Special Issue “Design, Synthesis and Applications of Macroporous, Mesoporous, and Microporous Materials”
1. Porous Inorganic Materials
2. Porous Organic and Hybrid Materials
Acknowledgments
Conflicts of Interest
References
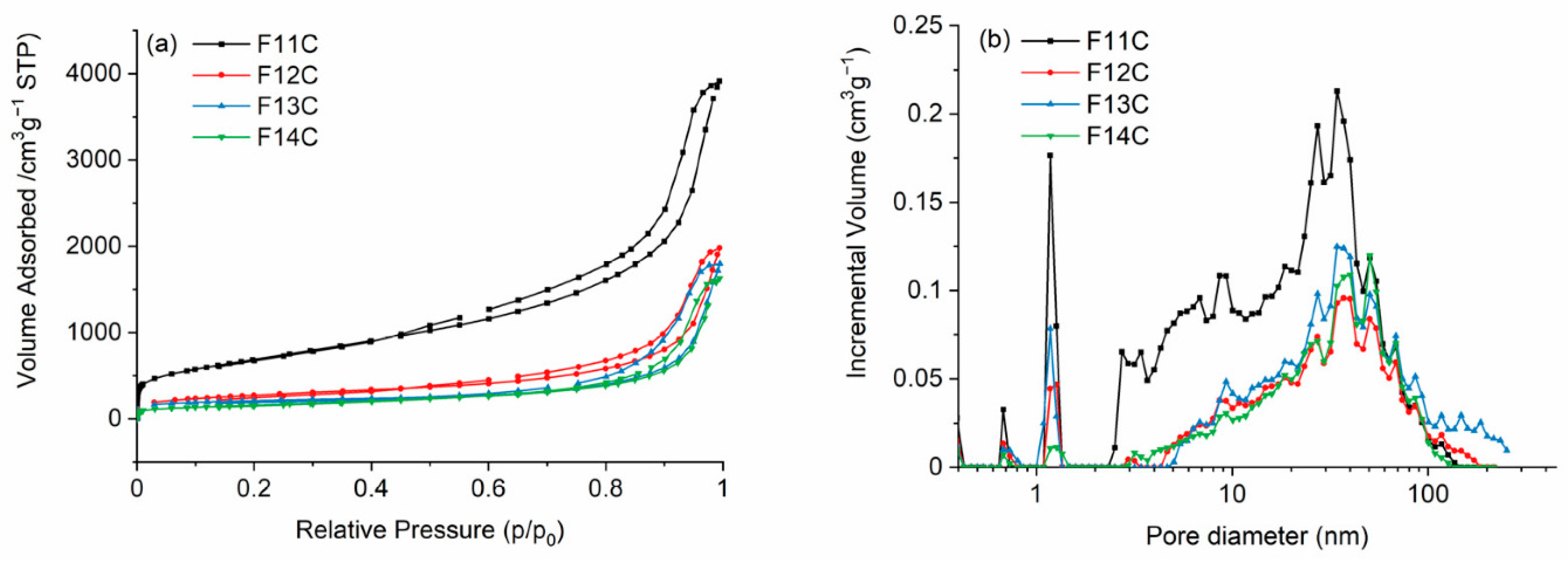
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Liu, T.; Liu, G. Porous Organic Materials Offer Vast Future Opportunities. Nat. Comm. 2020, 11, 4984. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.; Kim, J. Inverse Design of Porous Materials Using Artificial Neural Networks. Sci. Adv. 2020, 6, eaax9324. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Recent Developments in Processing Techniques and Morphologies of Bulk Macroporous Ceramics for Multifunctional Applications. Mater. Today Commun. 2024, 38, 107752. [Google Scholar] [CrossRef]
- Saeidi, B.; Derakhshandeh, M.R.; Chermahini, D.; Doostmohammadi, M.A. Novel Porous Barium Titanate/nano-bioactive Glass Composite with High Piezoelectric Coefficient for Bone R egeneration Applications. J. Mater. Eng. Perform. 2020, 29, 5420–5427. [Google Scholar] [CrossRef]
- Raja, N.; Sung, A.; Park, H.; Yun, H.S. Low-temperature Fabrication of Calcium Deficient Hydroxyapatite Bone Scaffold by Optimization of 3D Printing Conditions. Ceram. Int. 2021, 47, 7005–7016. [Google Scholar] [CrossRef]
- Sharma, S.; Talukdar, P. Thermo-mechanical Analysis of a Porous Volumetric Solar Receiver Subjected to Concentrated Solar Radiation. Sol. Energy 2022, 247, 41–54. [Google Scholar] [CrossRef]
- Baux, A.; Goillot, A.; Jacques, S.; Heisel, C.; Rochais, D.; Charpentier, L.; David, P.; Piquero, T.; Chartier, T.; Chollon, G. Synthesis and Properties of Macroporous SiC Ceramics Synthesized by 3D Printing and Chemical Vapor Infiltration/Deposition. J. Eur. Ceram. Soc. 2020, 40, 2834–2854. [Google Scholar] [CrossRef]
- Baux, A.; Jacques, S.; Allemand, A.; Vignoles, G.L.; David, P.; Piquero, T.; Stempin, M.P.; Chollon, G. Complex Geometry Macroporous SiC Ceramics Obtained by 3D-Printing, Polymer Impregnation and Pyrolysis (PIP) and Chemical Vapor Deposition (CVD). J. Eur. Ceram. Soc. 2021, 41, 3274–3284. [Google Scholar] [CrossRef]
- Barreto, G.; Canhoto, P.; Collares-Pereira, M. Parametric Analysis and Optimisation of Porous Volumetric Solar Receivers Made of Open-Cell SiC Ceramic Foam. Energy 2020, 200, 117476. [Google Scholar] [CrossRef]
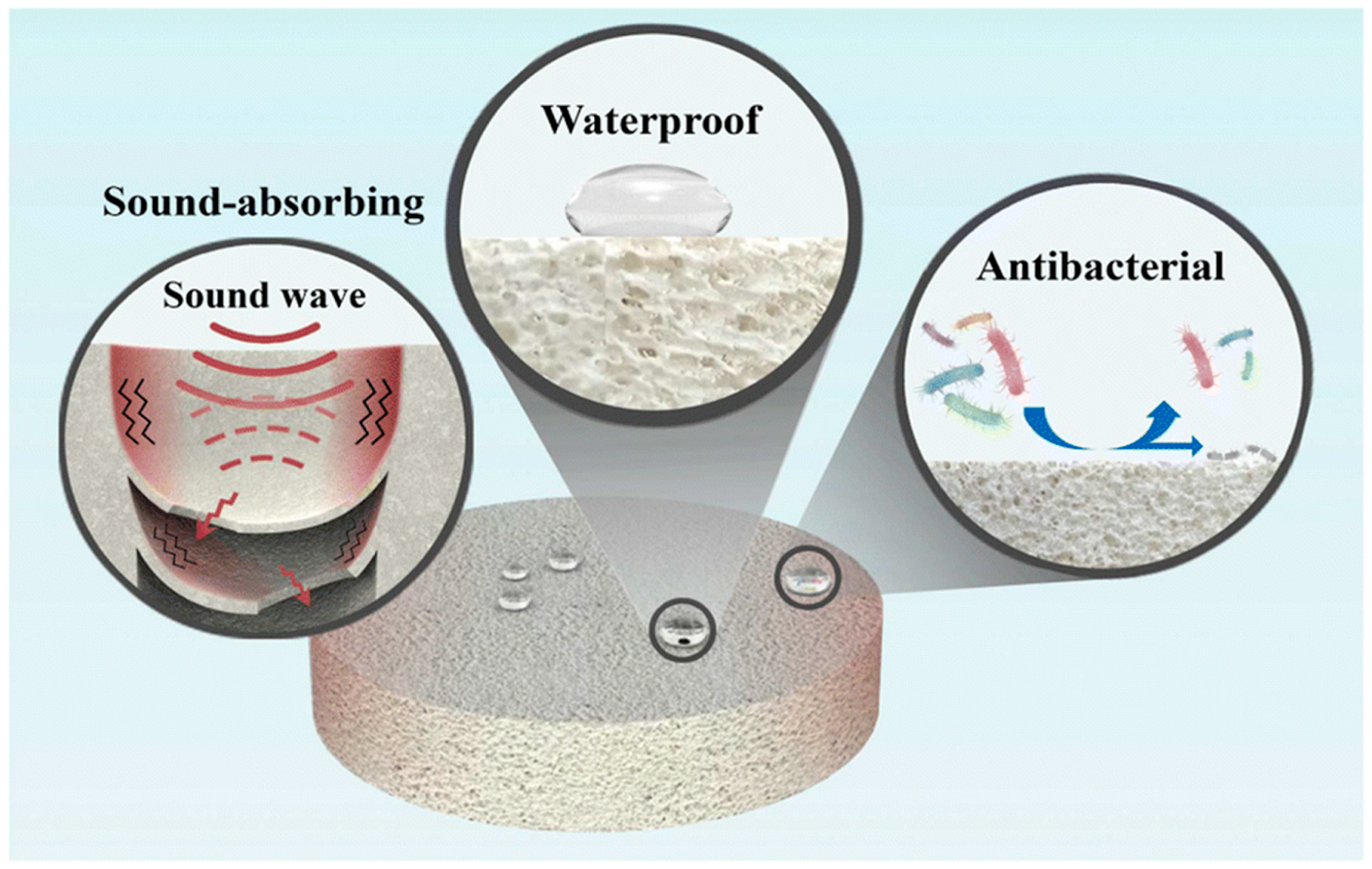
