Addition of Polyphenols to Drugs: The Potential of Controlling “Inflammaging” and Fibrosis in Human Senescent Lung Fibroblasts In Vitro
Abstract
:1. Introduction
2. Results
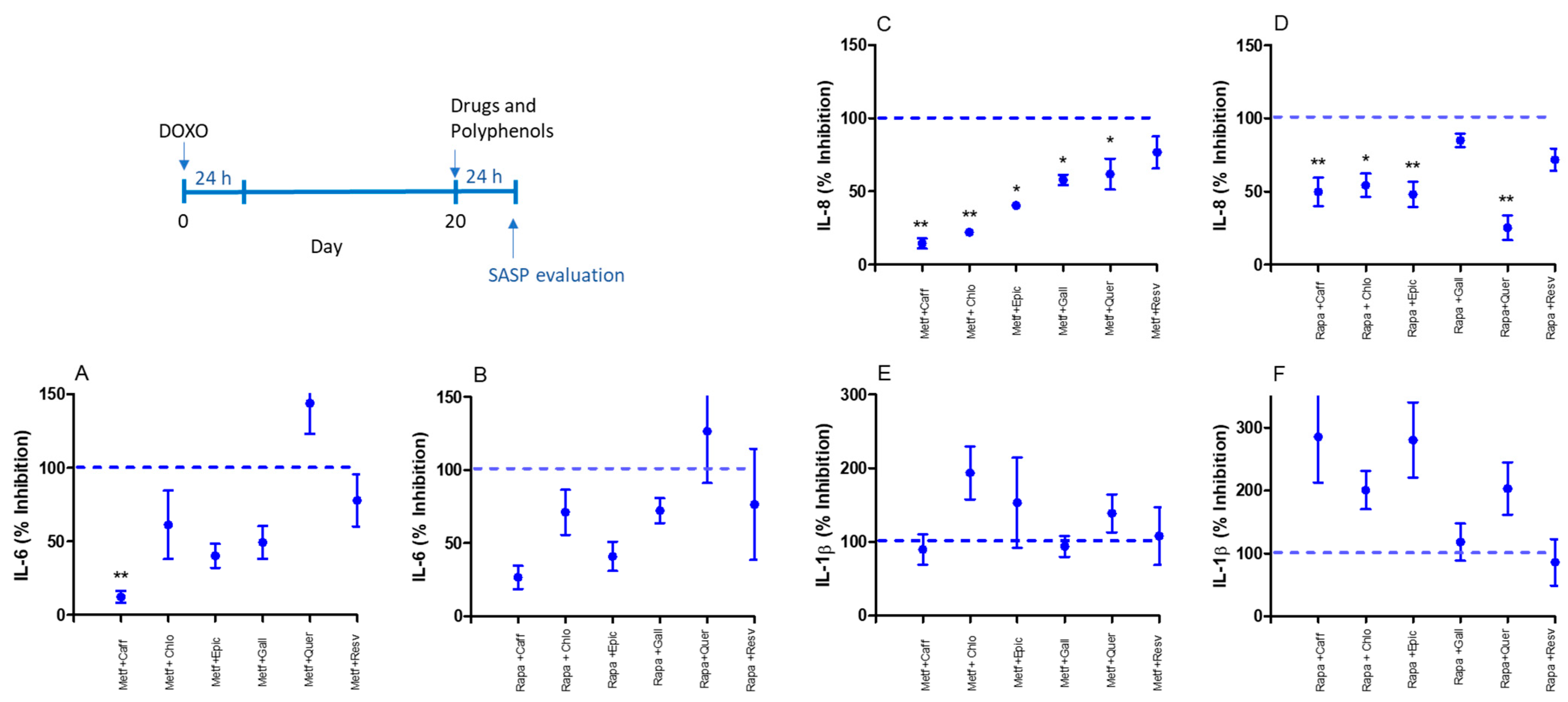
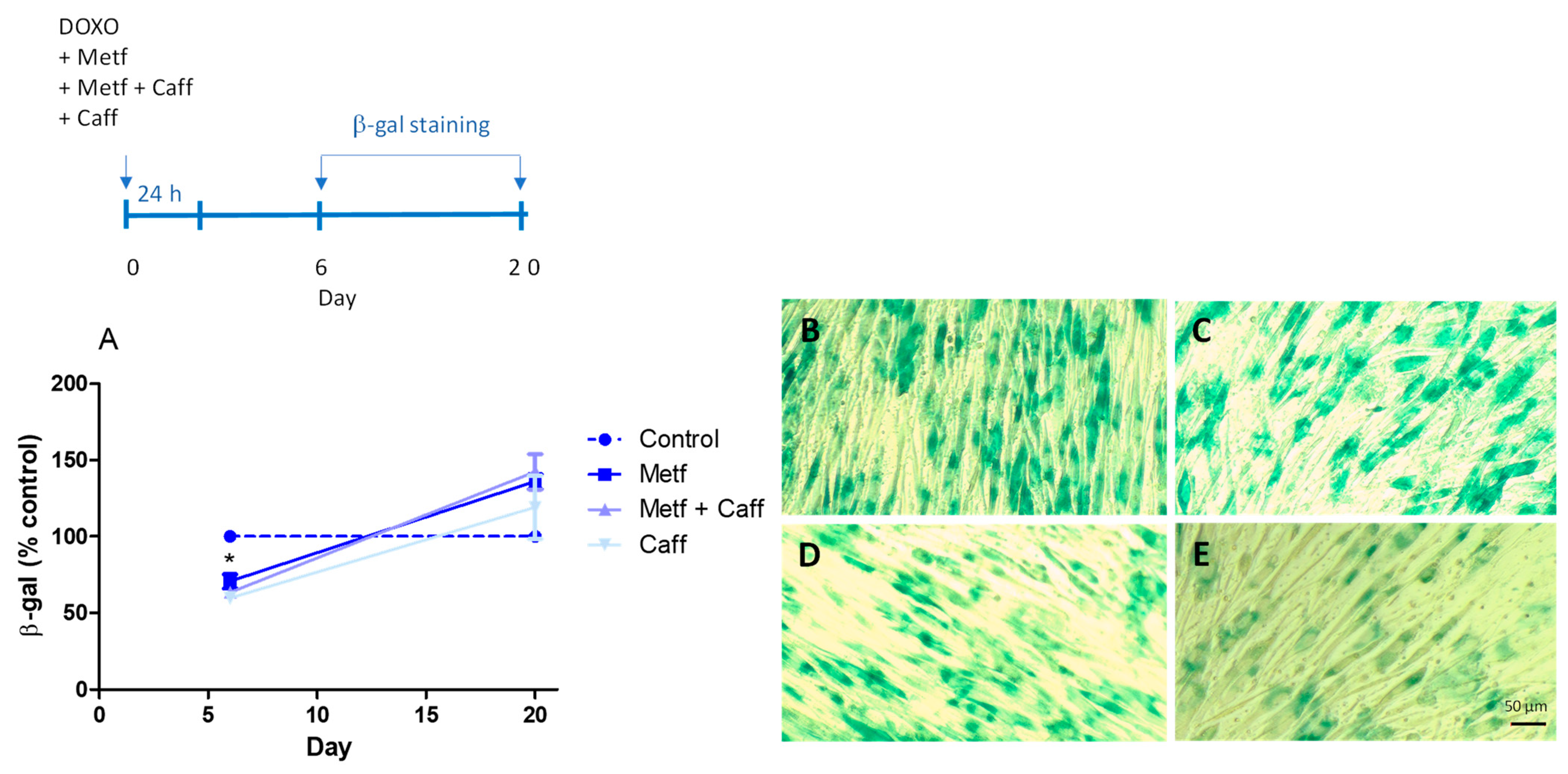
2.1. Senomorphic Effects of Drugs and Polyphenols
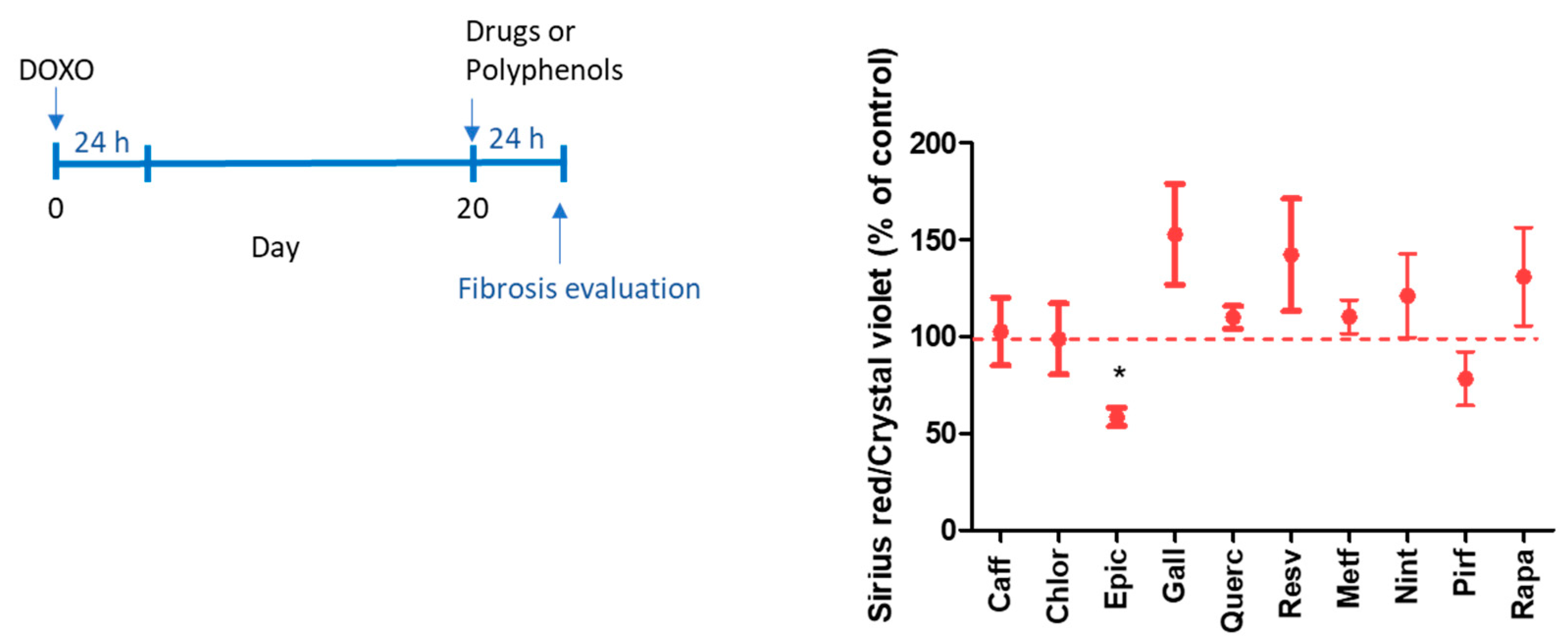
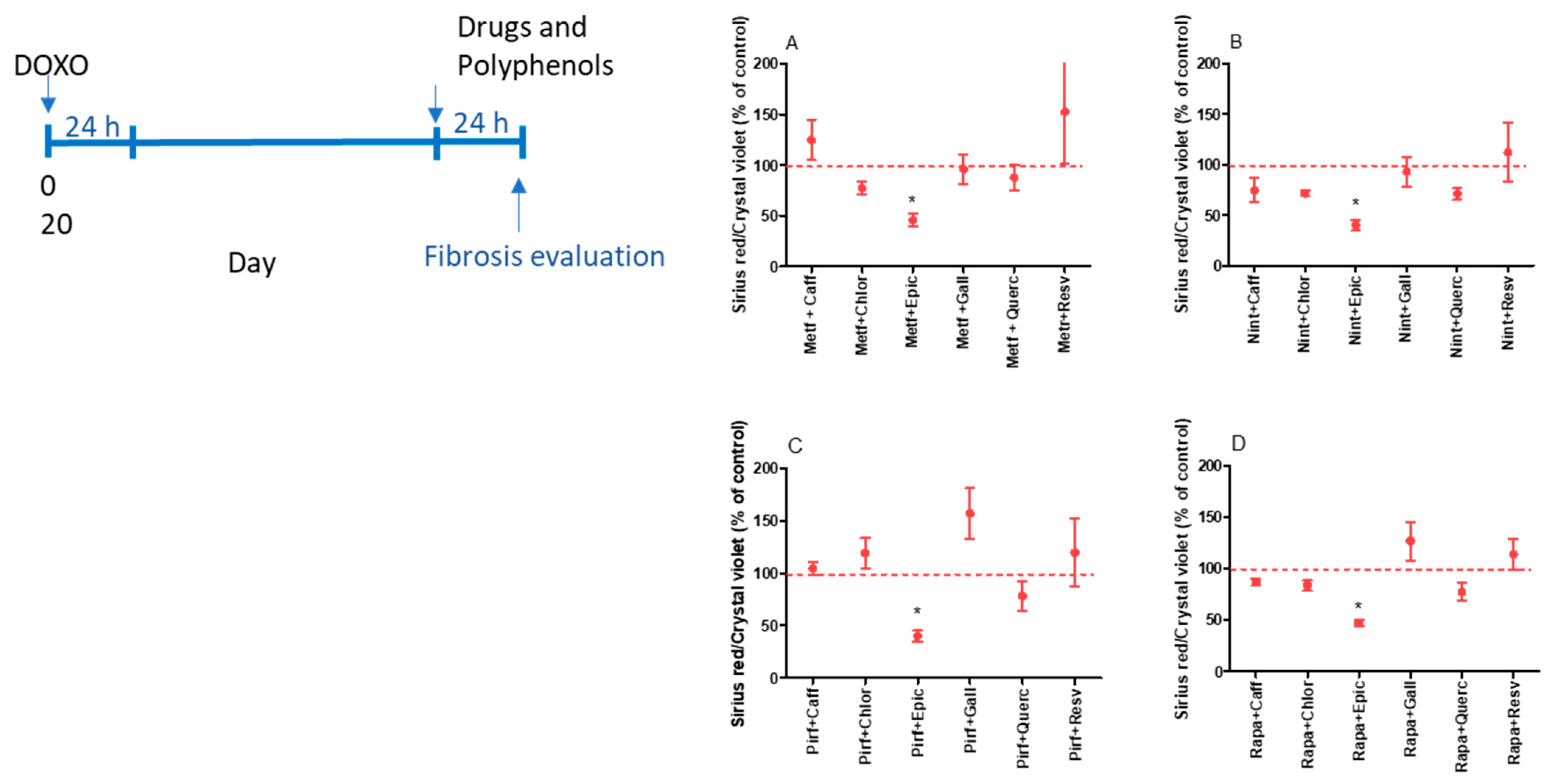
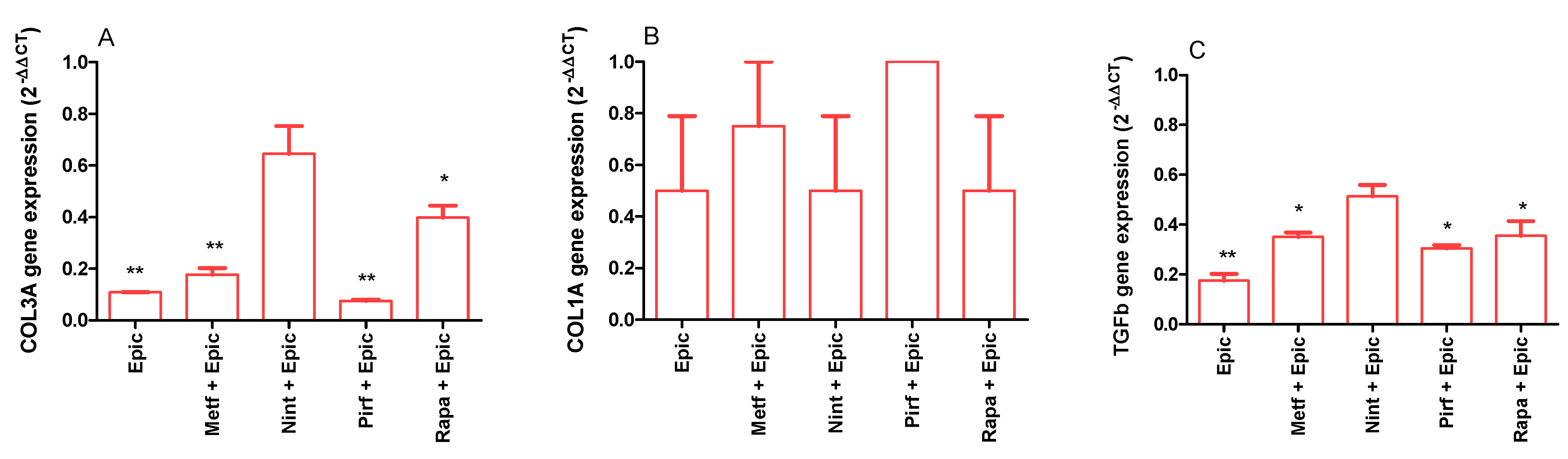
2.2. Evaluation of the Antifibrotic Activity of Drugs and Polyphenols
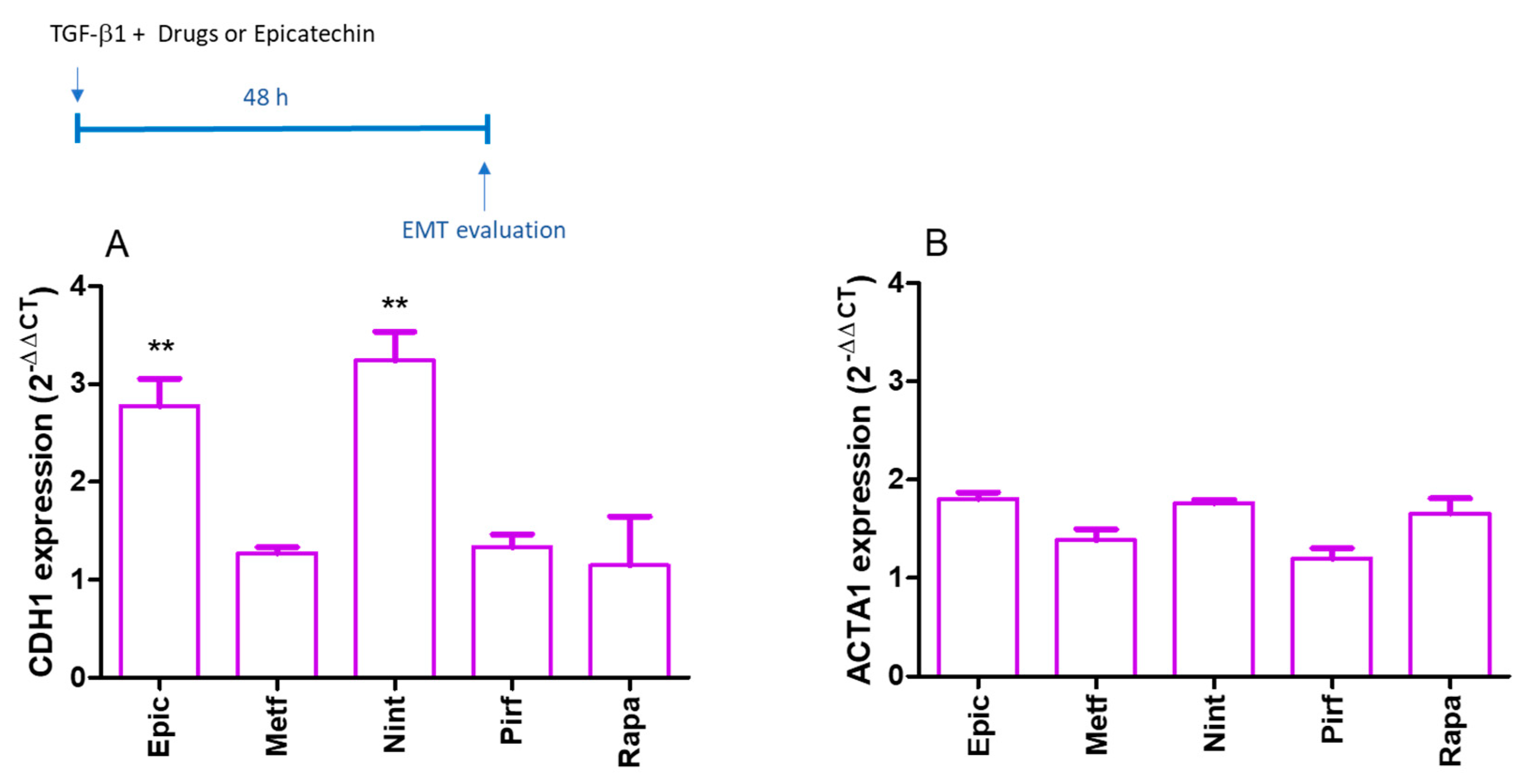
2.3. EMT Evaluation
3. Discussion
4. Materials and Methods
4.1. MRC-5 Cell Culture
4.2. MRC-5 Senescence Induced by Doxorubicin
4.3. Determination of Drug and Polyphenol Non-Toxic Concentrations
4.4. SASP Evaluation
4.5. Senescence-Associated β-Galactosidase (SA-β-gal) Assay
4.6. Quantitative Analysis of Gene Expression
4.7. Picrosirius Red and Crystal Violet Stains
4.8. Epithelial-Mesenchymal Transition (EMT) Assay Using A549 Cells
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Okuda, R.; Aoshiba, K.; Matsushima, H.; Ogura, T.; Okudela, K.; Ohashi, K. Cellular senescence and senescence-associated secretory phenotype: Comparison of idiopathic pulmonary fibrosis, connective tissue disease-associated interstitial lung disease, and chronic obstructive pulmonary disease. J. Thorac. Dis. 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Barnes, P.J. Senotherapy for lung diseases. Adv. Pharmacol. 