Pietrzak, R.H. FKBP5 Polymorphisms, Childhood Abuse, and PTSD Symptoms: Results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology 2016, 69, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Inslicht, S.S.; Metzler, T.J.; Neylan, T.C.; Ross, J.A. The Effects of Early Trauma and the FKBP5 Gene on PTSD and the HPA Axis in a Clinical Sample of Gulf War Veterans. Psychiatry Res. 2018, 270, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Bradley, R.G.; Liu, W.; Epstein, M.P.; Deveau, T.C.; Mercer, K.B.; Tang, Y.; Gillespie, C.F.; Heim, C.M.; Nemeroff, C.B.; et al. Association of FKBP5 Polymorphisms and Childhood Abuse with Risk of Posttraumatic Stress Disorder Symptoms in Adults. JAMA 2008, 299, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-Specific FKBP5 DNA Demethylation Mediates Gene-Childhood Trauma Interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Man, H.-Y.; Wang, Q.; Lu, W.-Y.; Ju, W.; Ahmadian, G.; Liu, L.; D’Souza, S.; Wong, T.P.; Taghibiglou, C.; Lu, J.; et al. Activation of PI3-Kinase Is Required for AMPA Receptor Insertion during LTP of MEPSCs in Cultured Hippocampal Neurons. Neuron 2003, 38, 611–624. [Google Scholar] [CrossRef]
- Sutton, G.; Chandler, L.J. Activity-dependent NMDA Receptor-mediated Activation of Protein Kinase B/Akt in Cortical Neuronal Cultures. J. Neurochem. 2002, 82, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.L.; Burks, D.J.; Pons, S.; Matter, W.F.; Vlahos, C.J.; White, M.F.; Sacks, D.B. Calmodulin Activates Phosphatidylinositol 3-Kinase. J. Biol. Chem. 1997, 272, 28183–28186. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Morales Silva, R.J.; Velazquez, B.; Hernández, A.; Colon, M.; Cruz, E.; Soler-Cedeño, O.; Porter, J.T. Dynamic Expression of FKBP5 in the Medial Prefrontal Cortex Regulates Resiliency to Conditioned Fear. Learn. Mem. 2017, 24, 145–152. [Google Scholar] [CrossRef]
- Sawamura, T.; Klengel, T.; Armario, A.; Jovanovic, T.; Norrholm, S.D.; Ressler, K.J.; Andero, R. Dexamethasone Treatment Leads to Enhanced Fear Extinction and Dynamic Fkbp5 Regulation in Amygdala. Neuropsychopharmacology 2016, 41, 832–846. [Google Scholar] [CrossRef]
- Li, H.; Su, P.; Lai, T.K.Y.; Jiang, A.; Liu, J.; Zhai, D.; Campbell, C.T.G.; Lee, F.H.F.; Yong, W.; Pasricha, S.; et al. The Glucocorticoid Receptor–FKBP51 Complex Contributes to Fear Conditioning and Posttraumatic Stress Disorder. J. Clin. Investig. 2020, 130, 877–889. [Google Scholar] [CrossRef]
