Blastic Plasmacytoid Dendritic Cell Neoplasm, from a Dermatological Point of View
Abstract
1. Introduction
2. Dendritic Cells in the Skin
3. Plasmacytoid Dendritic Cells in Dermatological Diseases
4. Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
4.1. Genetics
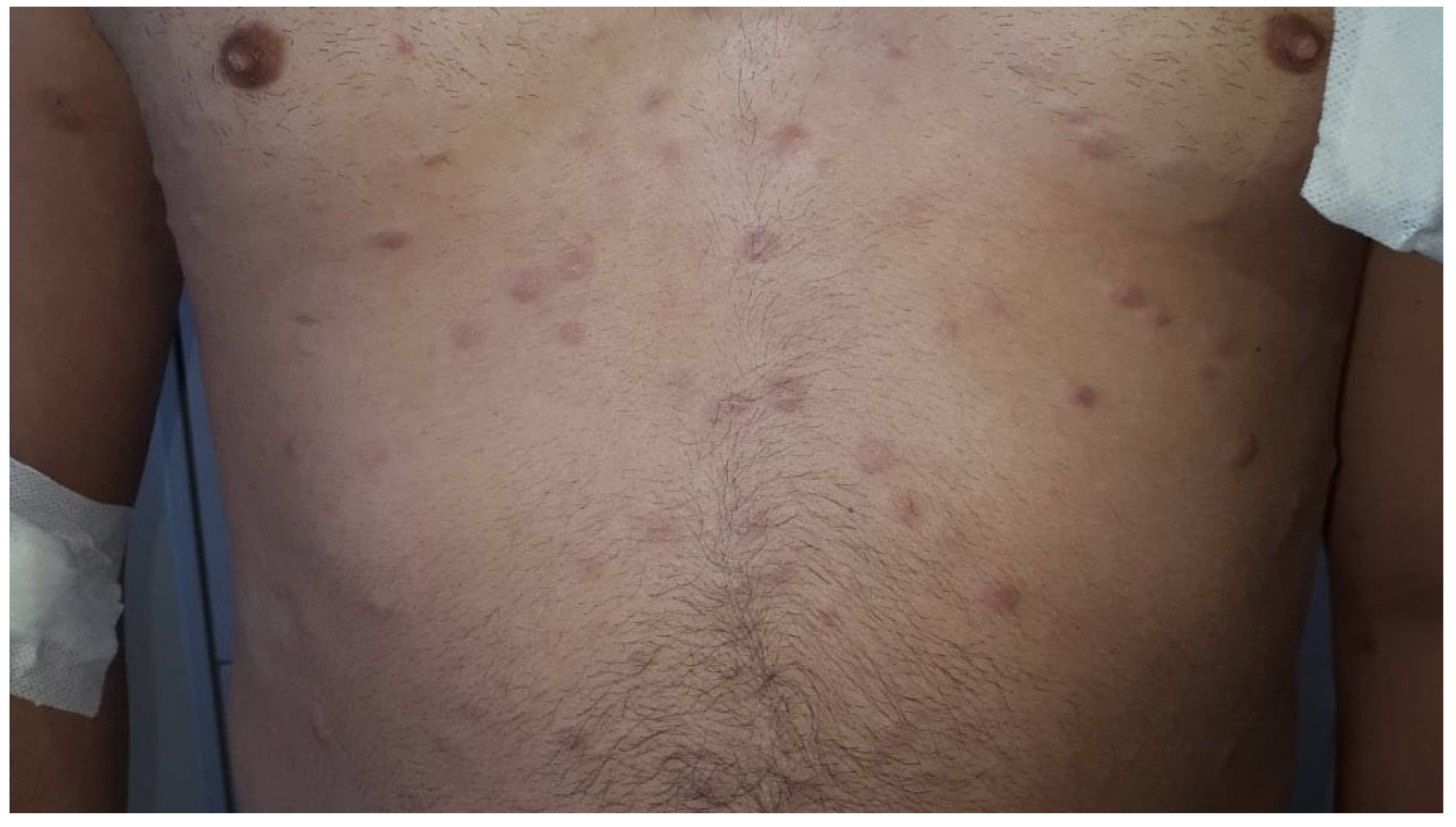
4.2. Clinical Features
4.3. Differential Diagnosis
4.4. Diagnosis
5. Pediatric BPDCN
6. Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.J.; Kim, Y.; Park, S.H.; Jo, J.C. Plasmacytoid dendritic cell neoplasms. Blood Res. 2023, 58 (Suppl. S1), 90–95. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Hu, Q.; Zhu, J.; Tao, Y.; Huang, L.; Niu, T. Incidence, prognostic factors, and survival outcomes in patients with blastic plasmacytoid dendritic cell neoplasm: A retrospective study in the Surveillance, Epidemiology, and End Results database. Eur. J. Haematol. 2023, 110, 743–753. [Google Scholar] [CrossRef]
- Jegalian, A.G.; Buxbaum, N.P.; Facchetti, F.; Raffeld, M.; Pittaluga, S.; Wayne, A.S.; Jaffe, E.S. Blastic plasmacytoid dendritic cell neoplasm in children: Diagnostic features and clinical implications. Haematologica 2010, 95, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Tanchiva, K.O.; Chavez, P.C.; Guevara, S.L.L.; Vicuna, C.R.Q.; Bhardwaj, N.; Lansigan, F.; Deconinck, E. A case report of blastic plasmacytoid dendritic cell neoplasm in a hispanic child. Leuk. Res. Rep. 2021, 16, 100262. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Nasr, A.; Kabir, B.; de Nanassy, J.; Tang, K.; Menzies-Toman, D.; Johnston, D.; El Demellawy, D. Pediatric Blastic Plasmacytoid Dendritic Cell Neoplasm: A Systematic Literature Review. J. Pediatr. Hematol. Oncol. 2017, 39, 528–537. [Google Scholar] [CrossRef]
- El Hussein, S.; Yabe, M.; Wang, W.; Pemmaraju, N.; Loghavi, S.; Jelloul, F.Z.; Fang, H.; Medeiros, L.J.; Burack, W.R.; Evans, A.G.; et al. Blastic plasmacytoid dendritic cell neoplasm (BPDCN) arising in the setting of polycythemia vera (PV): An illustration of the emerging role of flow cytometry analysis in monitoring progression of myeloproliferative neoplasms. eJHaem 2022, 3, 954–957. [Google Scholar] [CrossRef]
- Chamoun, K.; Loghavi, S.; Pemmaraju, N.; Konopleva, M.; Kroll, M.; Nguyen-Cao, M.; Hornbaker, M.; DiNardo, C.D.; Kadia, T.; Jorgensen, J.; et al. Early detection of transformation to BPDCN in a patient with MDS. Exp. Hematol. Oncol. 2018, 7, 26. [Google Scholar] [CrossRef]
- Alayed, K.; Patel, K.P.; Konoplev, S.; Singh, R.R.; Routbort, M.J.; Reddy, N.; Pemmaraju, N.; Zhang, L.; Al Shaikh, A.; Aladily, T.N.; et al. TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am. J. Hematol. 2013, 88, 1055–1061. [Google Scholar] [CrossRef]
- Batta, K.; Bossenbroek, H.M.; Pemmaraju, N.; Wilks, D.P.; Chasty, R.; Dennis, M.; Milne, P.; Collin, M.; Beird, H.C.; Taylor, J.; et al. Divergent clonal evolution of blastic plasmacytoid dendritic cell neoplasm and chronic myelomonocytic leukemia from a shared TET2-mutated origin. Leukemia 2021, 35, 3299–3303. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Chan, O.; Kerr, D.; Vincelette, N.D.; Idrees, A.; Mo, Q.; Sweet, K.; Lancet, J.E.; Kharfan-Dabaja, M.A.; Zhang, L.; et al. Survival outcomes in blastic plasmacytoid dendritic cell neoplasm by first-line treatment and stem cell transplant. Blood Adv. 2020, 4, 3435–3442. [Google Scholar] [CrossRef]
- Pagano, L.; Zinzani, P.L.; Pileri, S.; Quaglino, P.; Cuglievan, B.; Berti, E.; Pemmaraju, N.; Onida, F.; Willemze, R.; Orfao, A.; et al. Unmet Clinical Needs and Management Recommendations for Blastic Plasmacytoid Dendritic Cell Neoplasm: A Consensus-based Position Paper From an Ad Hoc International Expert Panel. Hemasphere 2023, 7, e841. [Google Scholar] [CrossRef] [PubMed]
- Klechevsky, E. Human dendritic cells—Stars in the skin. Eur. J. Immunol. 2013, 43, 3147–3155. [Google Scholar] [CrossRef]
- Zaba, L.C.; Fuentes-Duculan, J.; Steinman, R.M.; Krueger, J.G.; Lowes, M.A. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Investig. 2007, 117, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Banchereau, J.; Thompson-Snipes, L.; Zurawski, S.; Blanck, J.P.; Cao, Y.; Clayton, S.; Gorvel, J.P.; Zurawski, G.; Klechevsky, E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood 2012, 119, 5742–5749. [Google Scholar] [CrossRef]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef]