- Zhang, X.; Chen, X.; Min, W.; Liang, G.; Zhang, W.; Yao, S.; Zhong, X. Preparation of Multifunctional Ceramic Foams for Sound Absorption, Waterproofing, and Antibacterial Applications. RSC Adv. 2024, 14, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Vafaeenezhad, S.; Hanifi, A.R.; Laguna-Bercero, M.A.; Etsell, T.H.; Sarkar, P. Microstructure and Long-Term Stability of Ni-YSZ Anode Supported Fuel Cells: A Review. Mater. Futur. 2022, 1, 042101. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on Fabrication Techniques for Porous Electrodes of Solid Oxide Fuel Cells by Sacrificial Template Methods. Renew. Sustain. Energy Rev. 2017, 77, 1221–1239. [Google Scholar] [CrossRef]
- Chatterjee, S.; Jeevanandham, S.; Mukherjee, M.; Vo, D.-V.N.; Mishra, V. Significance of Re-engineered Zeolites in Climate Mitigation–A review for Carbon Capture and Separation. J. Environ. Chem. Eng. 2021, 9, 105957. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef] [PubMed]
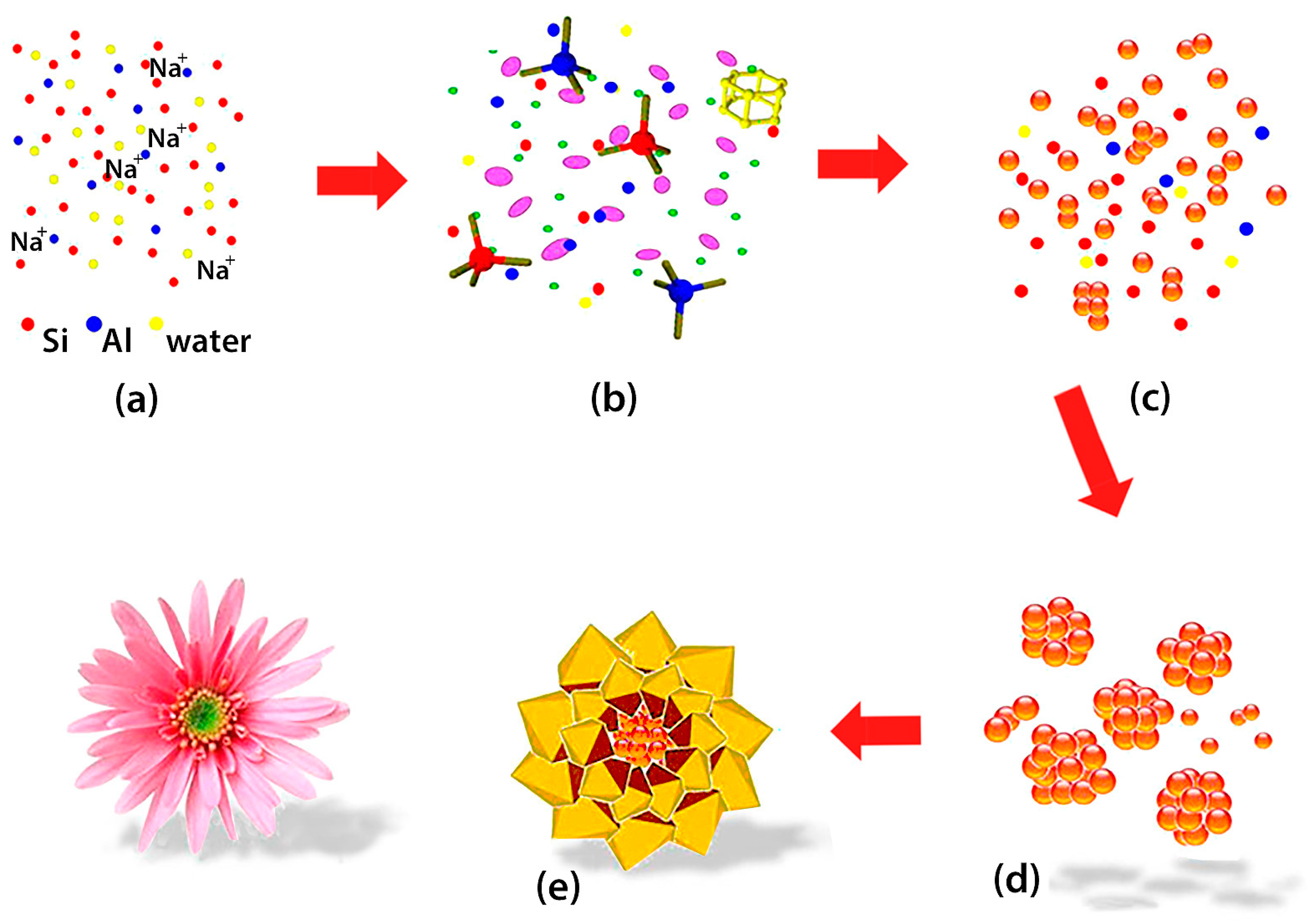
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Jia, X.; Wasim, K.; Zhijie Wu, Z.; Choi, J.; Yip, A.C.K. Modern Synthesis Strategies for Hierarchical Zeolites: Bottom-up versus Top-Down Strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Du, Y.; Kong, Q.; Gao, Z.; Wang, Z.; Zheng, J.; Qin, B.; Pan, M.; Li, W.; Li, R. Flowerlike Hierarchical Y with Dramatically Increased External Surface: A Potential Catalyst Contributing to Improving Pre-Cracking for Bulky Reactant Molecules. Ind. Eng. Chem. Res. 2018, 57, 7395–7403. [Google Scholar] [CrossRef]
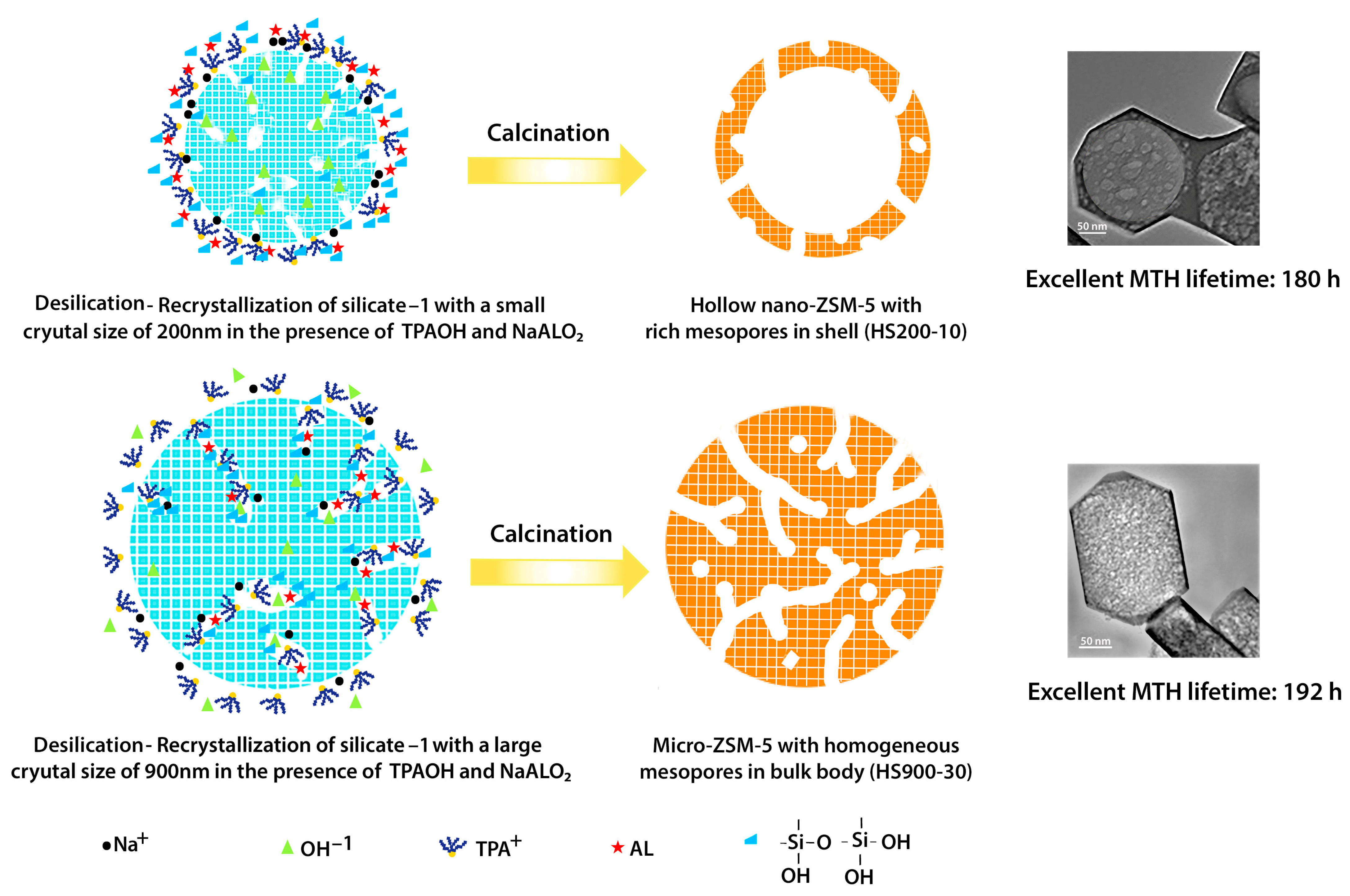
- Ma, Z.; Fu, T.; Wang, Y.; Shao, J.; Ma, Q.; Zhang, C.; Cui, L.; Li, Z. Silicalite-1 Derivational Desilication-Recrystallization to Prepare Hollow Nano-ZSM-5 and Highly Mesoporous Micro-ZSM-5 Catalyst for Methanol to Hydrocarbons. Ind. Eng. Chem. Res. 2019, 58, 2146–2158. [Google Scholar] [CrossRef]
- Liaquat, I.; Munir, R.; Abbasi, N.A.; Sadia, B.; Muneer, A.; Younas, F.; Sardar, M.F.; Zahid, M.; Noreen, S. Exploring Zeolite-Based Composites in Adsorption and Photocatalysis for Toxic Wastewater Treatment: Preparation, Mechanisms, and Future Perspectives. Environ. Pollut. 2024, 349, 123922. [Google Scholar] [CrossRef]
- Liang, D.; Liu, Y.; Zhang, R.; Xie, Q.; Zhang, L. A Review on the Influence Factors in the Synthesis of Zeolites and the Transformation Behavior of Silicon and Aluminum During the Process. Comments Inorg. Chem. 2024, 1–37. [Google Scholar] [CrossRef]
