Progress in the Study of Fra-2 in Respiratory Diseases
Abstract
1. Introduction
1.1. Structure of AP-1/Fra-2
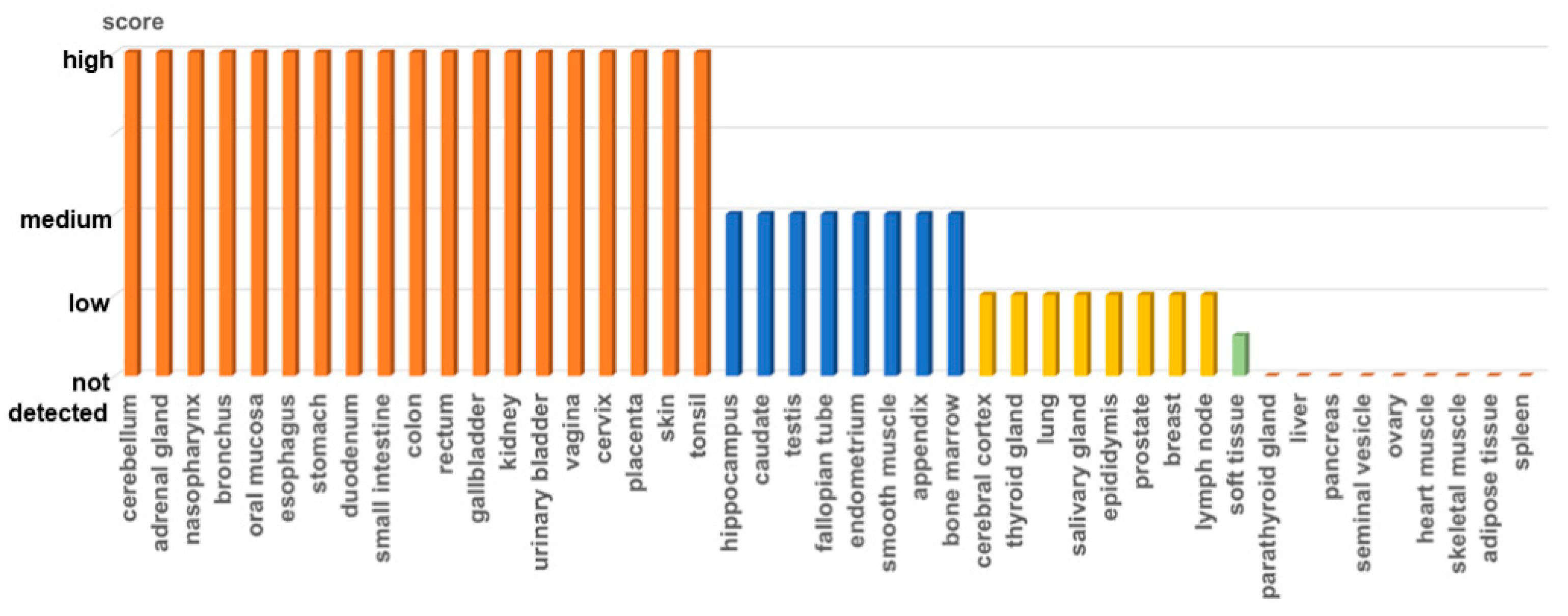
1.2. Expression of the Fra-2
1.3. Importance of Fra-2 in Tissue Development
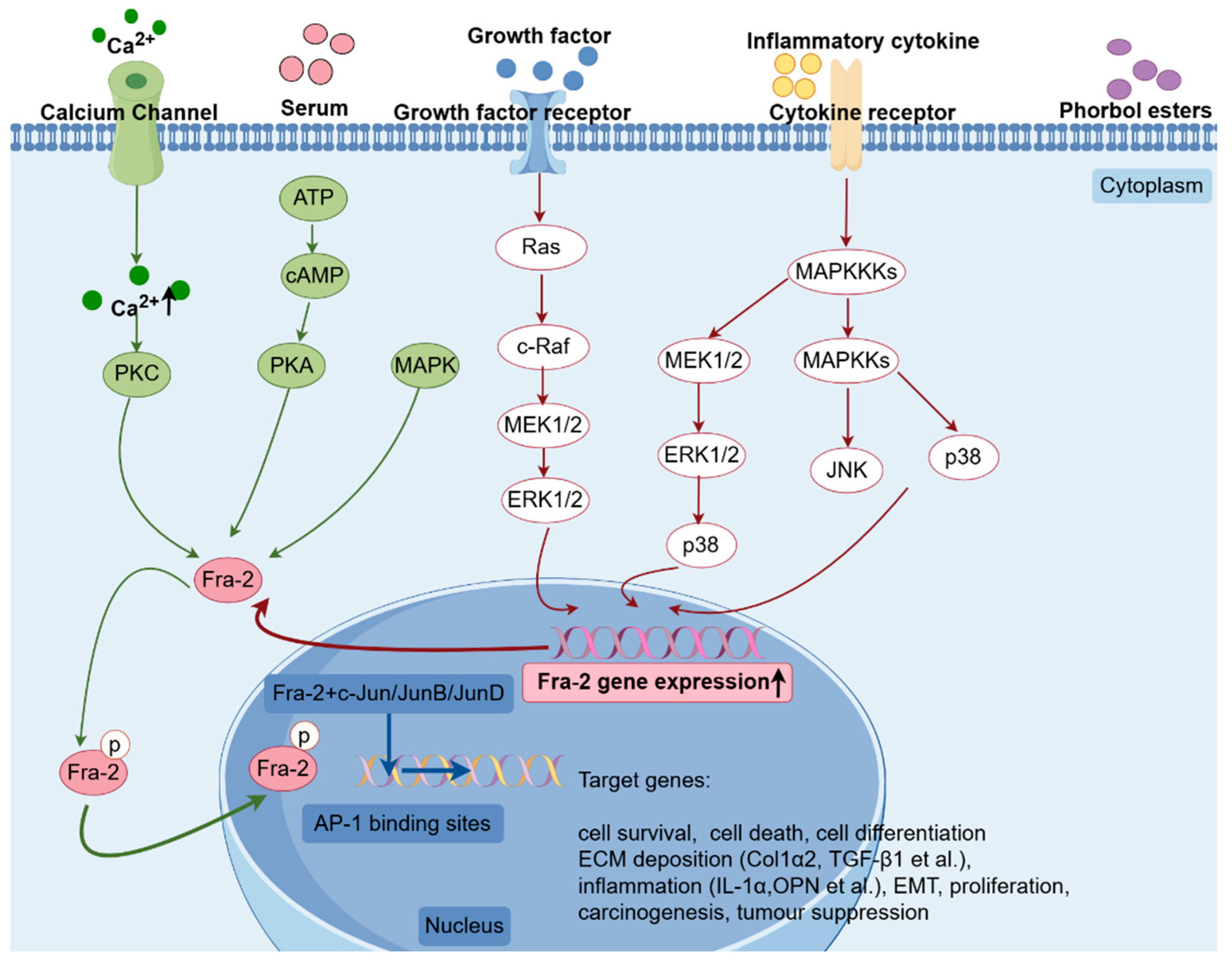
1.4. Regulation of Fra-2
2. Role of Fra-2 in the Development of Respiratory Diseases
2.1. Fra-2 and Chronic Obstructive Pulmonary Disease (COPD)
2.2. Fra-2 and Pulmonary Fibrosis
2.3. Fra-2 and Asthma
2.4. Fra-2 and Non-Small Cell Lung Cancer (NSCLC)
3. Characterization and Limitations of the Fra-2 Tg Mouse Model
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | activating protein-1 |
| Fra-1 (Fosl-1) | Fos-related antigen-1 |
| Fra-2 (Fosl-2) | Fos-related antigen-2 |
| SSc | systemic sclerosis |
| bZIP | basic leucine zipper |
| TAD | trans-activation domain |
| IHC | immunohistochemistry |
| LIF | leukemia inhibitory factor |
| ChIP | chromatin immunoprecipitation |
| IL | interleukin |
| Fra-2 Tg | Fra-2 transgenic |
| DZNep | 3-Deazaneplanocin A |
| TGF-β | transforming growth factor beta |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| MAP | mitogen-activated protein |
| JNK | c-Jun N-terminal kinase |
| MEKK1 | mitogen-activated protein kinase kinase kinase 1 |
| EMSA | electrophoretic mobility shift assay |
| COPD | chronic obstructive pulmonary disease |
| ECM | extracellular matrix |
| IPF | idiopathic pulmonary fibrosis |
| SSc-PH | SSc-associated pulmonary hypertension |
| α-SMA | α-smooth muscle actin |
| SMCs | smooth muscle cells |
| AT2 cells | alveolar type 2 cells |
| NSIP | nonspecific interstitial pneumonia |
| ColVI | collagen type VI |
| I/R | ischemia/reperfusion |
| Col6α1 | collagen type VI alpha 1 |
| TIMP1 | tissue inhibitor of metalloproteinase 1 |
| JAK3/STAT5 | Janus kinase 3/signal transducer and activator of transcription 5 |
| WT | wild-type |
| Muc5AC | mucin 5AC |
| Col1α2 | collagen type I alpha 2 |
| Col6α5 | collagen type VI alpha 5 |
| MMP12 | matrix metalloproteinases 12 |
| NSCLC | non-small cell lung cancer |
| EMT | epithelial–mesenchymal transition |
| HCC | hepatocellular carcinoma |
| LOXL4 | lysine oxidase-like 4 |
| TAMs | tumor-associated macrophages |
| OPN | osteopontin |
| MSCs | mesenchymal stem cells |
| ICOS | inducible costimulatory molecule |
| ECs | endothelial cells |
| ILD | interstitial lung disease |
| UIP | usual interstitial pneumonitis |
| IPAH | idiopathic pulmonary arterial hypertension |
References
- Angel, P.; Imagawa, M.; Chiu, R.; Stein, B.; Imbra, R.J.; Rahmsdorf, H.J.; Jonat, C.; Herrlich, P.; Karin, M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 1987, 49, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Haslinger, A.; Karin, M.; Tjian, R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature 1987, 325, 368–372. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, F.; Müller, R.; Curran, T.; Van Beveren, C.; Verma, I.M. Complete nucleotide sequence of a human c-onc gene: Deduced amino acid sequence of the human c-fos protein. Proc. Natl. Acad. Sci. USA 1983, 80, 3183–3187. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Ryder, K.; Lanahan, A.; Perez-Albuerne, E.; Nathans, D. jun-D: A third member of the jun gene family. Proc. Natl. Acad. Sci. USA 1989, 86, 1500–1503. [Google Scholar] [CrossRef]
- Hirai, S.I.; Ryseck, R.P.; Mechta, F.; Bravo, R.; Yaniv, M. Characterization of junD: A new member of the jun proto-oncogene family. EMBO J. 1989, 8, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Nishina, H.; Sato, H.; Suzuki, T.; Sato, M.; Iba, H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Aronheim, A.; Zandi, E.; Hennemann, H.; Elledge, S.J.; Karin, M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol. Cell Biol. 1997, 17, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Zenz, R.; Theussl, H.C.; Wagner, E.F. Simultaneous generation of fra-2 conditional and fra-2 knock-out mice. Genesis 2007, 45, 447–451. [Google Scholar] [CrossRef]
- Birnhuber, A.; Biasin, V.; Schnoegl, D.; Marsh, L.M.; Kwapiszewska, G. Transcription factor Fra-2 and its emerging role in matrix deposition, proliferation and inflammation in chronic lung diseases. Cell Signal. 2019, 64, 109408. [Google Scholar] [CrossRef]
