Implications of Genetic Factors Underlying Mouse Hydronephrosis: Cautionary Considerations on Phenotypic Interpretation in Genetically Engineered Mice
Abstract
:1. Introduction
2. Results
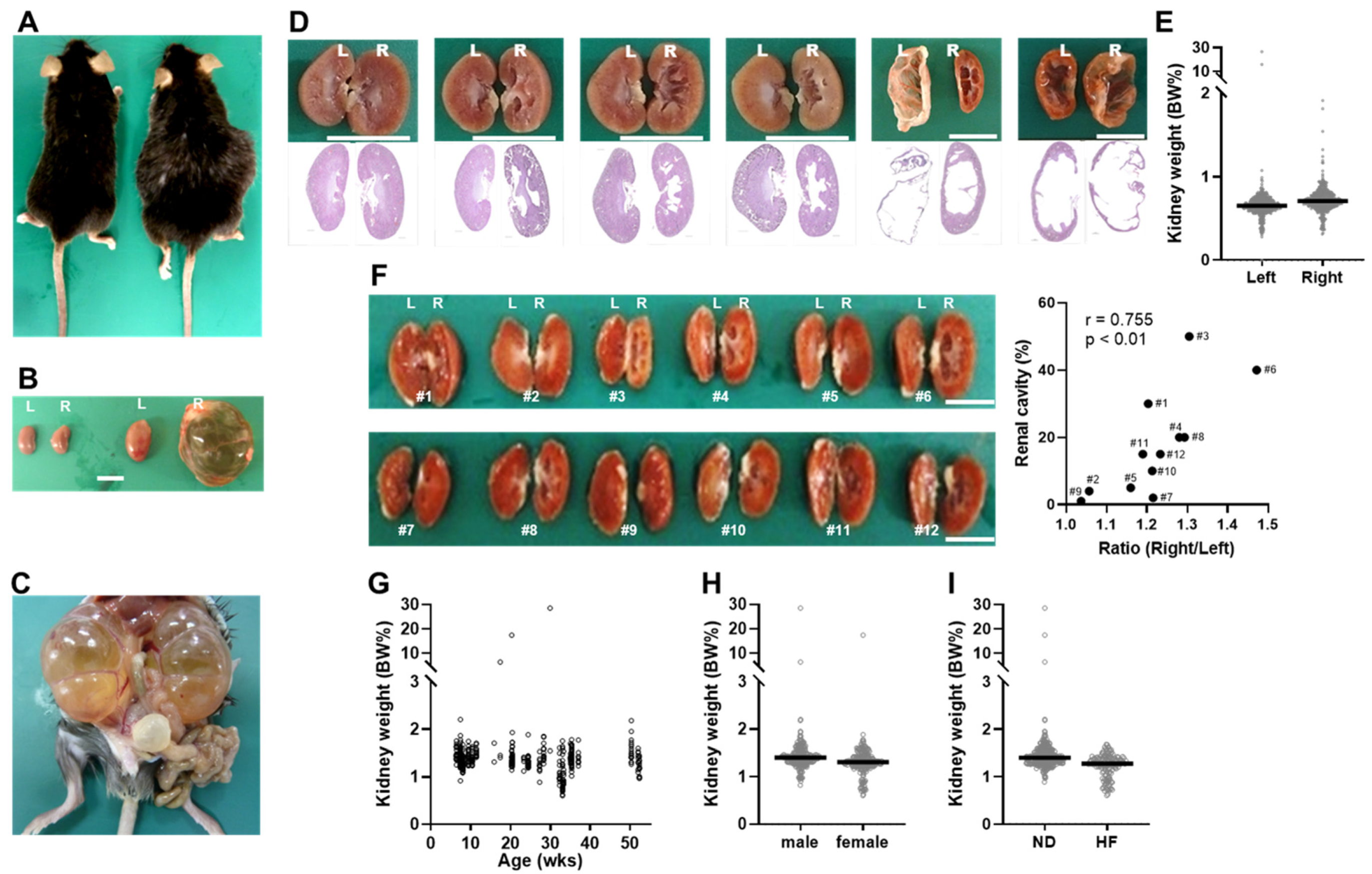
2.1. Discovery of Tspan7 Transgenic Mice with Kidney Abnormalities Resembling Hydronephrosis
2.2. Mice with Dilated Kidneys Exhibit Hyperammonemia and Atrophic Liver
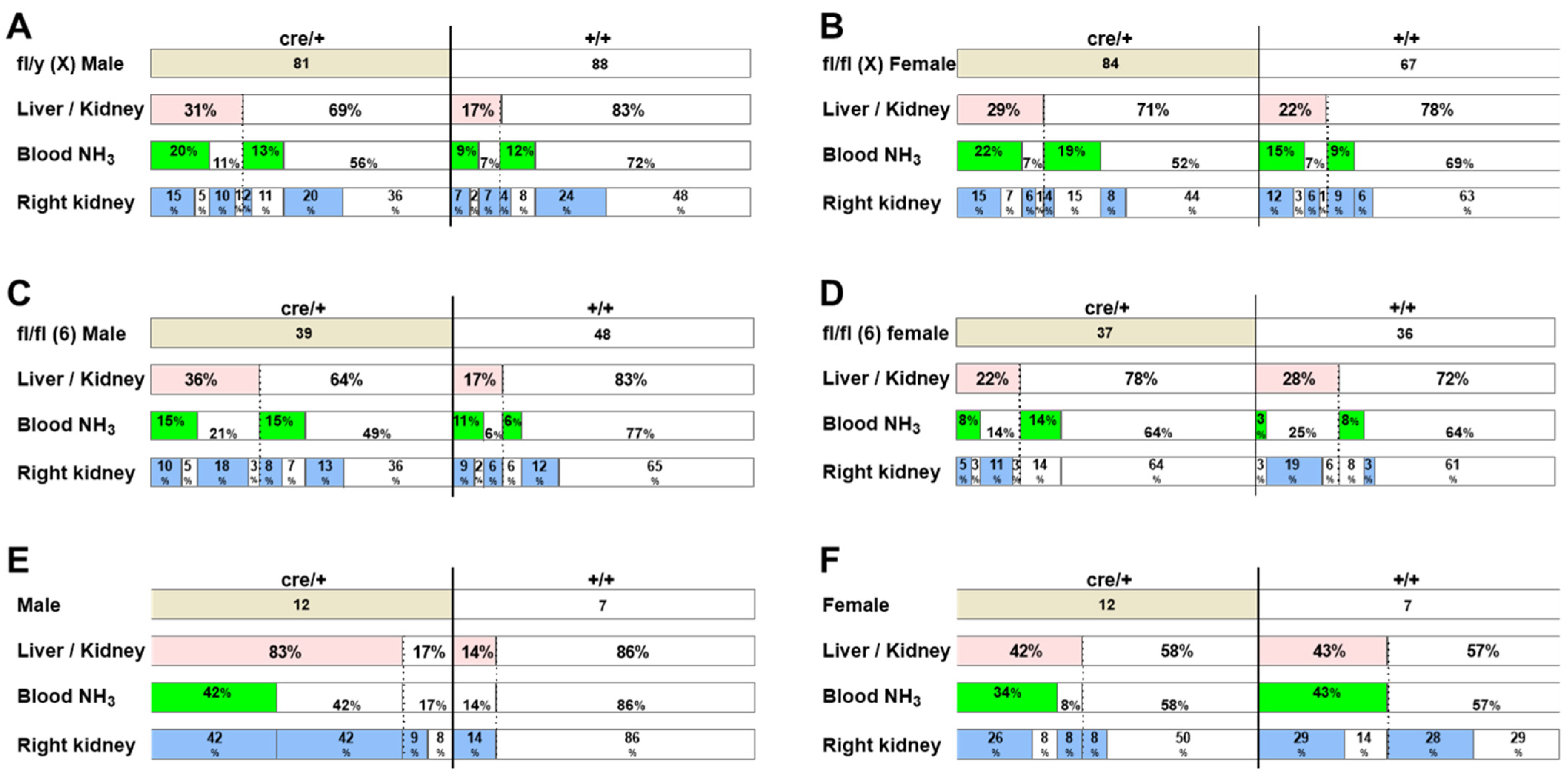
2.3. The Incidence of Hydronephrosis-like Pathology Is High in Genetically Engineered Mice
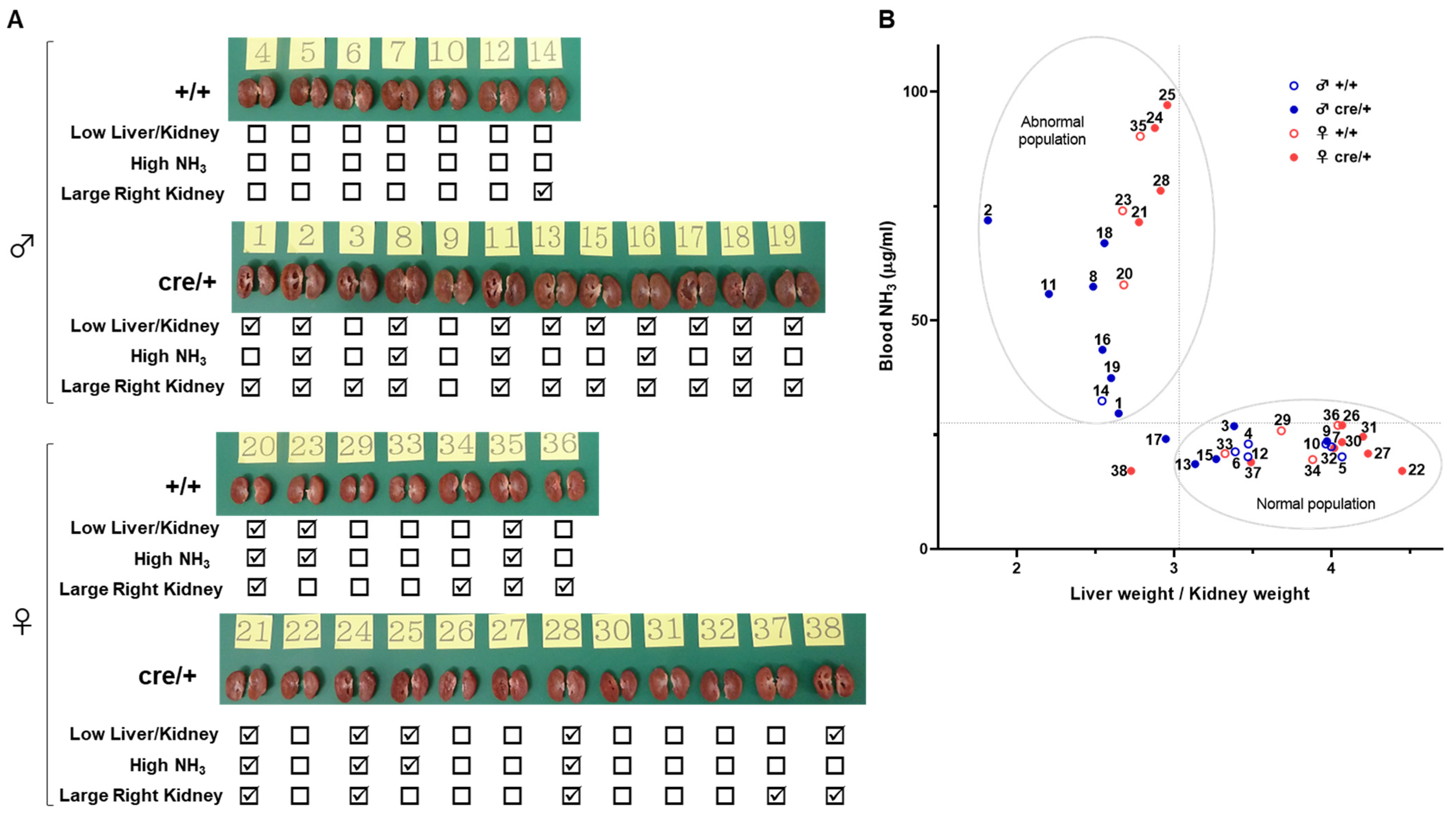
2.4. X Chromosome Mutations May Cause Hydronephrosis-like Pathology
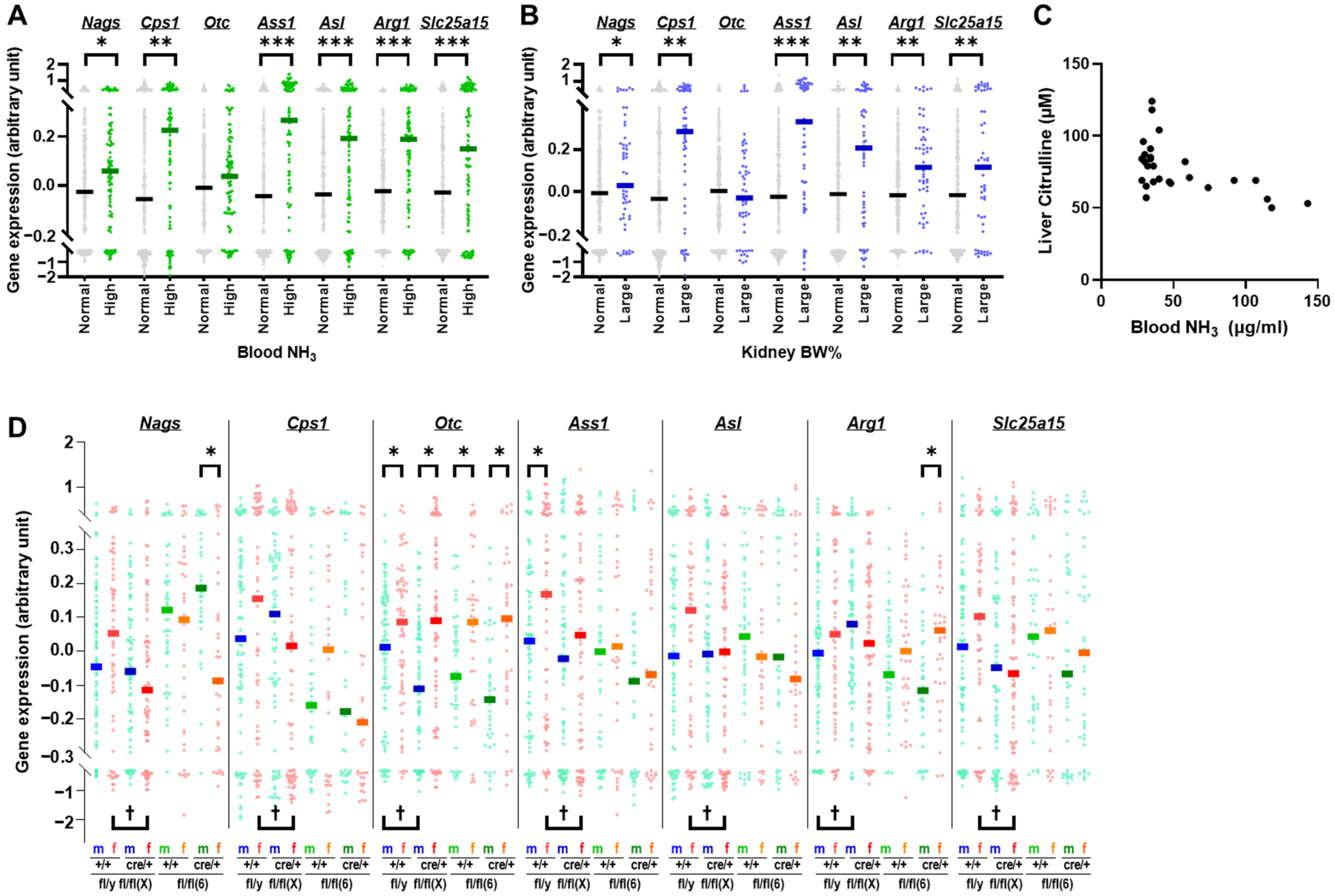
2.5. Otc and Urea Cycle Gene Expression Profiles in Mice with Hydronephrosis-like Pathology
2.6. Expression Levels of Genes Adjacent to Otc in Mice with Hydronephrosis-like Pathology
3. Discussion
4. Materials and Methods
4.1. Mice and Husbandary
4.2. Quantitation of Blood Ammonia
4.3. Quantitation of Citrulline
4.4. Histological Analysis
4.5. Tissue Preparation and RNA Isolation
4.6. Library Construction and Sequencing
4.7. Read Mapping and Analysis of Differentially Expressed Genes
