Ambient Particulate Matter Induces In Vitro Toxicity to Intestinal Epithelial Cells without Exacerbating Acute Colitis Induced by Dextran Sodium Sulfate or 2,4,6-Trinitrobenzenesulfonic Acid
Abstract
:1. Introduction
2. Results
2.1. In Vitro PM Exposure Demonstrated Toxicity in Intestinal Cells
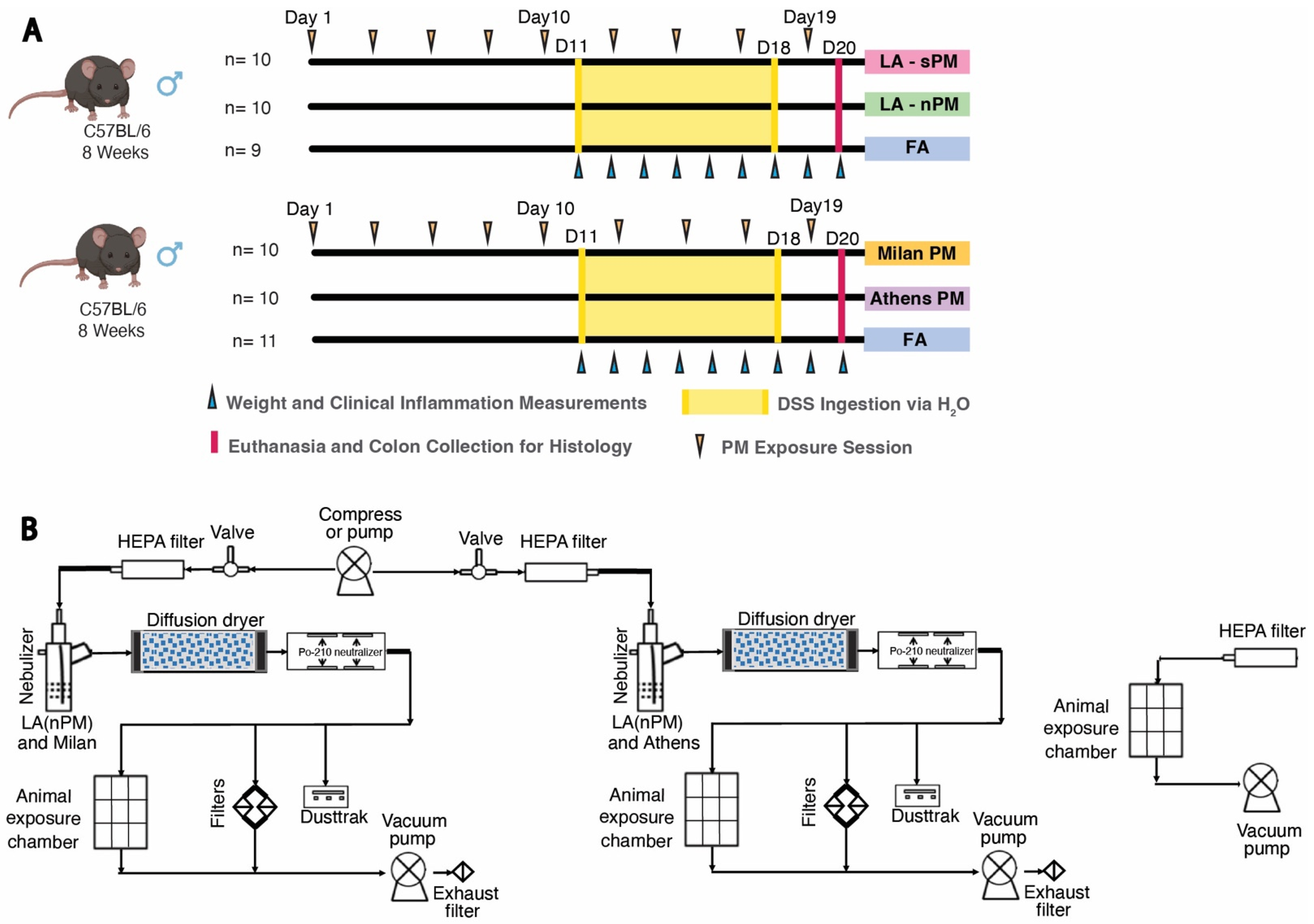
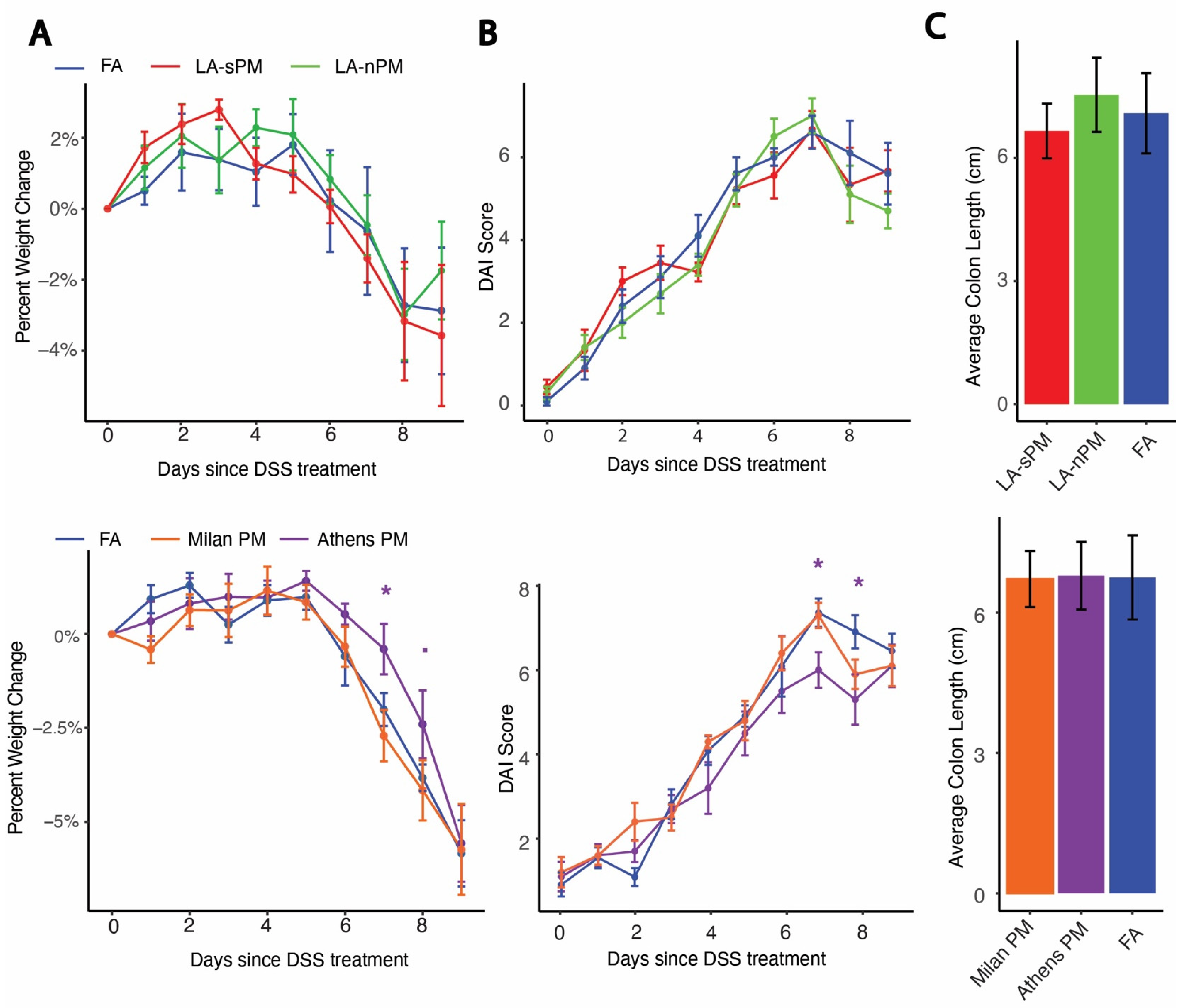
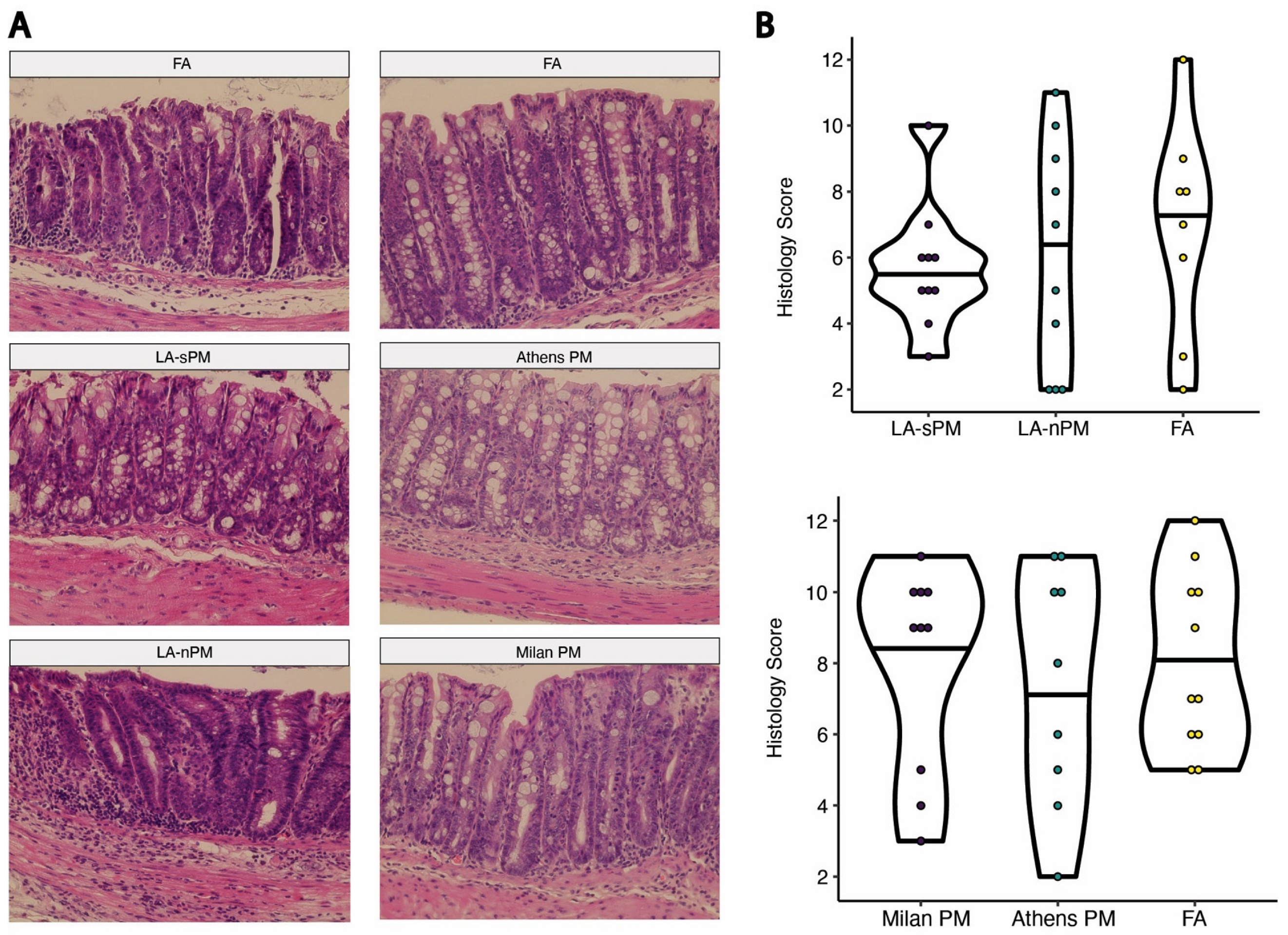
2.2. Inhalation Exposure to PM from Four Distinct Sources Did Not Exacerbate DSS Colitis Severity
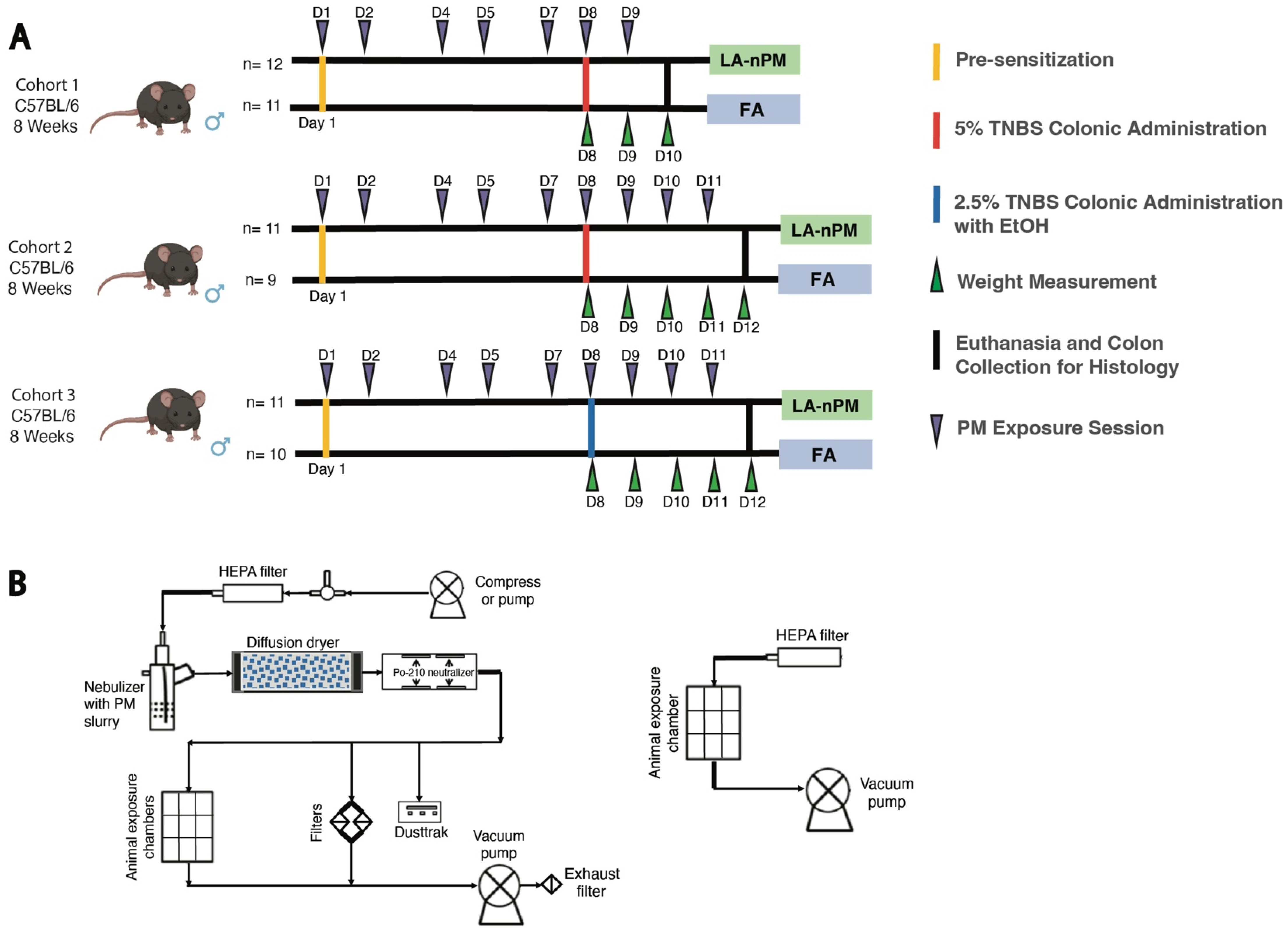
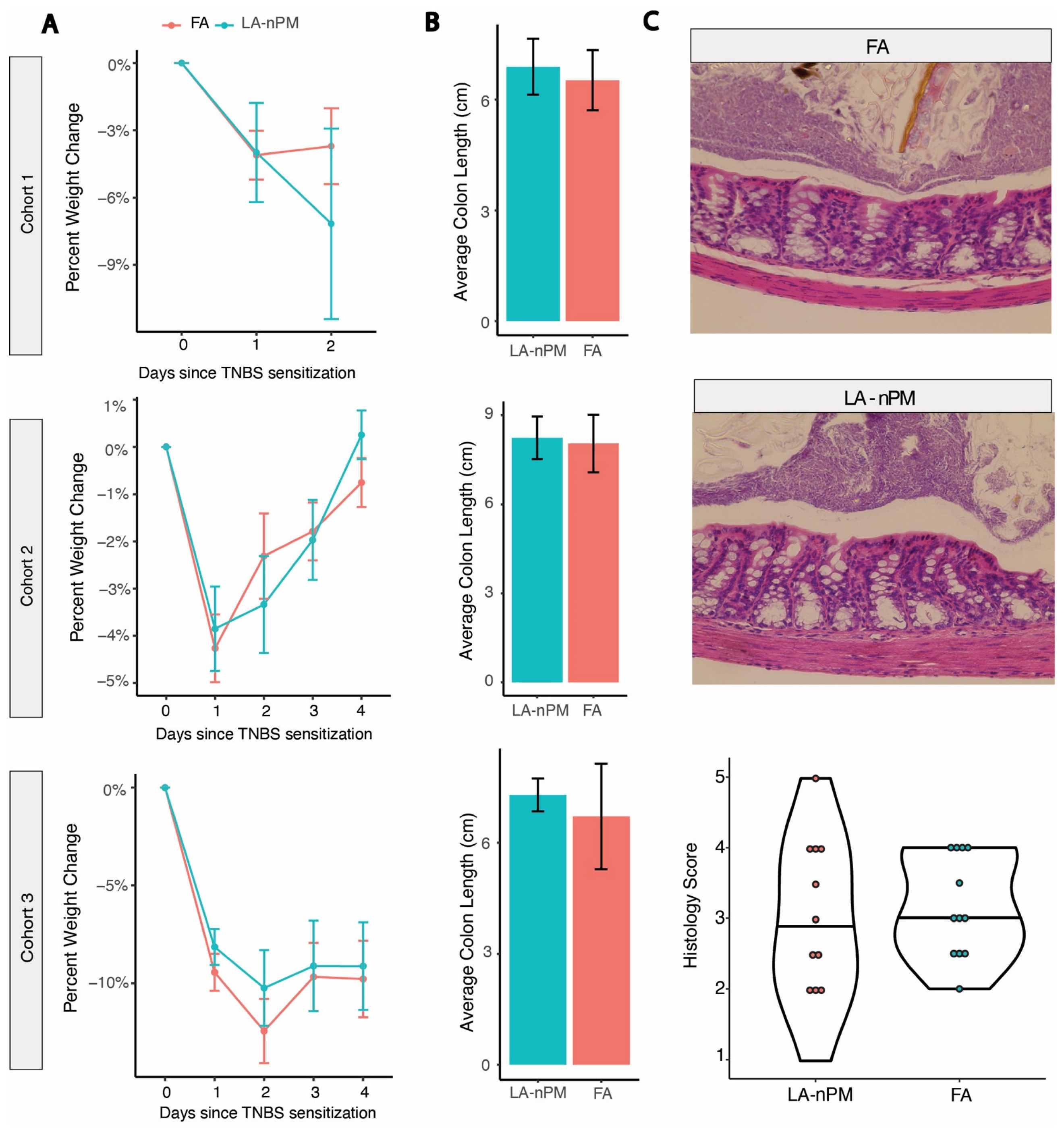
2.3. TNBS Colitis Severity Is Unaffected by PM Inhalation Exposure
3. Discussion
4. Materials and Methods
4.1. Animal Subjects
4.2. Collection of Particulate Matter
4.3. In Vitro Experiments
4.4. DSS PM Inhalation Exposures
4.5. DSS PM Characterization
4.6. TNBS PM Exposures
4.7. Clinical Measures of Inflammation Analysis
4.8. Histological Scoring
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology 2019, 157, 647–659.e644. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Ye, W.; Zhang, Y.; Tang, C.; Li, X.; Zhou, X.; Xie, Y. Urban–rural environmental exposure during childhood and subsequent risk of inflammatory bowel disease: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 591–602. [Google Scholar] [CrossRef]
- Soon, I.S.; Molodecky, N.A.; Rabi, D.M.; Ghali, W.A.; Barkema, H.W.; Kaplan, G.G. The relationship between urban environment and the inflammatory bowel diseases: A systematic review and meta-analysis. BMC Gastroenterol. 2012, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Hubbard, J.; Korzenik, J.; Sands, B.E.; Panaccione, R.; Ghosh, S.; Wheeler, A.J.; Villeneuve, P.J. The inflammatory bowel diseases and ambient air pollution: A novel association. Am. J. Gastroenterol. 2010, 105, 2412. [Google Scholar] [CrossRef]