- Maurer, B.; Reich, N.; Juengel, A.; Kriegsmann, J.; Gay, R.E.; Schett, G.; Michel, B.A.; Gay, S.; Distler, J.H.; Distler, O. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1382–1387. [Google Scholar] [CrossRef]
- Eferl, R.; Hasselblatt, P.; Rath, M.; Popper, H.; Zenz, R.; Komnenovic, V.; Idarraga, M.H.; Kenner, L.; Wagner, E.F. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 10525–10530. [Google Scholar] [CrossRef]
- Ucero, A.C.; Bakiri, L.; Roediger, B.; Suzuki, M.; Jimenez, M.; Mandal, P.; Braghetta, P.; Bonaldo, P.; Paz-Ares, L.; Fustero-Torre, C.; et al. Fra-2-expressing macrophages promote lung fibrosis in mice. J. Clin. Investig. 2019, 129, 3293–3309. [Google Scholar] [CrossRef]
- Glover, J.N.; Harrison, S.C. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature 1995, 373, 257–261. [Google Scholar] [CrossRef]
- Landschulz, W.H.; Johnson, P.F.; McKnight, S.L. The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science 1988, 240, 1759–1764. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Guinea-Viniegra, J.; Jimenez, M.; Wagner, E.F. Signalling in inflammatory skin disease by AP-1 (Fos/Jun). Clin. Exp. Rheumatol. 2015, 33 (Suppl. S92), S44–S49. [Google Scholar]
- Alber, T. Structure of the leucine zipper. Curr. Opin. Genet. Dev. 1992, 2, 205–210. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117 Pt 25, 5965–5973. [Google Scholar] [CrossRef]
- Jochum, W.; Passegué, E.; Wagner, E.F. AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef]
- Matsui, M.; Tokuhara, M.; Konuma, Y.; Nomura, N.; Ishizaki, R. Isolation of human fos-related genes and their expression during monocyte-macrophage differentiation. Oncogene 1990, 5, 249–255. [Google Scholar]
- Foletta, V.C.; Sonobe, M.H.; Suzuki, T.; Endo, T.; Iba, H.; Cohen, D.R. Cloning and characterisation of the mouse fra-2 gene. Oncogene 1994, 9, 3305–3311. [Google Scholar]
- Suzuki, T.; Okuno, H.; Yoshida, T.; Endo, T.; Nishina, H.; Iba, H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991, 19, 5537–5542. [Google Scholar] [CrossRef]
- Kerppola, T.K.; Curran, T. Selective DNA bending by a variety of bZIP proteins. Mol. Cell Biol. 1993, 13, 5479–5489. [Google Scholar]
- Molven, A.; Houge, G.; Berger, R. Chromosomal assignment of the human gene encoding the Fos-related antigen-2 (FRA2) to chromosome 2p22-p23. Genomics 1996, 38, 72–75. [Google Scholar] [CrossRef]
- Carrasco, D.; Bravo, R. Tissue-specific expression of the fos-related transcription factor fra-2 during mouse development. Oncogene 1995, 10, 1069–1079. [Google Scholar]
- Foletta, V.C. Transcription factor AP-1, and the role of Fra-2. Immunol. Cell Biol. 1996, 74, 121–133. [Google Scholar] [CrossRef]
- Bozec, A.; Bakiri, L.; Jimenez, M.; Rosen, E.D.; Catalá-Lehnen, P.; Schinke, T.; Schett, G.; Amling, M.; Wagner, E.F. Osteoblast-specific expression of Fra-2/AP-1 controls adiponectin and osteocalcin expression and affects metabolism. J. Cell Sci. 2013, 126 Pt 23, 5432–5440. [Google Scholar] [CrossRef]
- Luther, J.; Ubieta, K.; Hannemann, N.; Jimenez, M.; Garcia, M.; Zech, C.; Schett, G.; Wagner, E.F.; Bozec, A. Fra-2/AP-1 controls adipocyte differentiation and survival by regulating PPARγ and hypoxia. Cell Death Differ. 2014, 21, 655–664. [Google Scholar] [CrossRef]
- Wrann, C.D.; Eguchi, J.; Bozec, A.; Xu, Z.; Mikkelsen, T.; Gimble, J.; Nave, H.; Wagner, E.F.; Ong, S.E.; Rosen, E.D. FOSL2 promotes leptin gene expression in human and mouse adipocytes. J. Clin. Investig. 2012, 122, 1010–1021. [Google Scholar] [CrossRef]
- Karreth, F.; Hoebertz, A.; Scheuch, H.; Eferl, R.; Wagner, E.F. The AP1 transcription factor Fra2 is required for efficient cartilage development. Development 2004, 131, 5717–5725. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Bakiri, L.; Jimenez, M.; Schinke, T.; Amling, M.; Wagner, E.F. Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. J. Cell Biol. 2010, 190, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Bakiri, L.; Hoebertz, A.; Eferl, R.; Schilling, A.F.; Komnenovic, V.; Scheuch, H.; Priemel, M.; Stewart, C.L.; Amling, M.; et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 2008, 454, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Beranger, G.E.; Momier, D.; Guigonis, J.M.; Samson, M.; Carle, G.F.; Scimeca, J.C. Differential binding of poly(ADP-Ribose) polymerase-1 and JunD/Fra2 accounts for RANKL-induced Tcirg1 gene expression during osteoclastogenesis. J. Bone Miner. Res. 2007, 22, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ubieta, K.; Garcia, M.; Grötsch, B.; Uebe, S.; Weber, G.F.; Stein, M.; Ekici, A.; Schett, G.; Mielenz, D.; Bozec, A. Fra-2 regulates B cell development by enhancing IRF4 and Foxo1 transcription. J. Exp. Med. 2017, 214, 2059–2071. [Google Scholar] [CrossRef]