- Colomer, M.T.; Rubio, F. Textural Characteristics, Degree of Protonation, Water Uptake and Proton Transport Properties Relationships in Colloidal Sol-Gel Derived Micro- and Mesoporous Silica Membranes. Int. J. Hydrog. 2016, 41, 5748–5757. [Google Scholar] [CrossRef]
- Colomer, M.T.; Zenzinger, K. Mesoporous α-Fe2O3 Membranes as Proton Conductors: Synthesis by Microwave-Assisted Sol-Gel Route and Effect of their Textural Characteristics on Water Uptake and Proton Conductivity. Microp. Mesop. Mater. 2012, 161, 123–133. [Google Scholar] [CrossRef]
- Yu, X.; Williams, C.T. Recent Advances in the Applications of Mesoporous Silica in Heterogeneous Catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Colomer, M.T. Nanoporous Anatase Thin Films as Fast Proton-Conducting Materials. Adv. Mater. 2006, 18, 371–374. [Google Scholar] [CrossRef]
- Nogami, N.; Nagao, R.; Wong, C. Proton Conduction in Porous Silica Glasses with High Water Content. J. Phys. Chem. B 1998, 102, 5772–5775. [Google Scholar] [CrossRef]
- Celik, E.; Ma, Y.; Brezesinski, T.; Elm, M.T. Ordered Mesoporous Metal Oxides for Electrochemical Applications: Correlation between Structure, Electrical Properties and Device Performance. Phys. Chem. Chem. Phys. 2021, 23, 10706–10735. [Google Scholar] [CrossRef]
- Colomer, M.T.; Vattier, F. Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase. Int. J. Mol. Sci. 2022, 23, 13162. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfati, L. Mesoporous Ordered Titania Films: An Advanced Platform for Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2024, 58, 100646. [Google Scholar] [CrossRef]
- Innocenzi, P. Mesoporous Ordered Films via Self-Assembly: Trends and Perspectives. Chem. Sci. 2022, 13, 13264–13279. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Gialanella, S.; Pirola, C.; Ragaini, V. Tailored Anatase/Brookite Nanocrystalline TiO2: The Optimal Particle Features for Liquid- and Gas-Phase Photocatalytic Reactions. J. Phys. Chem. C 2007, 111, 13222–13231. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Yu, H.; Zhao, X. Ultrasonic Preparation of Mesoporous Titanium Dioxide Nanocrystalline Photocatalysts and Evaluation of Photocatalytic Activity. J. Mol. Catal. A Chem. 2005, 227, 75–80. [Google Scholar] [CrossRef]