- O’Leary, J.C.; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A New Anti-Depressive Strategy for the Elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6, e24840. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Mitra, R.; Shankaranarayana Rao, B.S.; Chattarji, S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D. Chronic Stress-Induced Hippocampal Vulnerability: The Glucocorticoid Vulnerability Hypothesis. Rev. Neurosci. 2008, 19, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Prolonged Glucocorticoid Exposure Reduces Hippocampal Neuron Number: Implications for Aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; O’Keane, V. How Does the Brain Deal with Cumulative Stress? A Review with Focus on Developmental Stress, HPA Axis Function and Hippocampal Structure in Humans. Neurobiol. Dis. 2013, 52, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Holt, W.G. Hippocampus and Pavlovian Fear Conditioning in Rats: Muscimol Infusions Into the Ventral, but Not Dorsal, Hippocampus Impair the Acquisition of Conditional Freezing to an Auditory Conditional Stimulus. Behav. Neurosci. 2004, 118, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.M.; Foilb, A.R.; Christianson, J.P. Inactivation of Ventral Hippocampus Interfered with Cued-Fear Acquisition but Did Not Influence Later Recall or Discrimination. Behav. Brain Res. 2016, 296, 249–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.N.; Bast, T.; Feldon, J. The Ventral Hippocampus and Fear Conditioning in Rats: Different Anterograde Amnesias of Fear after Infusion of N-Methyl-D-Aspartate or Its Noncompetitive Antagonist MK-801 into the Ventral Hippocampus. Behav. Brain Res. 2001, 126, 159–174. [Google Scholar] [CrossRef]
- Schaaf, M.J.M.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone Effects on BDNF Expression in the Hippocampus. Implications for Memory Formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Duvarci, S.; Paré, D. Glucocorticoids Enhance the Excitability of Principal Basolateral Amygdala Neurons. J. Neurosci. 2007, 27, 4482–4491. [Google Scholar] [CrossRef]
- Cordero, M.I.; Sandi, C. A Role for Brain Glucocorticoid Receptors in Contextual Fear Conditioning: Dependence upon Training Intensity. Brain Res. 1998, 786, 11–17. [Google Scholar] [CrossRef]
- Revest, J.-M.; Le Roux, A.; Roullot-Lacarrière, V.; Kaouane, N.; Vallée, M.; Kasanetz, F.; Rougé-Pont, F.; Tronche, F.; Desmedt, A.; Piazza, P.V. BDNF-TrkB Signaling through Erk1/2 MAPK Phosphorylation Mediates the Enhancement of Fear Memory Induced by Glucocorticoids. Mol. Psychiatry 2014, 19, 1001–1009. [Google Scholar] [CrossRef]
- Barsegyan, A.; Mackenzie, S.M.; Kurose, B.D.; McGaugh, J.L.; Roozendaal, B. Glucocorticoids in the Prefrontal Cortex Enhance Memory Consolidation and Impair Working Memory by a Common Neural Mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 16655–16660. [Google Scholar] [CrossRef]
- Revest, J.M.; Kaouane, N.; Mondin, M.; Le Roux, A.; Rougé-Pont, F.; Vallée, M.; Barik, J.; Tronche, F.; Desmedt, A.; Piazza, P.V. The Enhancement of Stress-Related Memory by Glucocorticoids Depends on Synapsin-Ia/Ib. Mol. Psychiatry 2010, 15, 1140–1151. [Google Scholar] [CrossRef]