- Matthews, K.; Chung, N.P.; Klasse, P.J.; Moore, J.P.; Sanders, R.W. Potent induction of antibody-secreting B cells by human dermal-derived CD14+ dendritic cells triggered by dual TLR ligation. J. Immunol. 2012, 189, 5729–5744. [Google Scholar] [CrossRef] [PubMed]
- Caux, C.; Massacrier, C.; Vanbervliet, B.; Dubois, B.; Durand, I.; Cella, M.; Lanzavecchia, A.; Banchereau, J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood 1997, 90, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Klechevsky, E.; Liu, M.; Morita, R.; Banchereau, R.; Thompson-Snipes, L.; Palucka, A.K.; Ueno, H.; Banchereau, J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum. Immunol. 2009, 70, 281–288. [Google Scholar] [CrossRef]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef]
- Jegalian, A.G.; Facchetti, F.; Jaffe, E.S. Plasmacytoid dendritic cells: Physiologic roles and pathologic states. Adv. Anat. Pathol. 2009, 16, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, F.; de Wolfe-Peeters, C.; van den Oord, J.J.; Desmet, V.J. Immunohistochemical visualization of plasmacytoid T cells in paraffin sections. Hum. Pathol. 1987, 18, 1300. [Google Scholar] [CrossRef] [PubMed]
- Grouard, G.; Rissoan, M.C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, N.; Antonenko, S.; Lau, J.Y.; Liu, Y.J. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 2000, 192, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Victora, G.D.; Schwickert, T.A.; Guermonprez, P.; Meredith, M.M.; Yao, K.; Chu, F.-F.; Randolph, G.J.; Rudensky, A.Y.; Nussenzweig, M. In vivo analysis of dendritic cell development and homeostasis. Science 2009, 324, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maraskovsky, E.; Brasel, K.; Teepe, M.; Roux, E.R.; Lyman, S.D.; Shortman, K.; McKenna, H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. J. Exp. Med. 1996, 184, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Karsunky, H.; Merad, M.; Cozzio, A.; Weissman, I.L.; Manz, M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 2003, 198, 305–313. [Google Scholar] [CrossRef]
- Facchetti, F.; De Wolf-Peeters, C.; van den Oord, J.J.; De vos, R.; Desmet, V.J. Plasmacytoid T cells: A cell population normally present in the reactive lymph node. An immunohistochemical and electronmicroscopic study. Hum. Pathol. 1988, 19, 1085–1092. [Google Scholar] [CrossRef]
- Facchetti, F.; Vermi, W.; Mason, D.; Colonna, M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003, 443, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Rissoan, M.C.; Soumelis, V.; Kadowaki, N.; Grouard, G.; Briere, F.; de Waal Malefyt, R.; Liu, Y.-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999, 283, 1183–1186. [Google Scholar] [CrossRef]
- Facchetti, F.; Candiago, E.; Vermi, W. Plasmacytoid monocytes express IL3-receptor alpha and differentiate into dendritic cells. Histopathology 1999, 35, 88–89. [Google Scholar] [CrossRef]
- Olweus, J.; BitMansour, A.; Warnke, R.; Thompson, P.A.; Carballido, J.; Picker, L.J.; Lund-Johansen, F. Dendritic cell ontogeny: A human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA 1997, 94, 12551–12556. [Google Scholar] [CrossRef] [PubMed]
- Herling, M.; Teitell, M.A.; Shen, R.R.; Medeiros, L.J.; Jones, D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood 2003, 101, 5007–5009. [Google Scholar] [CrossRef]
- Facchetti, F.; de Wolf-Peeters, C.; van den Oord, J.J.; Meijer, C.J.; Pals, S.T.; Desmet, V.J. Anti-high endothelial venule monoclonal antibody HECA-452 recognizes plasmacytoid T cells and delineates an “extranodular” compartment in the reactive lymph node. Immunol. Lett. 1989, 20, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Marafioti, T.; Paterson, J.C.; Ballabio, E.; Reichard, K.K.; Tedoldi, S.; Hollowood, K.; Dictor, M.; Hansmann, M.L.; Pileri, S.A.; Dyer, M.J.; et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood 2008, 111, 3778–3792. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef]