- Yu, J.; Godiksen, A.L.; Mamahkel, A.; Søndergaard-Pedersen, F.; Rios-Carvajal, T.; Marks, M.; Lock, N.; Rasmussen, S.B.; Iversen, B.B. Selective Catalytic Reduction of NO Using Phase-Pure Anatase, Rutile, and Brookite TiO2 Nanocrystals. Inorg. Chem. 2020, 59, 15324–15334. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Fornasiero, P. Brookite: Nothing New Under the Sun? Catalysts 2017, 7, 304. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.D.; Oskam, G. Phase-pure TiO2 Nanoparticles: Anatase, Brookite and Rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wu, L.; Liu, Y.; Gao, T.; Li, K.; Long, Y.; Zhang, R.; Zhang, L.; Qiao, Z.A.; Huo, Q.S.; et al. Controllable Synthesis of Mesoporous TiO2 Polymorphs with Tunable Crystal Structure for Enhanced Photocatalytic H2 Production. Adv. Energy Mater. 2019, 9, 1901634. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Yu, J. Direct Sonochemical Preparation and Characterization of Highly Active Mesoporous TiO2 with a Bicrystalline Framework. Chem. Mater. 2002, 14, 42647–44653. [Google Scholar] [CrossRef]
- Paul, R.; Kavinarmatha, K.; Parthiban, S. Tantalum Doped Titanium Dioxide Nanoparticles for Efficient Photocatalytic Degradation of Dyes. J. Mol. Struct. 2023, 1277, 134869. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzalka, M.; Blachnio, M.; Derylo-Marczewska, A.; Winter, S.; Maciejewska, M. Mesoporous Carbons and Highly Cross-Linking Polymers for Removal of Cationic Dyes from Aqueous Solutions—Studies on Adsorption Equilibrium and Kinetics. Materials 2024, 17, 1374. [Google Scholar] [CrossRef]
- Samejo, B.A.; Naseer, K.; Samejo, S.; Janjhi, F.A.; Memon, N.; Castro-Muñoz, N.; Boczkaj, G. MXene-based Composites for Capacitive Deionization—The Advantages, Progress, and their Role in Desalination—A Review. Water Resour. Ind. 2024, 31, 100230. [Google Scholar] [CrossRef]
- Wu, S.; Qu, X.; Zhu, J.; Liu, X.; Mao, H.; Wang, K.; Zhou, G.; Chi, J.; Wang, L. Recent Advances in Metal-Organic Frameworks Derived Electrocatalysts for Oxygen Reduction Reaction. J. Alloys Compd. 2024, 970, 172518. [Google Scholar] [CrossRef]
- Mazo, M.A.; Colomer, M.T.; Tamayo, A.; Rubio, J. Hierarchical Porous Fluorine-doped Silicon Oxycarbide Derived Materials: Physicochemical Characterization and Electrochemical Behaviour. Microp. Mesop. Mat. 2022, 330, 111604. [Google Scholar] [CrossRef]