- Lawson, V.J.; Maurice, D.; Silk, J.D.; Cerundolo, V.; Weston, K. Aberrant selection and function of invariant NKT cells in the absence of AP-1 transcription factor Fra-2. J. Immunol. 2009, 183, 2575–2584. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkhurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Shetty, A.; Bhosale, S.D.; Tripathi, S.K.; Buchacher, T.; Biradar, R.; Rasool, O.; Moulder, R.; Galande, S.; Lahesmaa, R. Interactome Networks of FOSL1 and FOSL2 in Human Th17 Cells. ACS Omega 2021, 6, 24834–24847. [Google Scholar] [CrossRef]
- Schwenger, G.T.; Kok, C.C.; Arthaningtyas, E.; Thomas, M.A.; Sanderson, C.J.; Mordvinov, V.A. Specific activation of human interleukin-5 depends on de novo synthesis of an AP-1 complex. J. Biol. Chem. 2002, 277, 47022–47027. [Google Scholar] [CrossRef]
- McHenry, J.Z.; Leon, A.; Matthaei, K.I.; Cohen, D.R. Overexpression of fra-2 in transgenic mice perturbs normal eye development. Oncogene 1998, 17, 1131–1140. [Google Scholar] [CrossRef]
- Robinson, G.A. Changes in the expression of transcription factors ATF-2 and Fra-2 after axotomy and during regeneration in rat retinal ganglion cells. Brain Res. Mol. Brain Res. 1996, 41, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xing, Y. Down-regulation of Fra-2 alleviates light-induced retina damage by inhibiting the PARP-1/AIF pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 4221–4229. [Google Scholar] [PubMed]
- Macián, F.; López-Rodríguez, C.; Rao, A. Partners in transcription: NFAT and AP-1. Oncogene 2001, 20, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Guru, S.C.; Heppner, C.; Erdos, M.R.; Collins, R.M.; Park, S.Y.; Saggar, S.; Chandrasekharappa, S.C.; Collins, F.S.; Spiegel, A.M.; et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 1999, 96, 143–152. [Google Scholar] [CrossRef]
- Yoshida, T.; Suzuki, T.; Sato, H.; Nishina, H.; Iba, H. Analysis of fra-2 gene expression. Nucleic Acids Res. 1993, 21, 2715–2721. [Google Scholar] [CrossRef]
- Biasin, V.; Marsh, L.M.; Egemnazarov, B.; Wilhelm, J.; Ghanim, B.; Klepetko, W.; Wygrecka, M.; Olschewski, H.; Eferl, R.; Olschewski, A.; et al. Meprin β, a novel mediator of vascular remodelling underlying pulmonary hypertension. J. Pathol. 2014, 233, 7–17. [Google Scholar] [CrossRef]
- Reich, N.; Maurer, B.; Akhmetshina, A.; Venalis, P.; Dees, C.; Zerr, P.; Palumbo, K.; Zwerina, J.; Nevskaya, T.; Gay, S.; et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum. 2010, 62, 280–290. [Google Scholar] [CrossRef]
- Kovary, K.; Bravo, R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: Differential role of Fos proteins. Mol. Cell Biol. 1992, 12, 5015–5023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tulchinsky, E. Fos family members: Regulation, structure and role in oncogenic transformation. Histol. Histopathol. 2000, 15, 921–928. [Google Scholar]
- Sonobe, M.H.; Yoshida, T.; Murakami, M.; Kameda, T.; Iba, H. fra-2 promoter can respond to serum-stimulation through AP-1 complexes. Oncogene 1995, 10, 689–696. [Google Scholar]
- Krämer, M.; Dees, C.; Huang, J.; Schlottmann, I.; Palumbo-Zerr, K.; Zerr, P.; Gelse, K.; Beyer, C.; Distler, A.; Marquez, V.E.; et al. Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Ann. Rheum. Dis. 2013, 72, 614–620. [Google Scholar] [CrossRef]
- Tang, W.; Yang, L.; Yang, Y.C.; Leng, S.X.; Elias, J.A. Transforming growth factor-beta stimulates interleukin-11 transcription via complex activating protein-1-dependent pathways. J. Biol. Chem. 1998, 273, 5506–5513. [Google Scholar] [CrossRef]