- Dzionek, A.; Inagaki, Y.; Okawa, K.; Nagafune, J.; Rock, J.; Sohma, Y.; Winkels, G.; Zysk, M.; Yamaguchi, Y.; Schmitz, J. Plasmacytoid dendritic cells: From specific surface markers to specific cellular functions. Hum. Immunol. 2002, 63, 1133–1148. [Google Scholar] [CrossRef]
- Comeau, M.R.; Van der Vuurst de Vries, A.R.; Maliszewski, C.R.; Galibert, L. CD123bright plasmacytoid predendritic cells: Progenitors undergoing cell fate conversion? J. Immunol. 2002, 169, 75–83. [Google Scholar] [CrossRef]
- Matsui, T.; Connolly, J.E.; Michnevitz, M.; Chaussabel, D.; Yu, C.I.; Glaser, C.; Tindle, S.; Pypaert, M.; Freitas, H.; Piqueras, B.; et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J. Immunol. 2009, 182, 6815–6823. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Ohba, Y.; Yanai, H.; Negishi, H.; Mizutani, T.; Takaoka, A.; Taya, C.; Taniguchi, T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 2005, 434, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L.; Beiske, K.; Lund-Johansen, F.; Brandtzaeg, P.; Jahnsen, F.L. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001, 159, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Wagner, M.; Gunther, S.; Towarowski, A.; Tuma, E.; Rothenfusser, S.; Endres, S.; Hartmann, G. Plasmacytoid dendritic cells: A new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Investig. Dermatol. 2002, 119, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.-J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Majorana, A.; Roversi, L.; Gentili, F.; Marrelli, S.; Vermi, W.; Bardellini, E.; Sapelli, P.; Facchetti, F. Recruitment of dendritic cells in oral lichen planus. J. Pathol. 2005, 205, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.; Wollenberg, B.; Rothenfusser, S.; Wagner, M.; Wellisch, D.; Mack, B.; Giese, T.; Gires, O.; Endres, S.; Hartmann, G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003, 63, 6478–6487. [Google Scholar] [PubMed]
- Urosevic, M.; Dummer, R.; Conrad, C.; Beyeler, M.; Laine, E.; Burg, G.; Gilliet, M. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J. Natl. Cancer Inst. 2005, 97, 1143–1153. [Google Scholar] [CrossRef]
- Vermi, W.; Bonecchi, R.; Facchetti, F.; Bianchi, D.; Sozzani, S.; Festa, S.; Berenzi, A.; Cella, M.; Colonna, M. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathol. 2003, 200, 255–268. [Google Scholar] [CrossRef]
- Pashenkov, M.; Goess, G.; Wagner, C.; Hormann, M.; Jandl, T.; Moser, A.; Britten, C.M.; Smolle, J.; Koller, S.; Mauch, C.; et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5716–5724. [Google Scholar] [CrossRef]
- Chaperot, L.; Blum, A.; Manches, O.; Lui, G.; Angel, J.; Molens, J.P.; Plumas, J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 2006, 176, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Stary, G.; Bangert, C.; Tauber, M.; Strohal, R.; Kopp, T.; Stingl, G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 2007, 204, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Hou, D.; Baban, B.; Lee, J.R.; Antonia, S.J.; Messina, J.L.; Chandler, P.; Koni, P.A.; Mellor, A.L. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Investig. 2004, 114, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Chaperot, L.; Bendriss, N.; Manches, O.; Gressin, R.; Maynadie, M.; Trimoreau, F.; Orfeuvre, H.; Corront, B.; Feuillard, J.; Sotto, J.J.; et al. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood 2001, 97, 3210–3217. [Google Scholar] [CrossRef] [PubMed]
- Ceribelli, M.; Hou, Z.E.; Kelly, P.N.; Huang, D.W.; Wright, G.; Ganapathi, K.; Evbuomwan, M.O.; Pittaluga, S.; Shaffer, A.L.; Marcucci, G.; et al. A Druggable TCF4- and BRD4-Dependent Transcriptional Network Sustains Malignancy in Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancer Cell 2016, 30, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B.; Idoyaga, J.; Dalod, M.; Barrat, F.; Naik, S.; Trinchieri, G.; Tussiwand, R.; Cella, M.; Colonna, M. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nat. Rev. Immunol. 2023, 23, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Jardin, F.; Ruminy, P.; Parmentier, F.; Troussard, X.; Vaida, I.; Stamatoullas, A.; Leprêtre, S.; Penther, D.; Duval, A.B.; Picquenot, J.; et al. TET2 and TP53 mutations are frequently observed in blastic plasmacytoid dendritic cell neoplasm. Br. J. Haematol. 2011, 153, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, M.R.; Pileri, S. Molecular Features of Blastic Plasmacytoid Dendritic Cell Neoplasm: DNA Mutations and Epigenetics. Hematol. Oncol. Clin. N. Am. 2020, 34, 511–521. [Google Scholar] [CrossRef]