2023, 98, 249–271. [Google Scholar]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Jha, S.K.; De Rubis, G.; Devkota, S.R.; Zhang, Y.; Adhikari, R.; Jha, L.A.; Bhattacharya, K.; Mehndiratta, S.; Gupta, G.; Singh, S.K.; et al. Cellular senescence in lung cancer: Molecular mechanisms and therapeutic interventions. Ageing Res. Rev. 2024, 97, 102315. [Google Scholar] [CrossRef]
- Lushchak, O.; Schosserer, M.; Grillari, J. Senopathies-Diseases Associated with Cellular Senescence. Biomolecules 2023, 13, 966. [Google Scholar] [CrossRef]
- Nambiar, A.; Kellogg, D., 3rd; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Kautz, T.F.; Palavicini, J.P.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.J.; Zare, H.; Xu, P.; Zhang, B.; et al. Senolytic therapy in mild Alzheimer’s disease: A phase 1 feasibility trial. Nat. Med. 2023, 29, 2481–2488. [Google Scholar] [CrossRef]
- Nehlin, J.O. Senolytic and senomorphic interventions to defy senescence-associated mitochondrial dysfunction. Adv. Protein Chem. Struct. Biol. 2023, 136, 217–247. [Google Scholar]
- Hansel, C.; Barr, S.; Schemann, A.V.; Lauber, K.; Hess, J.; Unger, K.; Zitzelsberger, H.; Jendrossek, V.; Klein, D. Metformin Protects against Radiation-Induced Acute Effects by Limiting Senescence of Bronchial-Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 7064. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Sun, C.; Shen, H.; Cui, X. Metformin attenuates lipopolysaccharide-induced epithelial cell senescence by activating autophagy. Cell Biol. Int. 2021, 45, 927–935. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun. Signal 2020, 18, 43. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Hou, J.; Wang, H.; Zheng, Y.; Li, H.; Cai, H.; Han, X.; Dai, J. Epithelial cell senescence induces pulmonary fibrosis through Nanog-mediated fibroblast activation. Aging 2019, 12, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Scisciola, L.; Olivieri, F.; Ambrosino, C.; Barbieri, M.; Rizzo, M.R.; Paolisso, G. On the wake of metformin: Do anti-diabetic SGLT2 inhibitors exert antiaging effects? Ageing Res. Rev. 2023, 92, 102131. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Antiaging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol. Biol. Rep. 2020, 47, 1021–1032. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, W.Y.; Li, L.L.; Hu, C.; Tang, Q.Z. Therapeutic Potential of Polyphenols in Cardiac Fibrosis. Front. Pharmacol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Jing, W.; Sun, B.; Miao, C.; Ren, L. Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can. J. Physiol. Pharmacol. 2013, 91, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Liu, Y.J.; Mao, Y.F.; Dong, W.W.; Zhu, X.Y.; Jiang, L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clin. Nutr. 2015, 34, 752–760. [Google Scholar] [CrossRef]