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brayton, C.F.; Treuting, P.M.; Ward, J.M. Pathobiology of aging mice and GEM: Background strains and experimental design. Vet. Pathol. 2012, 49, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Pettan-Brewer, C.; Treuting, P.M. Practical pathology of aging mice. Pathobiol. Aging Age Relat. Dis. 2011, 1, 3402. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Youssef, S.A.; Treuting, P.M. Why animals die: An introduction to the pathology of aging. Vet. Pathol. 2016, 53, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Nakajima, Y.; Onodera, T.; Imamura, K. Inheritance of hydronephrosis in the inbred mouse strain DDD. Lab. Anim. 1984, 18, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Imamura, K.; Onodera, T.; Motoi, Y.; Goto, N. Hydronephrosis in the inbred mouse strain DDD. Lab. Anim. 1983, 17, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Ogura, A.; Suzuki, O.; Noguchi, Y.; Yamamoto, Y.; Kurosawa, S.; Asano, T. Hereditary hydronephrosis in C57L/MsNrs mice. Jikken. Dobutsu 1993, 42, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Fraser, H. Hydronephrosis in inbred strains of mice with particular reference to the BRVR strain. Lab. Anim. 1973, 7, 229–236. [Google Scholar] [CrossRef]
- Warner, N.L. Spontaneous hydronephrosis in the inbred mouse strain NZC. Aust. J. Exp. Biol. Med. Sci. 1971, 49, 477–486. [Google Scholar] [CrossRef]