- Yin, C.C.; Pemmaraju, N.; You, M.J.; Li, S.; Xu, J.; Wang, W.; Tang, Z.; Alswailmi, O.; Bhalla, K.N.; Qazilbash, M.H.; et al. Integrated Clinical Genotype-Phenotype Characteristics of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers 2021, 13, 5888. [Google Scholar] [CrossRef]
- Bastidas Torres, A.N.; Cats, D.; Mei, H.; Fanoni, D.; Gliozzo, J.; Corti, L.; Paulli, M.; Vermeer, M.H.; Willemze, R.; Berti, E.; et al. Whole-genome analysis uncovers recurrent IKZF1 inactivation and aberrant cell adhesion in blastic plasmacytoid dendritic cell neoplasm. Genes Chromosomes Cancer 2020, 59, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Lasho, T.; Howard, M.; Finke, C.; Ketterling, R.L.; Al-Kali, A.; Pardanani, A.; Droin, N.; Gangat, N.; Tefferi, A.; et al. Biallelic inactivation of the retinoblastoma gene results in transformation of chronic myelomonocytic leukemia to a blastic plasmacytoid dendritic cell neoplasm: Shared clonal origins of two aggressive neoplasms. Blood Cancer J. 2018, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, Y.; Wang, W.; Yin, C.C.; Tang, G.; Aung, P.P.; Hu, S.; Lu, X.; Toruner, G.A.; Medeiros, L.J.; et al. Genomic aberrations involving 12p/ETV6 are highly prevalent in blastic plasmacytoid dendritic cell neoplasms and might represent early clonal events. Leuk. Res. 2018, 73, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Renosi, F.; Callanan, M.; Lefebvre, C. Genetics and Epigenetics in Neoplasms with Plasmacytoid Dendritic Cells. Cancers 2022, 14, 4132. [Google Scholar] [CrossRef] [PubMed]
- Leroux, D.; Mugneret, F.; Callanan, M.; Radford-Weiss, I.; Dastugue, N.; Feuillard, J.; Le Mée, F.; Plessis, G.; Talmant, P.; Gachard, N.; et al. CD4+, CD56+ DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: A study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood 2002, 99, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Emadali, A.; Hoghoughi, N.; Duley, S.; Hajmirza, A.; Verhoeyen, E.; Cosset, F.L.; Bertrand, P.; Roumier, C.; Roggy, A.; Suchaud-Martin, C.; et al. Haploinsufficiency for NR3C1, the gene encoding the glucocorticoid receptor, in blastic plasmacytoid dendritic cell neoplasms. Blood 2016, 127, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, M.; Novara, F.; Fiandrino, G.; Riboni, R.; Fanoni, D.; Arra, M.; Venegoni, L.; Nicola, M.; Dallera, E.; Arcaini, L.; et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: Focus on biallelic locus 9p21.3 deletion. Blood 2011, 118, 4591–4594. [Google Scholar] [CrossRef]
- Wiesner, T.; Obenauf, A.C.; Cota, C.; Fried, I.; Speicher, M.R.; Cerroni, L. Alterations of the cell-cycle inhibitors p27(KIP1) and p16(INK4a) are frequent in blastic plasmacytoid dendritic cell neoplasms. J. Investig. Dermatol. 2010, 130, 1152–1157. [Google Scholar] [CrossRef]
- Sapienza, M.R.; Fuligni, F.; Agostinelli, C.; Tripodo, C.; Righi, S.; Laginestra, M.A.; Pileri, A.; Mancini, M.; Rossi, M.; Ricci, F.; et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014, 28, 1606–1616. [Google Scholar] [CrossRef]
- Julia, F.; Petrella, T.; Beylot-Barry, M.; Bagot, M.; Lipsker, D.; Machet, L.; Joly, P.; Dereure, O.; Wetterwald, M.; D‘Incan, M.; et al. Blastic plasmacytoid dendritic cell neoplasm: Clinical features in 90 patients. Br. J. Dermatol. 2013, 169, 579–586. [Google Scholar] [CrossRef]
- Cota, C.; Vale, E.; Viana, I.; Requena, L.; Ferrara, G.; Anemona, L.; Metze, D.; Fink-Puches, R.; Wiesner, T.; Cerroni, L. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am. J. Surg. Pathol. 2010, 34, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Zhang, H.W.; Wang, L.; Bai, W.; Wang, J.F. Blastic plasmacytoid dendritic cell neoplasm with skin and bone marrow involvement: Report of three cases. World J. Clin. Cases 2021, 9, 10293–10299. [Google Scholar] [CrossRef] [PubMed]