- Opstelten, J.L.; Beelen, R.M.; Leenders, M.; Hoek, G.; Brunekreef, B.; van Schaik, F.D.; Siersema, P.D.; Eriksen, K.T.; Raaschou-Nielsen, O.; Tjønneland, A. Exposure to ambient air pollution and the risk of inflammatory bowel disease: A European nested case–control study. Dig. Dis. Sci. 2016, 61, 2963–2971. [Google Scholar] [CrossRef]
- Elten, M.; Benchimol, E.; Fell, D.; Lavigne, E. Air pollution and greenness, and their associated risk with pediatric inflammatory bowel disease. Environ. Epidemiol. 2019, 3, 111. [Google Scholar]
- Halfvarson, J.; Bodin, L.; Tysk, C.; Lindberg, E.; Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: A long-term follow-up of concordance and clinical characteristics. Gastroenterology 2003, 124, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: An ecologic analysis. Inflamm Bowel Dis 2011, 17, 1138–1145. [Google Scholar] [CrossRef]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef]
- Hart, J.E.; Laden, F.; Puett, R.C.; Costenbader, K.H.; Karlson, E.W. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ. Health Perspect. 2009, 117, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.; Dixon, E.; Panaccione, R.; Heitman, S.; Fong, A.; Chen, L.; Szyszkowicz, M.; MacLean, A.; Buie, D.; Villeneuve, P. Air Pollution and Appendicitis: A Novel Association: 292. Off. J. Am. Coll. Gastroenterol. ACG 2008, 103, S113. [Google Scholar] [CrossRef]
- Diaz, E.; Mariën, K.; Manahan, L.; Fox, J. Summary of Health Research on Ultrafine Particles; Washington State Department of Health, Environmental Public Health Division. Office of Environmental Public Health Sciences: Tumwater, WA, USA, 2019; pp. 334–454. [Google Scholar]