- Mérida, J.; Colomer, M.T.; Rubio, F.; Mazo, M.A. Highly Porous Carbon Materials Derived from Silicon Oxycarbides and Effect of the Pyrolysis Temperature on Their Electrochemical Response. Int. J. Mol. Sci. 2023, 24, 13868. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Hanaor, D.A.H.; Shen, X.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures- A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, 1907176. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Deng, C.; Gao, H.; Jiao, B.; Liu, Y.; Chen, F.; Deng, L.; Xiong, W. 3D Printing of Nanowrinkled Architectures via Laser Direct Assembly. Sci. Adv. 2022, 8, eabn9942. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Xu, X.; Lu, W.; Zhao, N.; Han, F.; Chen, S.C. Ultrafast 3D Nanofabrication Via Digital Holography. Nat. Commun. 2023, 14, 1716. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Zappitelli, K.M.; Singh, L.; Yuan, R.C.; Bemrose, M.; Brogden, V.; Miller, D.J.; Matthew, C.; Smear, M.C.; Cogan, S.F.; et al. Direct Laser Writing of 3D Electrodes on Flexible Substrates. Nat. Commun. 2023, 14, 3610. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shao, L.; Wu, Z.; Zhan, P.; Zhang, L. Design and Synthesis of Porous Organic Polymers: Promising Catalysts for Lignocellulose Conversion to 5-Hydroxymethylfurfural and Derivates. Polymers 2023, 15, 2630. [Google Scholar] [CrossRef]
- Serrano, J.M.; Tianyu, L.; Khan, A.U.; Botset, B.; Stovall, B.J.; Xu, Z.; Guo, D.; Cao, K.; Hao, X.; Cheng, S.; et al. Composition Design of Block Copolymers for Porous Carbon Fibers. Chem. Mater. 2019, 31, 8898–8907. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Guo, Y.; Guo, Y.; Guo, D.; Guoliang, L. Block Copolymer Derived Uniform Mesopores Enable Ultrafast Electron and Ion Transport at High Mass Loadings. Nat. Comm. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sohail, M.; El Jery, A.; Al-Zaydi, K.M.; Raza, S.; Ali, H.; Al-Hadeethi, Y.; Taha, T.A.; Din, I.U.; Khan, M.A.; et al. Recent Advances in Ground-Breaking Conjugated Microporous Polymers-Based Materials, Their Synthesis, Modification and Potential Applications. Mater. Today Commun. 2023, 64, 180–208. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, R.; Gao, Y.-Y.; Zhou, Y.; Zhu, J.-J. Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks. Nano-Micro Lett. 2024, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wu, Y.; Lin, X.; Zeb, A.; Xu, X.; Luo, Y.; Liu, J. Application of MOF-Derived Transition Metal Oxides and Composites as Anodes for Lithium-ion Batteries. Inorg. Chem. Front. 2020, 7, 4939–4955. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Schneemann, A.; Fischer, R.A.; Zhu, Y.; Xia, Y. MOF Derived Porous ZnO/C Nanocomposites for Efficient Dye Photodegradation. ACS Appl. Energy Mater. 2018, 1, 4695–4707. [Google Scholar] [CrossRef]