- Yue, J.; Mulder, K.M. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a Smad-dependent pathway. J. Biol. Chem. 2000, 275, 30765–30773. [Google Scholar] [CrossRef]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gruda, M.C.; Kovary, K.; Metz, R.; Bravo, R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene 1994, 9, 2537–2547. [Google Scholar]
- Zoumpourlis, V.; Papassava, P.; Linardopoulos, S.; Gillespie, D.; Balmain, A.; Pintzas, A. High levels of phosphorylated c-Jun, Fra-1, Fra-2 and ATF-2 proteins correlate with malignant phenotypes in the multistage mouse skin carcinogenesis model. Oncogene 2000, 19, 4011–4021. [Google Scholar] [CrossRef]
- Fuchs, S.Y.; Dolan, L.; Davis, R.J.; Ronai, Z. Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene 1996, 13, 1531–1535. [Google Scholar]
- Acquaviva, C.; Ferrara, P.; Bossis, G.; Brockly, F.; Salvat, C.; Jariel-Encontre, I.; Piechaczyk, M. Degradation of cellular and viral Fos proteins. Biochimie 2001, 83, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.D.; Uhlik, M.T.; Garrington, T.P.; Johnson, G.L. MEKK1 regulates the AP-1 dimer repertoire via control of JunB transcription and Fra-2 protein stability. Oncogene 2005, 24, 801–809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomard, T.; Jariel-Encontre, I.; Basbous, J.; Bossis, G.; Moquet-Torcy, G.; Piechaczyk, M. Fos family protein degradation by the proteasome. Biochem. Soc. Trans. 2008, 36 Pt 5, 858–863. [Google Scholar] [CrossRef]
- Luo, Y.; Grötsch, B.; Hannemann, N.; Jimenez, M.; Ipseiz, N.; Uluckan, O.; Lin, N.; Schett, G.; Wagner, E.F.; Bozec, A. Fra-2 Expression in Osteoblasts Regulates Systemic Inflammation and Lung Injury through Osteopontin. Mol. Cell Biol. 2018, 38, e00022-18. [Google Scholar] [CrossRef]
- Davies, J.S.; Klein, D.C.; Carter, D.A. Selective genomic targeting by FRA-2/FOSL2 transcription factor: Regulation of the Rgs4 gene is mediated by a variant activator protein 1 (AP-1) promoter sequence/CREB-binding protein (CBP) mechanism. J. Biol. Chem. 2011, 286, 15227–15239. [Google Scholar] [CrossRef] [PubMed]
- Adiseshaiah, P.; Li, J.; Vaz, M.; Kalvakolanu, D.V.; Reddy, S.P. ERK signaling regulates tumor promoter induced c-Jun recruitment at the Fra-1 promoter. Biochem. Biophys. Res. Commun. 2008, 371, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Bakiri, L.; Matsuo, K.; Wisniewska, M.; Wagner, E.F.; Yaniv, M. Promoter specificity and biological activity of tethered AP-1 dimers. Mol. Cell Biol. 2002, 22, 4952–4964. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Azad, A.; Schnitt, R.; He, G.; Weigert, C.; Ichijo, H.; Sen, C.K. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc. Res. 2010, 87, 647–655. [Google Scholar] [CrossRef]
- Virolle, T.; Monthouel, M.N.; Djabari, Z.; Ortonne, J.P.; Meneguzzi, G.; Aberdam, D. Three activator protein-1-binding sites bound by the Fra-2.JunD complex cooperate for the regulation of murine laminin alpha3A (lama3A) promoter activity by transforming growth factor-beta. J. Biol. Chem. 1998, 273, 17318–17325. [Google Scholar] [CrossRef]
- Birnhuber, A.; Crnkovic, S.; Biasin, V.; Marsh, L.M.; Odler, B.; Sahu-Osen, A.; Stacher-Priehse, E.; Brcic, L.; Schneider, F.; Cikes, N.; et al. IL-1 receptor blockade skews inflammation towards Th2 in a mouse model of systemic sclerosis. Eur. Respir. J. 2019, 54, 1900154. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Singer, C.F.; Hudelist, G.; Dampier, B.; Kaserer, K.; Vinatzer, U.; Pehamberger, H.; Zielinski, C.; Kubista, E.; Schreibner, M. Jun and Fos family protein expression in human breast cancer: Correlation of protein expression and clinicopathological parameters. Eur. J. Gynaecol. Oncol. 2006, 27, 345–352. [Google Scholar]
- Wang, Z.Q.; Ovitt, C.; Grigoriadis, A.E.; Möhle-Steinlein, U.; Rüther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef]