- Hussein, T.; Puustinen, A.; Aalto, P.P.; Mäkelä, J.M.; Hämeri, K.; Kulmala, M. Urban aerosol number size distributions. Atmos. Chem. Phys. 2004, 4, 391–411. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Y.; Chen, R.; Zhou, Z.; Chen, B.; Kan, H. Size-fractionated particle number concentrations and daily mortality in a Chinese city. Environ. Health Perspect. 2013, 121, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Shaw, C.A.; Langrish, J.P. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012, 8, 577–602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Verma, M.K.; Srivastava, A.K. Ultrafine particles in urban ambient air and their health perspectives. Rev. Environ. Health 2013, 28, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Devlin, R.B.; Smith, C.B.; Schmitt, M.T.; Rappold, A.G.; Hinderliter, A.; Graff, D.; Carraway, M.S. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol. Sci. 2014, 140, 61–72. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part. Fibre Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Usatyuk, P.V.; Samet, J.M.; Geyh, A.S.; Breysse, P.N. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part. Fibre Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- van EEDEN, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Haussinger, K.; Winkler-Heil, R.; Stahlhofen, W.; Meyer, T.; Hofmann, W.; Heyder, J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J. Appl. Physiol. 2004, 97, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Vidgren, M.; Waldrep, J.; Arppe, J.; Black, M.; Rodarte, J.; Cole, W.; Knight, V. A study of 99mtechnetium-labelled beclomethasone dipropionate dilauroylphosphatidylcholine liposome aerosol in normal volunteers. Int. J. Pharm. 1995, 115, 209–216. [Google Scholar] [CrossRef]