- Dubal, D.P.; Schneemann, A.; Ranc, V.; Kment, S.; Dubal, O.D.P.; Schneemann, A.; Ranc, V.; Kment, S.; Tomanec, O.; Petr, M.; et al. Ultrafine TiO2 Nanoparticle Supported Nitrogen-Rich Graphitic Porous Carbon as an Efficient Anode Material for Potassium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2100042. [Google Scholar] [CrossRef]
- Aijaz, A.; Masa, J.; Rösler, C.; Xia, W.; Weide, P.; Botz, A.J.R.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 Encapsulated in Carbon Nanotube-Grafted Nitrogen-Doped Carbon Polyhedra as an Advanced Bifunctional Oxygen Electrode. Angew. Chem. Int. Ed. 2016, 55, 4087–4091. [Google Scholar] [CrossRef]
- Zhai, Z.-B.; Huang, K.-J.; Wu, X.; Hu, H.; Xu, Y.; Chai, R.-M. Metal–organic Framework Derived Small Sized Metal Sulfide Nanoparticles Anchored on N-doped Carbon Plates for High-Capacity Energy Storage. Dalton Trans. 2019, 48, 4712–4718. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Masa, J.; Tomanec, O.; Peeters, D.; Ranc, V.; Schneemann, A.; Zboril, R.; Schuhmann, W.; Fischer, R.A. Nanoporous Nitrogen-Doped Graphene Oxide/Nickel Sulfide Composite Sheets Derived from a Metal-Organic Framework as an Efficient Electrocatalyst for Hydrogen and Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 1700451. [Google Scholar] [CrossRef]
- Zheng, X.X.; Shen, L.J.; Chen, X.P.; Zheng, X.H.; Au, C.T.; Jiang, L.L. Amino-Modified Fe-Terephthalate Metal-Organic Framework as an Efficient Catalyst for the Selective Oxidation of H2S. Inorg. Chem. 2018, 57, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.K.; Reimer, N.; Liu, J.; Cheng, S.Y.; Yiu, S.M.; Weber, J.; Stock, N.; Xu, Z. Effective Mercury Sorption by Thiol-Laced Metal-Organic Frameworks: In Strong Acid and the Vapor Phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of Highly Efficient MnOx Catalyst for Low-Temperature NH3-SCR Prepared from Mn-MOF-74 Template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Huang, W.; Pan, Z.; Zhou, H. Metal Organic Frameworks-Assisted Fabrication of CuO/Cu2O for Enhanced Selective Catalytic Reduction of NOx by NH3 at Low Temperatures. J. Hazard. Mater. 