- Hirner, J.P.; O’Malley, J.T.; LeBoeuf, N.R. Blastic Plasmacytoid Dendritic Cell Neoplasm: The Dermatologist’s Perspective. Hematol. Oncol. Clin. N. Am. 2020, 34, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Zhou, S.; El Jamal, S.M.; Lane, A.A.; Mascarenhas, J. Blastic Plasmacytoid Dendritic Cell Neoplasm-Current Insights. Clin. Lymphoma Myeloma Leuk. 2019, 19, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.; Bagot, M.; Willemze, R.; Beylot-Barry, M.; Vergier, B.; Delaunay, M.; Meijer, C.J.; Courville, P.; Joly, P.; Grange, F.; et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): A review. Am. J. Clin. Pathol. 2005, 123, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, C.; Bellolio, E.; Lopez, E.; Goldberg, I.; Geller, S.; Navarrete-Dechent, C. Dermoscopy of blastic plasmacytoid dendritic cell neoplasm in two patients. Clin. Exp. Dermatol. 2021, 46, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Denizon, N.; Besancon, A.; Farhi, J.; Lemaire, P.; Sandrini, J.; Truong, C.; Ghnaya, H.; de Materre, A.B. Blastic Plasmacytoid Dendritic Cell Neoplasm: From Origin of the Cell to Targeted Therapies. Biol. Blood Marrow Transplant. 2016, 22, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Haddadin, M.; Upadhyay, V.A.; Grussie, E.; Mehta-Shah, N.; Brunner, A.M.; Louissaint, A.; Lovitch, S.B.; Dogan, A.; Fathi, A.T.; et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood 2019, 134, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Feuillard, J.; Jacob, M.C.; Valensi, F.; Maynadie, M.; Gressin, R.; Chaperot, L.; Arnoulet, C.; Brignole-Baudouin, F.; Drénou, B.; Duchayne, E.; et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood 2002, 99, 1556–1563. [Google Scholar] [CrossRef]
- Pileri, A.; Delfino, C.; Grandi, V.; Agostinelli, C.; Pileri, S.A.; Pimpinelli, N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): The cutaneous sanctuary. G. Ital. Dermatol. Venereol. 2012, 147, 603–608. [Google Scholar]
- Hashikawa, K.; Niino, D.; Yasumoto, S.; Nakama, T.; Kiyasu, J.; Sato, K.; Kimura, Y.; Takeuchi, M.; Sugita, Y.; Hashimoto, T.; et al. Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. J. Am. Acad. Dermatol. 2012, 66, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, K.; Wang, Z. Atypical presentation of blastic plasmacytoid dendritic cell neoplasm: A potential diagnostic pitfall in nasal cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, e212–e214. [Google Scholar] [CrossRef]
- Thompson, L.D. Small round blue cell tumors of the sinonasal tract: A differential diagnosis approach. Mod. Pathol. 2017, 30 (Suppl. S1), S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.J.; Iriarte, C.; Moslehi, D.; Sheets, A.R.; LeBoeuf, N.R. Blastic plasmacytoid dendritic cell neoplasm mimicking dermatomyositis. JAAD Case Rep. 2023, 39, 70–73. [Google Scholar] [CrossRef]
- Chang, H.J.; Lee, M.D.; Yi, H.G.; Lim, J.H.; Lee, M.H.; Shin, J.H.; Choi, S.J.; Moon, Y.; Nahm, C.H.; Kim, C.S. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res. Treat. 2010, 42, 239–243. [Google Scholar] [CrossRef]
- Nomura, H.; Egami, S.; Kasai, H.; Yokoyama, T.; Fujimoto, A.; Sugiura, M.; Yagi, H.; Iwafuchi, H.; Kudo, K. Blastic plasmacytoid dendritic cell neoplasm in a 7-year-old girl with a solitary skin lesion mimicking traumatic purpura. Acta Derm. Venereol. 2015, 95, 231–232. [Google Scholar] [CrossRef][Green Version]
- Garnache-Ottou, F.; Vidal, C.; Biichle, S.; Renosi, F.; Poret, E.; Pagadoy, M.; Desmarets, M.; Roggy, A.; Seilles, E.; Soret, L.; et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019, 3, 4238–4251. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Fragliasso, V.; Broggi, G.; Palicelli, A.; Loscocco, G.G.; Cresta, C.; Caprera, C.; Corsi, M.; et al. Skin Involvement by Hematological Neoplasms with Blastic Morphology: Lymphoblastic Lymphoma, Blastoid Variant of Mantle Cell Lymphoma and Differential Diagnoses. Cancers 2023, 15, 3928. [Google Scholar] [CrossRef]
- Sukswai, N.; Aung, P.P.; Yin, C.C.; Li, S.; Wang, W.; Wang, S.A.; Ortega, V.; Lyapichev, K.; Nagarajan, P.; Alfattal, R.; et al. Dual Expression of TCF4 and CD123 Is Highly Sensitive and Specific For Blastic Plasmacytoid Dendritic Cell Neoplasm. Am. J. Surg. Pathol. 2019, 43, 1429–1437. [Google Scholar] [CrossRef]