- Raio, C.M.; Phelps, E.A. Neurobiology of Stress The in Fl Uence of Acute Stress on the Regulation of Conditioned Fear. Neurobiol. Stress 2015, 1, 134–146. [Google Scholar] [CrossRef]
- Raio, C.M.; Brignoni-Perez, E.; Goldman, R.; Phelps, E.A. Acute Stress Impairs the Retrieval of Extinction Memory in Humans. Neurobiol. Learn. Mem. 2014, 112, 212–221. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Humphreys, A.G.; Lehman, J.C.; Diamond, D.M.; Rose, G.M.; Meaney, M.J. Enduring Effects of Chronic Corticosterone Treatment on Spatial Learning, Synaptic Plasticity, and Hippocampal Neuropathology in Young and Mid-Aged Rats. J. Neurosci. 1995, 15 Pt 1, 61–69. [Google Scholar] [CrossRef]
- Dorey, R.; Piérard, C.; Chauveau, F.; David, V.; Béracochéa, D. Stress-Induced Memory Retrieval Impairments: Different Time-Course Involvement of Corticosterone and Glucocorticoid Receptors in Dorsal and Ventral Hippocampus. Neuropsychopharmacology 2012, 37, 2870–2880. [Google Scholar] [CrossRef]
- De Kloet, E.R. From Receptor Balance to Rational Glucocorticoid Therapy. Endocrinology 2014, 155, 2754–2769. [Google Scholar] [CrossRef]
- Joëls, M.; de Kloet, E.R. Effect of Corticosteroid Hormones on Electrical Activity in Rat Hippocampus. J. Steroid Biochem. Mol. Biol. 1991, 40, 83–86. [Google Scholar] [CrossRef]
- Kim, H.; Yi, J.H.; Choi, K.; Hong, S.; Shin, K.S.; Kang, S.J. Regional Differences in Acute Corticosterone-Induced Dendritic Remodeling in the Rat Brain and Their Behavioral Consequences. BMC Neurosci. 2014, 15, 65. [Google Scholar] [CrossRef][Green Version]
- Morales-Medina, J.C.; Sanchez, F.; Flores, G.; Dumont, Y.; Quirion, R. Morphological Reorganization after Repeated Corticosterone Administration in the Hippocampus, Nucleus Accumbens and Amygdala in the Rat. J. Chem. Neuroanat. 2009, 38, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Tse, Y.C.; Bagotu, R.C.; Wong, T.P. Dynamic Regulation of NMDAR Function in the Adult Brain by the Stress Hormone Corticosterone. Front. Cell Neurosci. 2012, 6, 9. [Google Scholar] [CrossRef]
- Kaouane, N.; Porte, Y.; Vallée, M.; Brayda-Bruno, L.; Mons, N.; Calandreau, L.; Marighetto, A.; Piazza, P.V.; Desmedt, A. Glucocorticoids Can Induce PTSD-Like Memory Impairments in Mice. Science 2012, 335, 1510–1513. [Google Scholar] [CrossRef]
- Haggerty, D.L.; Grecco, G.G.; Reeves, K.C.; Atwood, B. Adeno-Associated Viral Vectors in Neuroscience Research. Mol. Ther. Methods Clin. Dev. 2020, 17, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Bierwirth, P.; Sperl, M.F.J.; Antov, M.I.; Stockhorst, U. Prefrontal Theta Oscillations Are Modulated by Estradiol Status During Fear Recall and Extinction Recall. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, Z.S.; Milad, M.R. Fear Extinction Learning Modulates Large-Scale Brain Connectivity. Neuroimage 2021, 238, 118261. [Google Scholar] [CrossRef]