2019, 364, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Qian, H.; Chi, J.; Chen, B.; Wang, S.; Chen, C.; Zhang, N. Preferential CO Oxidation Over CuO-CeO2 Catalyst Synthesized from MOF with Nitrogen Containing Ligand as Precursor. Int. J. Hydrogen Energy 2018, 43, 23299–23309. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.; Qin, Y.H.; Chen, B.Z. Structure and Denitration Performance of Carbon-Based Catalysts Prepared from Cu-BTC Precursor. Trans. Nonferrous Met. Soc. China 2018, 28, 980–988L. [Google Scholar] [CrossRef]
- Rong, F.Q.J.; Zhang, T.; Zhu, Y.; Xu, J.C.; Guo, Q.; Peng, X.M. Non-Noble Metal@ Carbon Nanosheet Derived from Exfoliated MOF Crystal as Highly Reactive and Stable Heterogeneous Catalyst. Appl. Surf. Sci. 2018, 447, 222234. [Google Scholar] [CrossRef]
- Yong, S.; Xiaoyu, S.; Wang, Z.; Chun-Yan, L.; Qidong, Z.; Jinsuo, G.; Xinyong, L. Synthesis and Characterization of Cu+/MIL-100(Fe) with Enhanced Catalytic Performance for NH3-SCR. Ferroelectrics 2018, 522, 20–28. [Google Scholar] [CrossRef]
- Han, Y.; Xu, H.; Su, Y.; Xu, Z.-L.; Wang, K.; Wang, W. Noble Metal (Pt, Au@Pd) Nanoparticles Supported on Metal Organic Framework (MOF-74) Nanoshuttles as High-Selectivity CO2 Conversion Catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Xin, Z.; Chen, X.; Wang, Q.; Chen, Q.; Zhang, Q. Nanopolyhedrons and Mesoporous Supra-Structures of Zeolitic Imidazolate Framework with High Adsorption Performance. Microp. Mesop. Mater. 2013, 169, 218–221. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Zhang, Y.; Ren, N.; Tang, Y. Microwave-Assisted Hydrothermal Synthesis of Nanozeolites with Controllable Size. Microp. Mesop. Mater. 2009, 119, 306–314. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Facile Syntheses of Metal-Organic Framework Cu3(BTC)2(H2O)3 Under Ultrasound. Bull. Korean Chem. Soc. 2009, 30, 2921–2926. [Google Scholar] [CrossRef]
- Julien, P.A.; Uzarevic, K.; Katsenis, A.D.; Kimber, S.A. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water Treatment and Environmental Remediation Applications of Two-dimensional Metal Carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, C.; Shi, P. Ultrathin MXene-micropattern-based Field-Effect Transistor for Probing Neural Activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, Y.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, J.