- Calverley, P.M.; Walker, P. Chronic obstructive pulmonary disease. Lancet 2003, 362, 1053–1061. [Google Scholar] [CrossRef]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. New concepts in chronic obstructive pulmonary disease. Annu. Rev. Med. 2003, 54, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Kent, L.; Smyth, L.; Clayton, C.; Scott, L.; Cook, T.; Stephens, R.; Fox, S.; Hext, P.; Farrow, S.; Singh, D. Cigarette smoke extract induced cytokine and chemokine gene expression changes in COPD macrophages. Cytokine 2008, 42, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.J.; Hunninghake, G.W. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001, 345, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; III, J.P.L.; Martinez, F.J. Mechanisms of Pulmonary Fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Distler, J.H.; Distler, O. The Fra-2 transgenic mouse model of systemic sclerosis. Vascul Pharmacol. 2013, 58, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Guignabert, C.; Hoffmann-Vold, A.M.; Ruiz, B.; Dorfmuller, P.; Pezet, S.; Amar, O.; Tu, L.; Van Wassenhove, J.; Sadoine, J.; et al. Role of Stromelysin 2 (Matrix Metalloproteinase 10) as a Novel Mediator of Vascular Remodeling Underlying Pulmonary Hypertension Associated With Systemic Sclerosis. Arthritis Rheumatol. 2017, 69, 2209–2221. [Google Scholar] [CrossRef]
- Tabeling, C.; Wienhold, S.M.; Birnhuber, A.; Brack, M.C.; Nouailles, G.; Kershaw, O.; Firsching, T.C.; Gruber, A.D.; Lienau, J.; Marsh, L.M.; et al. Pulmonary fibrosis in Fra-2 transgenic mice is associated with decreased numbers of alveolar macrophages and increased susceptibility to pneumococcal pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L916–L925. [Google Scholar] [CrossRef]
- Gungl, A.; Biasin, V.; Wilhelm, J.; Olschewski, A.; Kwapiszewska, G.; Marsh, L.M. Fra2 Overexpression in Mice Leads to Non-allergic Asthma Development in an IL-13 Dependent Manner. Front. Immunol. 2018, 9, 2018. [Google Scholar] [CrossRef]
- Tsujino, K.; Li, J.T.; Tsukui, T.; Ren, X.; Bakiri, L.; Wagner, E.; Sheppard, D. Fra-2 negatively regulates postnatal alveolar septation by modulating myofibroblast function. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L878–L888. [Google Scholar] [CrossRef]
- Cheng, F.; Li, Y.; Feng, L.; Li, S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant. Proc. 2008, 40, 2167–2170. [Google Scholar] [CrossRef]
- Furuichi, K.; Gao, J.L.; Murphy, P.M. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am. J. Pathol. 2006, 169, 372–387. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef]
- Leask, A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc. Res. 2007, 74, 207–212. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Verrecchia, F.; Chu, M.L.; Mauviel, A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001, 276, 17058–17062. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, O.; González-Santamaría, J.; Lagares, D.; Guinea-Viniegra, J.; Pichol-Thievend, C.; Muller, L.; Rodríguez-Pascual, F. LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling. Mol. Cell Biol. 2013, 33, 2388–2401. [Google Scholar] [CrossRef]
- Carthy, J.M.; Sundqvist, A.; Heldin, A.; van Dam, H.; Kletsas, D.; Heldin, C.H.; Moustakas, A. Tamoxifen Inhibits TGF-β-Mediated Activation of Myofibroblasts by Blocking Non-Smad Signaling Through ERK1/2. J. Cell Physiol. 2015, 230, 3084–3092. [Google Scholar] [CrossRef]
- Seidenberg, J.; Stellato, M.; Hukara, A.; Ludewig, B.; Klingel, K.; Distler, O.; Błyszczuk, P.; Kania, G. The AP-1 Transcription Factor Fosl-2 Regulates Autophagy in Cardiac Fibroblasts during Myocardial Fibrogenesis. Int. J. Mol. Sci. 2021, 22, 1861. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Padem, N.; Saltoun, C. Classification of asthma. Allergy Asthma Proc. 2019, 40, 385–388. [Google Scholar] [CrossRef]
- Jacques, E.; Semlali, A.; Boulet, L.P.; Chakir, J. AP-1 overexpression impairs corticosteroid inhibition of collagen production by fibroblasts isolated from asthmatic subjects. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L281–L287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, S.; Wang, J.; Liu, F.; Dong, L. Alternatively activated macrophages promote airway inflammation through JAK3–STAT5–Fra2 in asthma. Inflamm. Res. 2022, 71, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Gensch, E.; Gallup, M.; Sucher, A.; Li, D.; Gebremichael, A.; Lemjabbar, H.; Mengistab, A.; Dasari, V.; Hotchkiss, J.; Harkema, J.; et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J. Biol. Chem. 2004, 279, 39085–39093. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Thomas, A.; Liu, S.V.; Subramaniam, D.S.; Giaccone, G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat. Rev. Clin. Oncol. 2015, 12, 511–526. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, E.; Schwarz, H.; Deiner, E.M.; Leitner, I.; Eilers, M.; Berger, J.; Busslinger, M.; Beug, H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell 1992, 71, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Fialka, I.; Schwarz, H.; Reichmann, E.; Oft, M.; Busslinger, M.; Beug, H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J. Cell Biol. 1996, 132, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K. The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 2005, 41, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, X.D.; Wang, X.Y.; Fei, B.Y. Fos-like antigen 2 (FOSL2) promotes metastasis in colon cancer. Exp. Cell Res. 2018, 373, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Z.; Sun, J.; Li, J.; Dong, Z.; Zhang, Y.; Chen, J.; Kan, Q.; Yu, Z. MiR-133a acts as an anti-oncogene in Hepatocellular carcinoma by inhibiting FOSL2 through TGF-β/Smad3 signaling pathway. Biomed. Pharmacother. 2018, 107, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dai, G.; Yu, L.; Hu, Q.; Chen, J.; Guo, W. miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci. Rep. 2018, 8, 606. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Kaur, H.; Sharma, N.; Saluja, D.; Bharti, A.C.; Das, B.C. Selective participation of c-Jun with Fra-2/c-Fos promotes aggressive tumor phenotypes and poor prognosis in tongue cancer. Sci. Rep. 2015, 5, 16811. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, D.; Wang, Y.; Ren, F.; Pang, S.; Wang, D.; Xu, S. FOSL2 positively regulates TGF-β1 signalling in non-small cell lung cancer. PLoS ONE 2014, 9, e112150. [Google Scholar] [CrossRef]
- He, J.; Mai, J.; Li, Y.; Chen, L.; Xu, H.; Zhu, X.; Pan, Q. miR-597 inhibits breast cancer cell proliferation, migration and invasion through FOSL2. Oncol. Rep. 2017, 37, 2672–2678. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, C.D.; Chung, J.H.; Kwon, K.H. Association Study of FOS-Like Antigen-2 Promoter Polymorphisms With Papillary Thyroid Cancer in Korean Population. Clin. Exp. Otorhinolaryngol. 2014, 7, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hu, W.; Fu, W.; Dai, L.; Jiang, Z.; Zhong, S.; Deng, B.; Zhao, J. HGF/MET Regulated Epithelial-Mesenchymal Transitions And Metastasis By FOSL2 In Non-Small Cell Lung Cancer. Onco Targets Ther. 2019, 12, 9227–9237. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Guo, Y.N.; Zeng, J.H.; Ma, F.C.; Luo, J.; Zhu, H.W.; Xia, S.; Wei, K.L.; Chen, G. The expression, significance and function of cancer susceptibility candidate 9 in lung squamous cell carcinoma: A bioinformatics and in vitro investigation. Int. J. Oncol. 2019, 54, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Janke, S.; Wagner, I.; Schröder, C.; Streichert, T.; Bamberger, A.M.; Jänicke, F.; Löning, T. Role of Fra-2 in breast cancer: Influence on tumor cell invasion and motility. Breast Cancer Res. Treat. 2008, 107, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Röder, H.; Andritzky, B.; Aslan, B.; Hemminger, G.; Brinkmann, A.; Bamberger, C.M.; Löning, T.; Bamberger, A.M. The role of the AP-1 transcription factors c-Fos, FosB, Fra-1 and Fra-2 in the invasion process of mammary carcinomas. Breast Cancer Res. Treat. 2004, 86, 139–152. [Google Scholar] [CrossRef]
- Skrypek, N.; Goossens, S.; De Smedt, E.; Vandamme, N.; Berx, G. Epithelial-to-Mesenchymal Transition: Epigenetic Reprogramming Driving Cellular Plasticity. Trends Genet. 2017, 33, 943–959. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J. Cell Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Song, L.N.; Qiao, G.L.; Yu, J.; Yang, C.M.; Chen, Y.; Deng, Z.F.; Song, L.H.; Ma, L.J.; Yan, H.L. Hsa_circ_0003998 promotes epithelial to mesenchymal transition of hepatocellular carcinoma by sponging miR-143-3p and PCBP1. J. Exp. Clin. Cancer Res. 2020, 39, 114. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Kortland, J.; Maltseva, D.V.; Nersisyan, S.A.; Samatov, T.R.; Lezius, S.; Tonevitsky, A.G.; Milde-Langosch, K.; Wicklein, D.; Schumacher, U.; et al. Fra-2 overexpression upregulates pro-metastatic cell-adhesion molecules, promotes pulmonary metastasis, and reduces survival in a spontaneous xenograft model of human breast cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 1525–1542. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Venkatesh, P.; Sunkoju, M.; Godugu, C. An Adaptogen: Withaferin A Ameliorates in Vitro and in Vivo Pulmonary Fibrosis by Modulating the Interplay of Fibrotic, Matricelluar Proteins, and Cytokines. Front. Pharmacol. 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yang, K.M.; Park, Y.; Hong, E.; Hong, C.P.; Park, J.; Pang, K.; Lee, J.; Park, B.; Lee, S.; et al. Identification of Epithelial-Mesenchymal Transition-related Target Genes Induced by the Mutation of Smad3 Linker Phosphorylation. J. Cancer Prev. 2018, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rakaee, M.; Busund, L.R.; Jamaly, S.; Paulsen, E.E.; Richardsen, E.; Andersen, S.; Al-Saad, S.; Bremnes, R.M.; Donnem, T.; Kilvaer, T.K. Prognostic Value of Macrophage Phenotypes in Resectable Non-Small Cell Lung Cancer Assessed by Multiplex Immunohistochemistry. Neoplasia 2019, 21, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Schmall, A.; Al-Tamari, H.M.; Herold, S.; Kampschulte, M.; Weigert, A.; Wietelmann, A.; Vipotnik, N.; Grimminger, F.; Seeger, W.; Pullamsetti, S.S.; et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am. J. Respir. Crit. Care Med. 2015, 191, 437–447. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Kojonazarov, B.; Storn, S.; Gall, H.; Salazar, Y.; Wolf, J.; Weigert, A.; El-Nikhely, N.; Ghofrani, H.A.; Krombach, G.A.; et al. Lung cancer-associated pulmonary hypertension: Role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci. Transl. Med. 2017, 9, eaai9048. [Google Scholar] [CrossRef]
- Sarode, P.; Zheng, X.; Giotopoulou, G.A.; Weigert, A.; Kuenne, C.; Günther, S.; Friedrich, A.; Gattenlöhner, S.; Stiewe, T.; Bruene, B.; et al. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: A potential treatment of lung cancer. Sci. Adv. 2020, 6, eaaz6105. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, L.; Xie, X.; Hu, F.; Yang, Q.; Hu, R.; Jiang, L.; Ding, F.; Mei, J.; Liu, J.; et al. Hsa_circ_0001869 promotes NSCLC progression via sponging miR-638 and enhancing FOSL2 expression. Aging 2020, 12, 23836–23848. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakajima, S.; Ohnuki, K.; Ogawa, S.; Yamashita, M.; Nakayama, T.; Murakami, Y.; Tanabe, K.; Abe, R. AP-1 is involved in ICOS gene expression downstream of TCR/CD28 and cytokine receptor signaling. Eur. J. Immunol. 2012, 42, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Renoux, F.; Stellato, M.; Haftmann, C.; Vogetseder, A.; Huang, R.; Subramaniam, A.; Becker, M.O.; Blyszczuk, P.; Becher, B.; Distler, J.H.W.; et al. The AP1 Transcription Factor Fosl2 Promotes Systemic Autoimmunity and Inflammation by Repressing Treg Development. Cell Rep. 2020, 31, 107826. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sugimoto, K.; Lu, H.; Yang, W.Y.; Liu, J.Y.; Yang, H.Y.; Song, Y.B.; Yan, D.; Zou, T.Y.; Shen, S. HDAC1-mediated deacetylation of HIF1α prevents atherosclerosis progression by promoting miR-224-3p-mediated inhibition of FOSL2. Mol. Ther. Nucleic Acids 2021, 23, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, Y.; Ning, M.; Li, T. KLRD1, FOSL2 and LILRB3 as potential biomarkers for plaques progression in acute myocardial infarction and stable coronary artery disease. BMC Cardiovasc. Disord. 2021, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Drosos, I.; Chalikias, G.; Pavlaki, M.; Kareli, D.; Epitropou, G.; Bougioukas, G.; Mikroulis, D.; Konstantinou, F.; Giatromanolaki, A.; Ritis, K.; et al. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: Possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin. Res. Cardiol. 2016, 105, 887–900. [Google Scholar] [CrossRef]
- Choi, J.; Jang, Y.J.; Dabrowska, C.; Iich, E.; Evans, K.V.; Hall, H.; Janes, S.M.; Simons, B.D.; Koo, B.K.; Kim, J.; et al. Release of Notch activity coordinated by IL-1β signalling confers differentiation plasticity of airway progenitors via Fosl2 during alveolar regeneration. Nat. Cell Biol. 2021, 23, 953–966. [Google Scholar] [CrossRef]