- Milad, M.R.; Furtak, S.C.; Greenberg, J.L.; Keshaviah, A.; Im, J.J.; Falkenstein, M.J.; Jenike, M.; Rauch, S.L.; Wilhelm, S. Deficits in Conditioned Fear Extinction in Obsessive-Compulsive Disorder and Neurobiological Changes in the Fear Circuit. JAMA Psychiatry 2013, 70, 608. [Google Scholar] [CrossRef]
- Pan, H.Q.; Liu, X.X.; He, Y.; Zhou, J.; Liao, C.Z.; You, W.J.; Jiang, S.Y.; Qin, X.; Chen, W.B.; Fei, E.K.; et al. Prefrontal GABAA(δ)R Promotes Fear Extinction through Enabling the Plastic Regulation of Neuronal Intrinsic Excitability. J. Neurosci. 2022, 42, 5755–5770. [Google Scholar] [CrossRef]
- Russo, A.S.; Parsons, R.G. Neural Activity in Afferent Projections to the Infralimbic Cortex Is Associated with Individual Differences in the Recall of Fear Extinction. Sci. Rep. 2022, 12, 13703. [Google Scholar] [CrossRef]
- Milad, M.R.; Pitman, R.K.; Ellis, C.B.; Gold, A.L.; Shin, L.M.; Lasko, N.B.; Zeidan, M.A.; Handwerger, K.; Orr, S.P.; Rauch, S.L. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol. Psychiatry 2009, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xu, Y.; Wang, J.; Liu, M.; Dou, L.; Deng, R.; Wang, C.; Williams, K.E.; Stewart, R.B.; Xie, Z.; et al. Loss of FKBP5 Affects Neuron Synaptic Plasticity: An Electrophysiology Insight. Neuroscience 2019, 402, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sarapas, C.; Cai, G.; Bierer, L.M.; Golier, J.A.; Galea, S.; Ising, M.; Rein, T.; Schmeidler, J.; Müller-Myhsok, B.; Uhr, M.; et al. Genetic Markers for PTSD Risk and Resilience among Survivors of the World Trade Center Attacks. Dis. Markers 2011, 30, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Cai, G.; Golier, J.A.; Sarapas, C.; Galea, S.; Ising, M.; Rein, T.; Schmeidler, J.; Müller-Myhsok, B.; Holsboer, F.; et al. Gene Expression Patterns Associated with Posttraumatic Stress Disorder Following Exposure to the World Trade Center Attacks. Biol. Psychiatry 2009, 66, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Bajaj, T.; Klengel, C.; Chatzinakos, C.; Ebert, T.; Dedic, N.; McCullough, K.M.; Lardenoije, R.; Joëls, M.; Meijer, O.C.; et al. Mineralocorticoid Receptors Dampen Glucocorticoid Receptor Sensitivity to Stress via Regulation of FKBP5. Cell Rep. 2021, 35, 109185. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Segal, M. Differential Corticosteroid Modulation of Inhibitory Synaptic Currents in the Dorsal and Ventral Hippocampus. J. Neurosci. 2009, 29, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Kaouane, N.; Ducourneau, E.G.; Marighetto, A.; Segal, M.; Desmedt, A. False Opposing Fear Memories Are Produced as a Function of the Hippocampal Sector Where Glucocorticoid Receptors Are Activated. Front. Behav. Neurosci. 2020, 14, 144. [Google Scholar] [CrossRef]