- Albanawany, N.M.; Samy, D.M.; Zahran, N.; El-Moslemany, R.M.; Elsawy, S.M.; Abou Nazel, M.W. Histopathological, physiological and biochemical assessment of resveratrol nanocapsules efficacy in bleomycin-induced acute and chronic lung injury in rats. Drug Deliv. 2022, 29, 2592–2608. [Google Scholar] [CrossRef]
- Wang, L.; Shao, M.; Jiang, W.; Huang, Y. Resveratrol alleviates bleomycin-induced pulmonary fibrosis by inhibiting epithelial-mesenchymal transition and down-regulating TLR4/NF-kappaB and TGF-beta1/smad3 signalling pathways in rats. Tissue Cell 2022, 79, 101953. [Google Scholar] [CrossRef]
- Ghonim, M.A.; Boyd, D.F.; Flerlage, T.; Thomas, P.G. Pulmonary inflammation and fibroblast immunoregulation: From bench to bedside. J. Clin. Investig. 2023, 133, e170499. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; Lopez Fernandez, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef]
- He, H.; Ji, X.; Cao, L.; Wang, Z.; Wang, X.; Li, X.M.; Miao, M. Medicine Targeting Epithelial-Mesenchymal Transition to Treat Airway Remodeling and Pulmonary Fibrosis Progression. Can. Respir. J. 2023, 2023, 3291957. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Caramori, G.; Cari, L.; Chung, K.F.; Diamant, Z.; Eguiluz-Gracia, I.; et al. Metabolic pathways in immune senescence and inflammaging: Novel therapeutic strategy for chronic inflammatory lung diseases. An EAACI position paper from the Task Force for Immunopharmacology. Allergy 2023, 79, 1089–1122. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- de Oliveira Neto, L.; Tavares, V.D.O.; Agricola, P.M.D.; de Oliveira, L.P.; Sales, M.C.; de Sena-Evangelista, K.C.M.; Gomes, I.C.; Galvao-Coelho, N.L.; Pedrosa, L.F.C.; Lima, K.C. Factors associated with inflamm-aging in institutionalized older people. Sci. Rep. 2021, 11, 18333. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, D.A.; Kolosova, E.D.; Pukhalskaia, T.V.; Levchuk, K.A.; Demidov, O.N.; Belotserkovskaya, E.V. The Differential Effect of Senolytics on SASP Cytokine Secretion and Regulation of EMT by CAFs. Int. J. Mol. Sci. 2024, 25, 4031. [Google Scholar] [CrossRef] [PubMed]
- Al Dubayee, M.; Alshahrani, A.; Almalk, M.; Hakami, A.; Homoud, B.; Alzneidi, N.; Aldhalaan, J.; Aljbli, G.; Nasr, A.; Farahat, A.I.; et al. Metformin alters peripheral blood mononuclear cells (PBMC) senescence biomarkers gene expression in type 2 diabetic patients. J. Diabetes Complicat. 2021, 35, 107758. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Sreedharan, S.; Nair, V.; Cisneros-Zevallos, L. Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells. Nutrients 2023, 15, 2965. [Google Scholar] [CrossRef]
- Rebecca Roy, J.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Veeraraghavan, V.P.; Krishnamoorthy, K.; Prasad, M. Carica Papaya Reduces High Fat Diet and Streptozotocin-Induced Development of Inflammation in Adipocyte via IL-1beta/IL-6/TNF-alpha Mediated Signaling Mechanisms in Type-2 Diabetic Rats. Curr. Issues Mol. Biol. 2023, 45, 852–884. [Google Scholar] [CrossRef]