- Maurer, B.; Busch, N.; Jüngel, A.; Pileckyte, M.; Gay, R.E.; Michel, B.A.; Schett, G.; Gay, S.; Distler, J.; Distler, O. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation 2009, 120, 2367–2376. [Google Scholar] [CrossRef]
- Huang, J.; Maier, C.; Zhang, Y.; Soare, A.; Dees, C.; Beyer, C.; Harre, U.; Chen, C.W.; Distler, O.; Schett, G.; et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1941–1948. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.; Zhang, Y. The role of c-Jun in the AP-1 activation induced by naturally occurring isothiocyanates. Food Chem. Toxicol. 2005, 43, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Won, J.S.; Kim, Y.H.; Song, D.K.; Huh, S.O.; Lee, J.K.; Suh, H.W. Stimulation of astrocyte-enriched culture with arachidonic acid increases proenkephalin mRNA: Involvement of proto-oncoprotein and mitogen activated protein kinases. Brain Res. Mol. Brain Res. 2000, 76, 396–406. [Google Scholar] [CrossRef] [PubMed]
| Gene | Full Name | Details |
|---|---|---|
| OPN | Osteopontin | Luo et al. [61] validated this by ChIP and luciferase reporter assay in mouse osteoblasts. |
| Fra-2 | Fos-related antigen-2 | Davies et al. [62] validated these by ChIP and electrophoretic mobility shift assay (EMSA) in rat pineal body tissue. |
| Rgs4 | Regulator of G protein signaling 4 | |
| Atf4 | Activating transcription factor 4 | |
| Cox6a2 | Cytochrome c oxidase subunit 6A2 | |
| Nr4a1 | Nuclear receptor subfamily 4 group A member 1 | |
| Mt1a | Metallothionein 1 A | |
| Opn1sw | Opsin 1, short-wave sensitive | |
| Dio2 | Iodothyronine deiodinase 2 | |
| CD24 | CD24 molecule | |
| Fra-1 | Fos-related antigen-1 | Adiseshaiah et al. [63] validated this by ChIP in A549 cells. |
| CCND1 | Cyclin D1 | Bakiri et al. [64] validated these by luciferase reporter assay and EMSA in NIH 3T3 cells. |
| CCNA2 | Cyclin A2 | |
| TGF-β1 | Transforming growth factor beta 1 | Fichtner-Feigl et al. [54] validated this by luciferase reporter assay and EMSA in THP-1 and MonoMac6 cells. Roy et al. [65] validated this by luciferase reporter assay in mouse cardiac fibroblasts. |
| Lama3A | Laminin alpha3A | Virolle et al. [66] validated this by β-galactosidase assay and EMSA in PAM212 cells. |
| Col1α2 | Collagen type I alpha 2 chain | Bozec et al. [32] validated this by ChIP and luciferase reporter assay in mouse osteoblasts. |
| Oc | Osteocalcin | |
| IL-1α | Interleukin 1 alpha | Birnhuber et al. [67] validated this by EMSA in primary human parenchymal fibroblasts and mice. |
| Respiratory Diseases | Regulation of Fra-2 | Reference |
|---|---|---|
| COPD | Up-regulation | Kent et al. [73] |
| Pulmonary fibrosis | Up-regulation | Eferl et al. [12] Ucero et al. [13] Birnhuber et al. [67] |
| SSc-PH | Up-regulation | Maurer et al. [11] Biasin et al. [46] |
| Asthma | Up-regulation | Gungl et al. [81] Huang et al. [95] |
| NSCLC | Up-regulation | Wang et al. [109] Yin et al. [112] Gao et al. [113] Sarode et al. [131] Xu et al. [132] |
| Inferred Drugs for Fra-2 Gene | ||
|---|---|---|
| Name | Status | Mechanism of Action |
| Diethylstilbestrol | Approved, investigational, withdrawn | Small molecule, hormone replacement agents |
| D-leucine | Experimental, investigational | - |
| Compounds for Fra-2/AP-1 | ||
| Name | Reference | |
| T-5224 (AP-1 inhibition) | Ucero et al. [13] | |
| N-acetylcysteine | Li et al. [141] | |
| PD98059 | Won et al. [142] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Liu, Y. Progress in the Study of Fra-2 in Respiratory Diseases. Int. J. Mol. Sci. 2024, 25, 7143. https://doi.org/10.3390/ijms25137143
Zheng S, Liu Y. Progress in the Study of Fra-2 in Respiratory Diseases. International Journal of Molecular Sciences. 2024; 25(13):7143. https://doi.org/10.3390/ijms25137143
Chicago/Turabian StyleZheng, Shuping, and Yun Liu. 2024. "Progress in the Study of Fra-2 in Respiratory Diseases" International Journal of Molecular Sciences 25, no. 13: 7143. https://doi.org/10.3390/ijms25137143
APA StyleZheng, S., & Liu, Y. (2024). Progress in the Study of Fra-2 in Respiratory Diseases. International Journal of Molecular Sciences, 25(13), 7143. https://doi.org/10.3390/ijms25137143