- Agliari, E.; De Marzo, G. Tolerance versus Synaptic Noise in Dense Associative Memories. Eur. Phys. J. Plus 2020, 135, 883. [Google Scholar] [CrossRef]
- Ryu, H.; Cheon, M.; Chung, C. The Impact of FKBP5 Deficiency in Glucocorticoid Receptor Mediated Regulation of Synaptic Transmission in the Medial Prefrontal Cortex. Neuroscience 2021, 457, 20–26. [Google Scholar] [CrossRef]
- Jin, X.; Lu, Y.; Yang, X.; Ma, L.; Li, B. Glucocorticoid Receptors in the Basolateral Nucleus of Amygdala Are Required for Postreactivation Reconsolidation of Auditory Fear Memory. Eur. J. Neurosci. 2007, 25, 3702–3712. [Google Scholar] [CrossRef]
- Kjelstrup, K.G.; Tuvnes, F.A.; Steffenach, H.A.; Murison, R.; Moser, E.I.; Moser, M.B. Reduced Fear Expression after Lesions of the Ventral Hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Su, K.; Goldberg, A.R.; Luna, V.M.; Biane, J.S.; Ordek, G.; Zhou, P.; Ong, S.K.; Wright, M.A.; Zweifel, L.; et al. Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron 2018, 97, 670–683.e6. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Beyeler, A.; Seo, C.; Leppla, C.A.; Wildes, C.P.; Tye, K.M. BLA to VHPC Inputs Modulate Anxiety-Related Behaviors. Neuron 2013, 79, 658–664. [Google Scholar] [CrossRef]
- Kondev, V.; Bluett, R.; Najeed, M.; Rosas-Vidal, L.E.; Grueter, B.A.; Patel, S. Ventral Hippocampal Diacylglycerol Lipase-Alpha Deletion Decreases Avoidance Behaviors and Alters Excitation-Inhibition Balance. Neurobiol. Stress 2023, 22, 100510. [Google Scholar] [CrossRef]
- Touma, C.; Gassen, N.C.; Herrmann, L.; Cheung-Flynn, J.; Bull, D.R.; Ionescu, I.A.; Heinzmann, J.M.; Knapman, A.; Siebertz, A.; Depping, A.M.; et al. FK506 Binding Protein 5 Shapes Stress Responsiveness: Modulation of Neuroendocrine Reactivity and Coping Behavior. Biol. Psychiatry 2011, 70, 928–936. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary- Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, Glucocorticoids and Memory: Implications for Treating Fear-Related Disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Kara, N.; Ratsika, A.; Levone, B.R.; van de Wouw, M.; Tan, L.A.; Cunningham, J.I.; Sanchez, C.; Cryan, J.F.; O’Leary, O.F. Inhibition of FKBP51 Induces Stress Resilience and Alters Hippocampal Neurogenesis. Mol. Psychiatry 2022, 27, 4928–4938. [Google Scholar] [CrossRef]
- van Doeselaar, L.; Stark, T.; Mitra, S.; Yang, H.; Bordes, J.; Stolwijk, L.; Engelhardt, C.; Kovarova, V.; Narayan, S.; Brix, L.M.; et al. Sex-Specific and Opposed Effects of FKBP51 in Glutamatergic and GABAergic Neurons: Implications for Stress Susceptibility and Resilience. Proc. Natl. Acad. Sci. USA 2023, 120, e2300722120. [Google Scholar] [CrossRef]
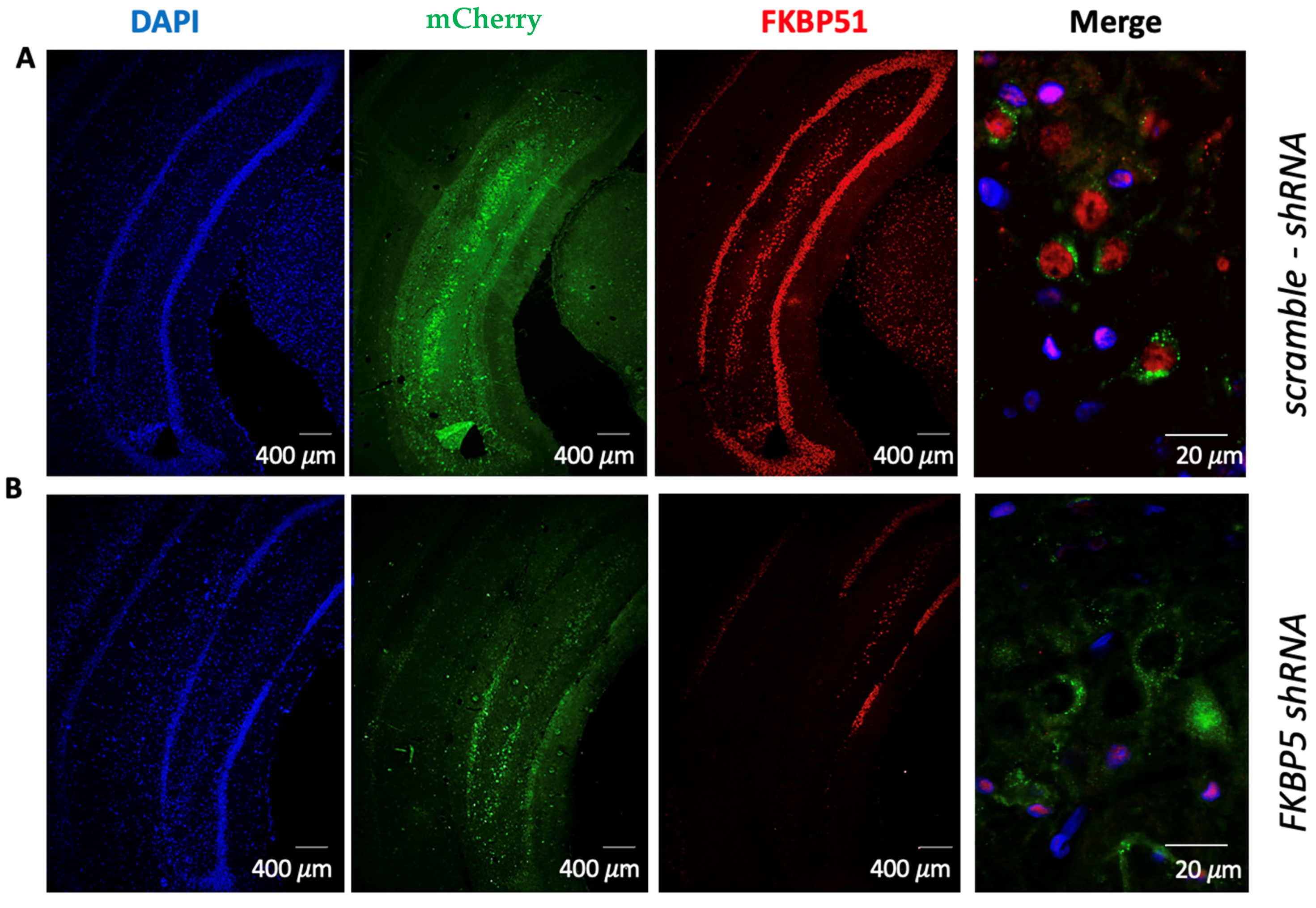