- Wright, J.R.; Lacy, P.E. Spontaneous hydronephrosis in C57BL/KsJ mice. J. Comp. Pathol. 1988, 99, 449–454. [Google Scholar] [CrossRef]
- Hydronephrosis. Available online: https://www.kidney.org/atoz/content/hydronephrosis (accessed on 6 January 2024).
- Alshoabi, S.A.; Alhamodi, D.S.; Alhammadi, M.A.; Alshamrani, A.F. Etiology of hydronephrosis in adults and children: Ultrasonographic assessment in 233 patients. Pak. J. Med. Sci. 2021, 37, 1326–1330. [Google Scholar] [CrossRef]
- Thotakura, R.; Anjum, F. Hydronephrosis and hydroureter. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Al-Salem, A.H. Hydronephrosis in infants and children. In Atlas of Pediatric Surgery: Principles and Treatment; Springer International Publishing: Cham, Switzerland, 2020; pp. 829–840, ISBN 978-3-030-29211-9. [Google Scholar]
- Nguyen, H.T.; Benson, C.B.; Bromley, B.; Campbell, J.B.; Chow, J.; Coleman, B.; Cooper, C.; Crino, J.; Darge, K.; Herndon, C.D.; et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J. Pediatr. Urol. 2014, 10, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.Y.; Lee, R.S. Ureteropelvic junction obstruction/hydronephrosis. Urol. Clin. N. Am. 2023, 50, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Yousefichaijan, P.; Safi, F.; Saleh Jafari, A.; Movahedkia, R.; Rezagholizamnjany, M.; Rafiei, M.; Arjmand, A. Etiology of hydronephrosis in neonates. Nephro.-Urol. Mon. 2018, 10, e65307. [Google Scholar] [CrossRef]