- Kreyling, W.; Blanchard, J.; Godleski, J.; Haeussermann, S.; Heyder, J.; Hutzler, P.; Schulz, H.; Sweeney, T.; Takenaka, S.; Ziesenis, A. Anatomic localization of 24-and 96-h particle retention in canine airways. J. Appl. Physiol. 1999, 87, 269–284. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.M.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.; Hoet, P.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Beamish, L.A.; Osornio-Vargas, A.R.; Wine, E. Air pollution: An environmental factor contributing to intestinal disease. J. Crohn’s Colitis 2011, 5, 279–286. [Google Scholar] [CrossRef]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Gänzle, M.; Rioux, K.P.; Thiesen, A.; Barkema, H.W.; Wine, E. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef]
- Salim, S.Y.; Jovel, J.; Wine, E.; Kaplan, G.G.; Vincent, R.; Thiesen, A.; Barkema, H.W.; Madsen, K.L. Exposure to Ingested Airborne Pollutant Particulate Matter Increases Mucosal Exposure to Bacteria and Induces Early Onset of Inflammation in Neonatal IL-10–Deficient Mice. Inflamm. Bowel Dis. 2014, 20, 1129–1138. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Saffari, A.; Jacobs, J.; Baek, K.I.; Hough, G.; Larauche, M.H.; Ma, J.; Jen, N.; Moussaoui, N. Ambient ultrafine particle ingestion alters gut microbiota in association with increased atherogenic lipid metabolites. Sci. Rep. 2017, 7, 42906. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C.; Zhao, J.; Li, D.; Chen, J. Exposure to concentrated ambient PM2. 5 (CAPM) induces intestinal disturbance via inflammation and alternation of gut microbiome. Environ. Int. 2022, 161, 107138. [Google Scholar] [CrossRef]