| Figure | Measured Condition | Factor | Statistics | Value |
|---|---|---|---|---|
| Figure 2B | Freezing % during COND | Treatment | Two-way ANOVA | F (1, 17) = 17.21, p = 0.007 |
| Animal | F (17, 23) = 2.69, p = 0.004 | |||
| Tone | F (19, 323) = 13.79, p < 0.001 | |||
| Tone x Treatment | F (19, 323) = 2.69, p = 0.0002 | |||
| Freezing % during COND 3 | Treatment | Two-way ANOVA, Uncorrected Fisher’s LSD | p = 0.0102 | |
| Freezing % during EXT 1 | p < 0.0001 | |||
| Freezing % during EXT 2 | p < 0.0001 | |||
| Freezing % during EXT 3 | p = 0.0005 | |||
| Freezing % during EXT 4 | p = 0.0027 | |||
| Figure 2C | Freezing% during RECALL | Treatment | Mann–Whitney test | p = 0.0282 |
| Figure 2D | Extinction Retention Index | Treatment | Mann–Whitney test | p = 0.3950 |
| Figure | Measured Condition | Factor | Statistics | Value |
|---|---|---|---|---|
| Figure 3B–D | OFT: Time in Center | Treatment | Unpaired t-test | p = 0.1326 |
| OFT: Distance | Treatment | Unpaired t-test | p = 0.0654 | |
| OFT: Mean Speed | Treatment | Unpaired t-test | p = 0.4048 | |
| Figure 3E–G | EZM: Time in Open Arms | Treatment | Unpaired t-test | p = 0.8685 |
| EZM: Open Arms Entries | Treatment | Unpaired t-test | p = 0.7898 | |
| EZM: Mean Speed | Treatment | Unpaired t-test | p = 0.1445 | |
| Figure 3H–K | FST: Swimming | Treatment | Unpaired t-test | p = 0.1309 |
| FST: Struggling | Treatment | Unpaired t-test | p = 0.0168 | |
| FST: Diving | Treatment | Unpaired t-test | p = 0.7489 | |
| FST: Immobility | Treatment | Unpaired t-test | p = 0.0168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irizarry-Méndez, N.; Criado-Marrero, M.; Hernandez, A.; Colón, M.; Porter, J.T. Reducing FKBP51 Expression in the Ventral Hippocampus Decreases Auditory Fear Conditioning in Male Rats. Int. J. Mol. Sci. 2024, 25, 7097. https://doi.org/10.3390/ijms25137097
Irizarry-Méndez N, Criado-Marrero M, Hernandez A, Colón M, Porter JT. Reducing FKBP51 Expression in the Ventral Hippocampus Decreases Auditory Fear Conditioning in Male Rats. International Journal of Molecular Sciences. 2024; 25(13):7097. https://doi.org/10.3390/ijms25137097
Chicago/Turabian StyleIrizarry-Méndez, Nashaly, Marangelie Criado-Marrero, Anixa Hernandez, Maria Colón, and James T. Porter. 2024. "Reducing FKBP51 Expression in the Ventral Hippocampus Decreases Auditory Fear Conditioning in Male Rats" International Journal of Molecular Sciences 25, no. 13: 7097. https://doi.org/10.3390/ijms25137097
APA StyleIrizarry-Méndez, N., Criado-Marrero, M., Hernandez, A., Colón, M., & Porter, J. T. (2024). Reducing FKBP51 Expression in the Ventral Hippocampus Decreases Auditory Fear Conditioning in Male Rats. International Journal of Molecular Sciences, 25(13), 7097. https://doi.org/10.3390/ijms25137097