- McDill, B.W.; Li, S.Z.; Kovach, P.A.; Ding, L.; Chen, F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl. Acad. Sci. USA 2006, 103, 6952–6957. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Otaka, Y.; Wang, J.; Hiai, H.; Takai, T.; Ravetch, J.V.; Honjo, T. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b-/-Pdcd1-/- mice. J. Exp. Med. 2005, 202, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Valerius, M.T.; McMahon, A.P. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 2007, 134, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Carroll, T.J.; Rajagopal, J.; Kobayashi, A.; Ren, Q.; McMahon, A.P. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 2009, 136, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J.; Moon, J.A.; Sung, Y.H.; Baek, I.J.; Roh, J.I.; Ha, N.Y.; Kim, S.Y.; Bahk, Y.Y.; Lee, J.E.; et al. Transgenic overexpression of p23 induces spontaneous hydronephrosis in mice. Int. J. Exp. Pathol. 2011, 92, 251–259. [Google Scholar] [CrossRef]
- Maezawa, Y.; Binnie, M.; Li, C.; Thorner, P.; Hui, C.C.; Alman, B.; Taketo, M.M.; Quaggin, S.E. A new Cre driver mouse line, Tcf21/Pod1-Cre, targets metanephric mesenchyme. PLoS ONE 2012, 7, e40547. [Google Scholar] [CrossRef]