- Chang, C.; Gupta, R.; Sedighian, F.; Louie, A.; Gonzalez, D.; Le, C.; Cho, J.M.; Park, S.-K.; Castellanos, J.; Ting, T.-W. Subchronic inhalation exposure to ultrafine particulate matter alters the intestinal microbiome in various mouse models. Environ. Res. 2024, 248, 118242. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Gerasimidis, K.; Ho, S.-M.; Mayer, E.; Pollock, J.; Soni, S.; Wu, G.D.; Benyacoub, J.; Ali, B.; Favreau, A. Challenges in IBD Research 2024: Environmental Triggers. Inflamm. Bowel Dis. 2024, 30, S19–S29. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Shi, Y.; Cao, H.; Liu, L.; Washington, M.K.; Chaturvedi, R.; Israel, D.A.; Cao, H.; Wang, B. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G504–G514. [Google Scholar] [CrossRef]
- Saleh, M.; Trinchieri, G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 2011, 11, 9–20. [Google Scholar] [CrossRef]
- Britto, S.L.; Krishna, M.; Kellermayer, R. Weight loss is a sufficient and economical single outcome measure of murine dextran sulfate sodium colitis. FASEB BioAdv. 2019, 1, 493. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Sartor, R.B.; Tennyson, G.S.; Riddell, R.H. Experimental models of inflammatory bowel disease. Gastroenterology 1995, 109, 1344–1367. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Asano, N.; Murray, P.J.; Ozato, K.; Tailor, P.; Fuss, I.J.; Kitani, A.; Strober, W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Investig. 2008, 118, 545–559. [Google Scholar] [PubMed]