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene Interface Materials as a Schottky Catalyst with Enhanced Photocatalytic Activities and Anti-Photocorrosion Performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as Co-Catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Carné-Sánchez, A.; Craig, G.A.; Larpent, P.; Hirose, T.; Higuchi, M.; Kitagawa, S.; Matsuda, K.; Urayama, K.; Furukawa, S. Self-Assembly of Metal-Organic Polyhedra into Supramolecular Polymers with Intrinsic Microporosity. Nat. Commun. 2018, 9, 2506. [Google Scholar] [CrossRef]
- Legrand, A.; Craig, G.A.; Bonneau, M.; Minami, S.; Urayama, K.; Furukawa, S. Understanding the Multiscale Self-Assembly of Metal-Organic Polyhedra Towards Functionally Graded Porous Gels. Chem. Sci. 2019, 10, 10833–10842. [Google Scholar] [CrossRef]
- Legrand, A.; Liu, L.-H.; Royla, P.; Aoyama, T.; Craig, G.A.; Carné-Sánchez, A.; Urayama, K.; Weigand, J.J.; Lin, C.-H.; Furukawa, S. Spatiotemporal Control of Supramolecular Polymerization and Gelation of Metal-Organic Polyhedra. J. Am. Chem. Soc. 2021, 143, 3562–3570. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Sani, H.; Scheibe, B.; Dubal, D.P.; Schneemann, A.; Jayaramulu, K. Hierarchical Porous Metal-Organic Gels and Derived Materials: From Fundamentals to Potential Applications. Chem. Soc. Rev. 2022, 51, 9068–9126. [Google Scholar] [CrossRef] [PubMed]
- Schüth, F.; Sing, K.S.W.; Weitkamp, J. (Eds.) Handbook of Porous Solids; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- He, H.; Li, R.; Yang, Z.; Chai, L.; Jin, L.; Alhassan, S.I.; Ren, L.; Wang, H.; Huang, L. Preparation of MOFs and MOFs Derived Materials and their Catalytic Application in Air Pollution: A review. Catal. Today 2021, 375, 10–29. [Google Scholar] [CrossRef]
- Ramesha, B.M.; Vera Meynen, V. Advances and Challenges in the Creation of Porous Metal Phosphonates. Materials 2020, 13, 5366. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomer, M.T. Special Issue “Design, Synthesis and Applications of Macroporous, Mesoporous, and Microporous Materials”. Int. J. Mol. Sci. 2024, 25, 7127. https://doi.org/10.3390/ijms25137127
Colomer MT. Special Issue “Design, Synthesis and Applications of Macroporous, Mesoporous, and Microporous Materials”. International Journal of Molecular Sciences. 2024; 25(13):7127. https://doi.org/10.3390/ijms25137127
Chicago/Turabian StyleColomer, María Teresa. 2024. "Special Issue “Design, Synthesis and Applications of Macroporous, Mesoporous, and Microporous Materials”" International Journal of Molecular Sciences 25, no. 13: 7127. https://doi.org/10.3390/ijms25137127