- Davisson, M.T.; Cook, S.A.; Akeson, E.C.; Liu, D.; Heffner, C.; Gudis, P.; Fairfield, H.; Murray, S.A. Kidney adysplasia and variable hydronephrosis, a new mutation affecting the odd-skipped related 1 gene in the mouse, causes variable defects in kidney development and hydronephrosis. Am. J. Physiol. Renal. Physiol. 2015, 308, F1335–F1342. [Google Scholar] [CrossRef]
- Medrano, S.; Sequeira-Lopez, M.L.; Gomez, R.A. Deletion of the miR-143/145 cluster leads to hydronephrosis in mice. Am. J. Pathol. 2014, 184, 3226–3238. [Google Scholar] [CrossRef]
- Ichii, O.; Chihara, M.; Lee, S.H.; Nakamura, T.; Otsuka-Kanazawa, S.; Horino, T.; Elewa, Y.H.; Kon, Y. Hydronephrosis with ureteritis developed in C57BL/6N mice carrying the congenic region derived from MRL/MpJ-type chromosome 11. Autoimmunity 2017, 50, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Baker, N.E. E Proteins and ID proteins: Helix-loop-helix partners in development and disease. Dev. Cell 2015, 35, 269–280. [Google Scholar] [CrossRef]
- Abbott, B.D.; Birnbaum, L.S.; Pratt, R.M. TCDD-induced hyperplasia of the ureteral epithelium produces hydronephrosis in murine fetuses. Teratology 1987, 35, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, W.; Tohyama, C. Mechanisms of developmental toxicity of dioxins and related compounds. Int. J. Mol. Sci. 2019, 20, 617. [Google Scholar] [CrossRef]