- Espindola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Lim, H.; Park, B.K.; Shin, S.Y.; Kwon, Y.S.; Kim, H.P. Methyl caffeate and some plant constituents inhibit age-related inflammation: Effects on senescence-associated secretory phenotype (SASP) formation. Arch. Pharm. Res. 2017, 40, 524–535. [Google Scholar] [CrossRef]
- Hickson, L.J.; Eirin, A.; Conley, S.M.; Taner, T.; Bian, X.; Saad, A.; Herrmann, S.M.; Mehta, R.A.; McKenzie, T.J.; Kellogg, T.A.; et al. Diabetic Kidney Disease Alters the Transcriptome and Function of Human Adipose-Derived Mesenchymal Stromal Cells but Maintains Immunomodulatory and Paracrine Activities Important for Renal Repair. Diabetes 2021, 70, 1561–1574. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological Activity of Quercetin: An Updated Review. Evid. Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Xia, R.; Zheng, X.; Li, X.; Wu, S.; Zhang, Q.; Li, S.; Deng, Y.; Yao, Y.; et al. Component analysis and anti-pulmonary fibrosis effects of Rosa sterilis juice. Food Funct. 2022, 13, 12915–12924. [Google Scholar] [CrossRef] [PubMed]
- Desdiani, D.; Rengganis, I.; Djauzi, S.; Setiyono, A.; Sadikin, M.; Jusman, S.W.A.; Siregar, N.C.; Suradi, S.; Eyanoer, P.C. Fibropreventive and Antifibrotic Effects of Uncaria gambir on Rats with Pulmonary Fibrosis. Evid. Based Complement. Altern. Med. 2022, 2022, 6721958. [Google Scholar] [CrossRef] [PubMed]