- Amendola, A.; Butera, A.; Sanchez, M.; Strober, W.; Boirivant, M. Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms. Mucosal Immunol. 2014, 7, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, E.; Taghvaee, S.; Sioutas, C. Characterization of organic compounds and oxidative potential of aqueous PM2. 5 suspensions collected via an aerosol-into-liquid collector for use in toxicology studies. Atmos. Environ. 2020, 241, 117839. [Google Scholar] [CrossRef]
- Farahani, V.J.; Altuwayjiri, A.; Pirhadi, M.; Verma, V.; Ruprecht, A.A.; Diapouli, E.; Eleftheriadis, K.; Sioutas, C. The oxidative potential of particulate matter (PM) in different regions around the world and its relation to air pollution sources. Environ. Sci. Atmos. 2022, 2, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Hakimzadeh, M.; Soleimanian, E.; Mousavi, A.; Borgini, A.; De Marco, C.; Ruprecht, A.A.; Sioutas, C. The impact of biomass burning on the oxidative potential of PM2. 5 in the metropolitan area of Milan. Atmos. Environ. 2020, 224, 117328. [Google Scholar] [CrossRef]
- Pirhadi, M.; Mousavi, A.; Taghvaee, S.; Shafer, M.M.; Sioutas, C. Semi-volatile components of PM2. 5 in an urban environment: Volatility profiles and associated oxidative potential. Atmos. Environ. 2020, 223, 117197. [Google Scholar] [CrossRef] [PubMed]
- Steerenberg, P.; Withagen, C.; Van Dalen, W.; Dorma, J.; Heisterkamp, S.; Loveren, H.v.; Cassee, F. Dose dependency of adjuvant activity of particulate matter from five european sites in three seasons in an ovalbumin–mouse model. Inhal. Toxicol. 2005, 17, 133–145. [Google Scholar] [CrossRef]