- Assaf, C.; Gellrich, S.; Whittaker, S.; Robson, A.; Cerroni, L.; Massone, C.; Kerl, H.; Rose, C.; Chott, A.; Chimenti, S.; et al. CD56-positive haematological neoplasms of the skin: A multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. J. Clin. Pathol. 2007, 60, 981–989. [Google Scholar] [CrossRef]
- Gholami, S.; Mohammadi, S.M.; Movasaghpour Akbari, A.; Abedelahi, A.; Alihemmati, A.; Fallahi, S.; Charoudeh, H.N. Terminal Deoxynucleotidyl Transferase (TdT) Inhibiti on of Cord Blood Derived B and T Cells Expansion. Adv. Pharm. Bull. 2017, 7, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, J.; Lindsey, K. Cytologic features of blastic plasmacytoid dendritic cell neoplasm involving liver: A case report and literature review. Diagn. Cytopathol. 2021, 49, E80–E83. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D., 2nd; Zhang, L.; Sokol, L. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr. Treat. Options Oncol. 2019, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saab Chalhoub, M.W.; Singh, D. Blastic Plasmacytoid Dendritic Cell Neoplasm; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jeong, D.; Choi, J.W.; Jeong, K.; Sokol, L. CT findings associated with blastic plasmacytoid dendritic cell neoplasm: A case report. Acta Radiol. Open 2016, 5, 2058460116657688. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kurokawa, R.; Amemiya, S.; Koyamaa, H.; Matsuda, K.; Honda, A.; Kurokawa, M.; Shinozaki-Ushiku, A.; Abe, O. Rapidly progressing blastic plasmacytoid dendritic cell neoplasm causing diffuse skin thickening: A case report with sequential computed tomography examinations. Radiol. Case Rep. 2021, 16, 2929–2933. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Chakraborty, S.; Faiz, K.; Shankar, J. Imaging of the Spontaneous Low Cerebrospinal Fluid Pressure Headache: A Review. Can Assoc. Radiol. J. 2020, 71, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Cuglievan, B.; Connors, J.; He, J.; Khazal, S.; Yedururi, S.; Dai, J.; Garces, S.; Quesada, A.E.; Roth, M.; Garcia, M.; et al. Blastic plasmacytoid dendritic cell neoplasm: A comprehensive review in pediatrics, adolescents, and young adults (AYA) and an update of novel therapies. Leukemia 2023, 37, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, Y.; Hama, A.; Muramatsu, H.; Nakatochi, M.; Gunji, M.; Ichikawa, D.; Hamada, M.; Taniguchi, R.; Kataoka, S.; et al. Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2017, 31, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Soza-Ried, C.; Hess, I.; Netuschil, N.; Schorpp, M.; Boehm, T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc. Natl. Acad. Sci. USA 2010, 107, 17304–17308. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Gonda, T.J. Role and potential for therapeutic targeting of MYB in leukemia. Leukemia 2013, 27, 269–277. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: An Italian multicenter study. Haematologica 2013, 98, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Saito, S.; Yanagisawa, R.; Tanaka, M.; Yoshikawa, K.; Hirabayashi, K.; Tsukahara, K.; Motobayashi, M.; Nakazawa, Y.; Koike, K. Usefulness of allogeneic hematopoietic stem cell transplantation in first complete remission for pediatric blastic plasmacytoid dendritic cell neoplasm with skin involvement: A case report and review of literature. Pediatr. Blood Cancer 2013, 60, E140–E142. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sweet, K. Blastic Plasmacytoid Dendritic Cell Neoplasm. J. Natl. Compr. Cancer Netw. 2023, 21, 515–521. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.; Yin, L.; Zhou, L.; Deng, Y.; Deng, H. Diagnosis, treatment, and genetic characteristics of blastic plasmacytoid dendritic cell neoplasm: A review. Medicine 2023, 102, e32904. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A. The 2016 WHO classification of acute myeloid leukemia: What the practicing clinician needs to know. Semin Hematol. 2019, 56, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Pemmaraju, N.; Medeiros, B.C.; Carraway, H.E.; Frankfurt, O.; Forman, S.J.; Yang, X.A.; Konopleva, M.; et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014, 124, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.R.; Lane, A.A. Tagraxofusp for blastic plasmacytoid dendritic cell neoplasm. Haematologica 2024, 109, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Valentini, C.G.; Pagano, L. Tagraxofusp for blastic plasmacytoid dendritic cell neoplasm: A 2-speed cure in the United States and European Union. Blood Adv. 2023, 7, 7084–7086. [Google Scholar] [CrossRef]