- Shariati, S.; Kalantar, H.; Pashmforoosh, M.; Mansouri, E.; Khodayar, M.J. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed. Pharmacother. 2019, 114, 108776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, R.; Zhou, Z.; Liu, R.; Wen, J. Efficacy and safety of caffeic acid tablets in the treatment of thrombocytopenia: A systematic review and meta-analysis. Medicine 2023, 102, e35353. [Google Scholar] [CrossRef]
- Zhongyin, Z.; Wei, W.; Juan, X.; Guohua, F. Epigallocatechin Gallate Relieved PM2.5-Induced Lung Fibrosis by Inhibiting Oxidative Damage and Epithelial-Mesenchymal Transition through AKT/mTOR Pathway. Oxid. Med. Cell Longev. 2022, 2022, 7291774. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Mitsuishi, Y.; Kato, M.; Takahashi, F.; Tajima, K.; Hayashi, T.; Hidayat, M.; Winardi, W.; Wirawan, A.; Hayakawa, D.; et al. Nintedanib inhibits epithelial-mesenchymal transition in A549 alveolar epithelial cells through regulation of the TGF-beta/Smad pathway. Respir. Investig. 2020, 58, 275–284. [Google Scholar] [CrossRef]
- de Godoy, M.C.X.; Macedo, J.A.; Gambero, A. Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line. Pharmaceuticals 2024, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Itahana, K.; Itahana, Y.; Dimri, G.P. Colorimetric detection of senescence-associated beta galactosidase. Methods Mol. Biol. 2013, 965, 143–156. [Google Scholar] [PubMed]