- Grahame, T.J.; Schlesinger, R.B. Health effects of airborne particulate matter: Do we know enough to consider regulating specific particle types or sources? Inhal. Toxicol. 2007, 19, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shkirkova, K.; Lamorie-Foote, K.; Connor, M.; Patel, A.; Babadjouni, R.; Huuskonen, M.; Montagne, A.; Baertsch, H.; Zhang, H. Air pollution particulate matter exposure and chronic cerebral hypoperfusion and measures of white matter injury in a murine model. Environ. Health Perspect. 2021, 129, 087006. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Pichavant, M.; Alleman, L.Y.; Djouina, M.; Dingreville, F.; Perdrix, E.; Waxin, C.; Ouali Alami, A.; Gower-Rousseau, C.; Desreumaux, P. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 2017, 14, 46. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.; Yang, H.; Sun, H.; Lu, R.; Gao, N.; Meng, Q.; Wu, S.; Wu, J.; Aschner, M. Colonic injuries induced by inhalational exposure to particulate-matter air pollution. Adv. Sci. 2019, 6, 1900180. [Google Scholar] [CrossRef]
- Misra, C.; Kim, S.; Shen, S.; Sioutas, C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. J. Aerosol Sci. 2002, 33, 735–752. [Google Scholar] [CrossRef]
- Taghvaee, S.; Mousavi, A.; Sowlat, M.H.; Sioutas, C. Development of a novel aerosol generation system for conducting inhalation exposures to ambient particulate matter (PM). Sci. Total Environ. 2019, 665, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lin, L.; Goudarzi, M.; Ruegger, P.; McGovern, D.P.; Fornace, A.J., Jr.; Borneman, J.; Xia, L.; Braun, J. Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency. Gut Microbes 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557. [Google Scholar] [PubMed]

| Period | Location | Dominant Emision Source | Collection Period |
|---|---|---|---|
| Campaign I | Los Angeles—sPM | Vehicular emissions | December 2020–January 2021 |
| Los Angeles—nPM | Vehicular emissions | September 2020–October 2020 | |