- Faustmann, P.; Schroeder, J.C.; Mix, L.; Harland, L.; Riedel, A.; Vogel, W.; Lengerke, C.; Wirths, S. Real-world evidence on tagraxofusp for blastic plasmacytoid dendritic cell neoplasm-collected cases from a single center and case reports. Front. Oncol. 2024, 14, 1384172. [Google Scholar] [CrossRef]
- Azad, F.; Zhang, J.; Miranda, C.J.; Gravina, M. Venetoclax and Azacitidine in the Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm Refractory to Conventional Therapy. Cureus 2022, 14, e33109. [Google Scholar] [CrossRef] [PubMed]
- Sibai, J.; Chen, R.; Nabhani, I.A.; Perusini, M.A.; Sibai, H. Foot gangrene following Tagraxofusp treatment for blastic plasmacytoid dendritic cell neoplasm: Case report. eJHaem 2022, 3, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Mouhayar, E.N.; Hammond, D.; Lopez-Mattei, J.; Banchs, J.; Konopleva, M.; Pemmaraju, N. Reversible Myocardial Edema Secondary to Tagraxofusp-Induced Capillary Leak Syndrome. JACC Cardio Oncol. 2021, 3, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Koerber, R.M.; Held, S.A.E.; Vonnahme, M.; Feldmann, G.; Wenzel, J.; Gutgemann, I.; Brossart, P.; Heine, A. Blastic plasmacytoid dendritic-cell neoplasia: A challenging case report. J. Cancer Res. Clin. Oncol. 2022, 148, 743–748. [Google Scholar] [CrossRef]
- Egger, A.; Coello, D.; Kirsner, R.S.; Brehm, J.E. A Case of Cutaneous Blastic Plasmacytoid Dendritic Cell Neoplasm Treated With a Bcl-2 Inhibitor. J. Drugs Dermatol. 2021, 20, 550–551. [Google Scholar] [PubMed]
- Acedo, R.D.; Muñoz, M.D.; Laguna, C.N.; Camacho, R.M.; Pilo, I.S.; Ruiz-Mateos, V.P.C.; Ramírez, M.Y.; de Medina, M.V.S.; Criado, S.A.; Pérez, A.R.; et al. Tagraxofusp as first-line treatment for blastic plasmacytoid dendritic cell neoplasm. Leuk. Lymphoma 2022, 63, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- Massone, C.; Raiola, A.M.; Dominietto, A.; Minetto, P.; Beltramini, S.; Cerroni, L.; Sola, S.; Angelucci, E. Blastic Plasmacytoid Dendritic Cell Neoplasm: Underlining the importance of an early diagnosis and the use of tagraxofusp therapy before wide dissemination. Australas J. Dermatol. 2021, 62, e316–e318. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, H.; Kim, Y.; Karras, N.; Pawlowska, A.; Toomey, D.; Kyono, W.; Gaynon, P.; Rosenthal, J.; Stein, A. First pediatric experience of SL-401, a CD123-targeted therapy, in patients with blastic plasmacytoid dendritic cell neoplasm: Report of three cases. J. Hematol. Oncol. 2018, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Y.; Wang, Y.L.; Pei, J.; Mansoor, N.; Styler, M.; Testa, J.R.; Nejati, R. Multiple Genomic Alterations, Including a Novel AFF4::IRF1 Fusion Gene, in a Treatment-Refractory Blastic Plasmacytoid Dendritic-Cell Neoplasm: A Case Report and Literature Review. Int. J. Mol. Sci. 2023, 25, 305. [Google Scholar] [CrossRef]
- Hu, X.; Ediriwickrema, A.; Saleem, A.; Tan, B.; Pemmaraju, N.; Mannis, G.N. CD38 and BCL2 expression guides treatment with daratumumab and venetoclax in tagraxofusp-refractory blastic plasmacytoid dendritic cell neoplasm (BPDCN) featuring dynamic loss of CD123. Leuk. Res. 2024, 139, 107479. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Deconinck, E.; Mehta, P.; Walker, I.; Herling, M.; Garnache-Ottou, F.; Gabarin, N.; Campbell, C.J.; Duell, J.; Moshe, Y.; et al. Recent Advances in the Biology and CD123-Directed Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm. Clin. Lymphoma Myeloma Leuk. 2024, 24, e130–e137. [Google Scholar] [CrossRef]
- Aoki, T.; Suzuki, R.; Kuwatsuka, Y.; Kako, S.; Fujimoto, K.; Taguchi, J.; Kondo, T.; Ohata, K.; Ito, T.; Kamoda, Y.; et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood 2015, 125, 3559–3562. [Google Scholar] [CrossRef]