- Trackman, P.C.; Saxena, D.; Bais, M.V. TGF-beta1- and CCN2-Stimulated Sirius Red Assay for Collagen Accumulation in Cultured Cells. Methods Mol. Biol. 2017, 1489, 481–485. [Google Scholar]
- Ding, H.; Chen, J.; Qin, J.; Chen, R.; Yi, Z. TGF-beta-induced alpha-SMA expression is mediated by C/EBPbeta acetylation in human alveolar epithelial cells. Mol. Med. 2021, 27, 22. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, M.C.X.d.; Monteiro, G.A.; Moraes, B.H.d.; Macedo, J.A.; Gonçalves, G.M.S.; Gambero, A. Addition of Polyphenols to Drugs: The Potential of Controlling “Inflammaging” and Fibrosis in Human Senescent Lung Fibroblasts In Vitro. Int. J. Mol. Sci. 2024, 25, 7163. https://doi.org/10.3390/ijms25137163
Godoy MCXd, Monteiro GA, Moraes BHd, Macedo JA, Gonçalves GMS, Gambero A. Addition of Polyphenols to Drugs: The Potential of Controlling “Inflammaging” and Fibrosis in Human Senescent Lung Fibroblasts In Vitro. International Journal of Molecular Sciences. 2024; 25(13):7163. https://doi.org/10.3390/ijms25137163
Chicago/Turabian StyleGodoy, Maria Carolina Ximenes de, Gabriela Arruda Monteiro, Bárbara Hakim de Moraes, Juliana Alves Macedo, Gisele Mara Silva Gonçalves, and Alessandra Gambero. 2024. "Addition of Polyphenols to Drugs: The Potential of Controlling “Inflammaging” and Fibrosis in Human Senescent Lung Fibroblasts In Vitro" International Journal of Molecular Sciences 25, no. 13: 7163. https://doi.org/10.3390/ijms25137163