| Campaign II | Athens PM | Secondary aerosols | August 2020–September 2020 |
| Milan PM | Residential heating (biomass) | December 2020–January 2021 |
| Sample ID | Location | Concentration (μg/mL) | Volume (mL) | Mass (mg) |
|---|---|---|---|---|
| 1 | Los Angeles—sPM | 65 | 355 | 23 |
| 2 | Los Angeles—nPM | 200 | 115 | 23 |
| 3 | Athens PM | 200 | 115 | 23 |
| 4 | Milan PM | 200 | 115 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Louie, A.; Zhou, Y.; Gupta, R.; Liang, F.; Xanthou, G.; Ereso, J.; Koletic, C.; Yang, J.C.; Sedighian, F.; et al. Ambient Particulate Matter Induces In Vitro Toxicity to Intestinal Epithelial Cells without Exacerbating Acute Colitis Induced by Dextran Sodium Sulfate or 2,4,6-Trinitrobenzenesulfonic Acid. Int. J. Mol. Sci. 2024, 25, 7184. https://doi.org/10.3390/ijms25137184
Chang C, Louie A, Zhou Y, Gupta R, Liang F, Xanthou G, Ereso J, Koletic C, Yang JC, Sedighian F, et al. Ambient Particulate Matter Induces In Vitro Toxicity to Intestinal Epithelial Cells without Exacerbating Acute Colitis Induced by Dextran Sodium Sulfate or 2,4,6-Trinitrobenzenesulfonic Acid. International Journal of Molecular Sciences. 2024; 25(13):7184. https://doi.org/10.3390/ijms25137184
Chicago/Turabian StyleChang, Candace, Allen Louie, Yi Zhou, Rajat Gupta, Fengting Liang, Georgina Xanthou, Jason Ereso, Carolina Koletic, Julianne Ching Yang, Farzaneh Sedighian, and et al. 2024. "Ambient Particulate Matter Induces In Vitro Toxicity to Intestinal Epithelial Cells without Exacerbating Acute Colitis Induced by Dextran Sodium Sulfate or 2,4,6-Trinitrobenzenesulfonic Acid" International Journal of Molecular Sciences 25, no. 13: 7184. https://doi.org/10.3390/ijms25137184