- Kharfan-Dabaja, M.A.; Al Malki, M.M.; Deotare, U.; Raj, R.V.; El-Jurdi, N.; Majhail, N.; Cherry, M.A.; Bashir, Q.; Darrah, J.; Nishihori, T.; et al. Haematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: A North American multicentre collaborative study. Br. J. Haematol. 2017, 179, 781–789. [Google Scholar] [CrossRef]
- Roos-Weil, D.; Dietrich, S.; Boumendil, A.; Polge, E.; Bron, D.; Carreras, E.; Atienza, A.I.; Arcese, W.; Beelen, D.W.; Cornelissen, J.J.; et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: A retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2013, 121, 440–446. [Google Scholar] [CrossRef]
- Murthy, H.S.; Zhang, M.J.; Chen, K.; Ahmed, S.; Deotare, U.; Ganguly, S.; Kansagra, A.; Michelis, F.V.; Nishihori, T.; Patnaik, M.; et al. Allogeneic hematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: A CIBMTR analysis. Blood Adv. 2023, 7, 7007–7016. [Google Scholar] [CrossRef]
- Montero, J.; Stephansky, J.; Cai, T.; Griffin, G.K.; Cabal-Hierro, L.; Togami, K.; Hogdal, L.J.; Galinsky, I.; Morgan, E.A.; Aster, J.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017, 7, 156–164. [Google Scholar] [CrossRef]
- Lane, A.A. Novel Therapies for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematol. Oncol. Clin. N. Am. 2020, 34, 589–600. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Konopleva, M.; Lane, A.A. More on Blastic Plasmacytoid Dendritic-Cell Neoplasms. N. Engl. J. Med. 2019, 380, 695–696. [Google Scholar] [CrossRef]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Stratigos, A.; Trakatelli, M.; Puig, L.; The EADV Burden of Skin Diseases Project Team. The journey of patients with skin diseases from the first consultation to the diagnosis in a representative sample of the European general population from the EADV burden of skin diseases study. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S7), 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rubsam, M.L.; Esch, M.; Baum, E.; Bosner, S. Diagnosing skin disease in primary care: A qualitative study of GPs’ approaches. Fam. Pract. 2015, 32, 591–595. [Google Scholar] [CrossRef] [PubMed]
| Mutated genes [58,59,60,61,62,63] | TET2 ASXL1 IKZF1 RB1 ETV6 NR3C1 MYC |
| Chromosomal losses [64,65,66] | 5q deletion 12p deletion, 13q deletion or monosomy 13 6q deletion 15q deletion or monosomy 15 monosomy 9 |
| Differential Diagnosis | |
|---|---|
| Non Neoplastic Diseases | Neoplastic Diseases |
| Systemic lupus erythematosus | AML/leukemia cutis |
| Psoriasis | NK/T-cell leukemia/lymphoma |
| Lichen planus | hematological neoplasms with blastic morphology |
| Contact dermatitis | Kaposi sarcoma |
| Traumatic purpura | angiosarcoma |
| Merkel cell carcinoma | |
| Clinical features |
|
| Dermoscopic features |
|
| Histologic features |
|
| Immunophenotype |
|
| Acute myeloid leukemia regimen |
|
| Acute lymphoblastic leukemia regimen |
|
| Lymphoma regimens |
|
| Biologic agent |
|
| Allo-HSCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Raimondo, C.; Lozzi, F.; Di Domenico, P.P.; Paganini, C.; Campione, E.; Galluzzo, M.; Bianchi, L. Blastic Plasmacytoid Dendritic Cell Neoplasm, from a Dermatological Point of View. Int. J. Mol. Sci. 2024, 25, 7099. https://doi.org/10.3390/ijms25137099
Di Raimondo C, Lozzi F, Di Domenico PP, Paganini C, Campione E, Galluzzo M, Bianchi L. Blastic Plasmacytoid Dendritic Cell Neoplasm, from a Dermatological Point of View. International Journal of Molecular Sciences. 2024; 25(13):7099. https://doi.org/10.3390/ijms25137099
Chicago/Turabian StyleDi Raimondo, Cosimo, Flavia Lozzi, Pier Paolo Di Domenico, Claudia Paganini, Elena Campione, Marco Galluzzo, and Luca Bianchi. 2024. "Blastic Plasmacytoid Dendritic Cell Neoplasm, from a Dermatological Point of View" International Journal of Molecular Sciences 25, no. 13: 7099. https://doi.org/10.3390/ijms25137099
APA StyleDi Raimondo, C., Lozzi, F., Di Domenico, P. P., Paganini, C., Campione, E., Galluzzo, M., & Bianchi, L. (2024). Blastic Plasmacytoid Dendritic Cell Neoplasm, from a Dermatological Point of View. International Journal of Molecular Sciences, 25(13), 7099. https://doi.org/10.3390/ijms25137099