- Hesketh, E.E.; Vernon, M.A.; Ding, P.; Clay, S.; Borthwick, G.; Conway, B.; Hughes, J. A murine model of irreversible and reversible unilateral ureteric obstruction. J. Vis. Exp. 2014, 20, e52559. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Luo, J.; Zhang, Q.; Xia, Y.; Shao, Q.; Sun, C.; Jiang, C.; Zhang, M.; Zhu, W. A modified relief of unilateral ureteral obstruction model. Ren. Fail. 2019, 41, 497–506. [Google Scholar] [CrossRef]
- Nemoto, S.; Kubota, T.; Ishikura, T.; Nakayama, M.; Kobayashi, A.; Yazaki, J.; Uchida, K.; Matsuda, M.; Kondo, T.; Ohara, O.; et al. Characterization of metabolic phenotypes and distinctive genes in mice with low-weight gain. FASEB J. 2024, 38, e23339. [Google Scholar] [CrossRef]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol 2017, 5, 34. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.; Li, Y.; Lin, X.; Xia, S.; Wanggou, S.; Li, X. Functional characterization of TSPAN7 as a novel indicator for immunotherapy in glioma. Front. Immunol. 2023, 14, 1105489. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 6 January 2024).
- McPheron, M.; Lah, M. Survival of a male infant with a familial Xp11.4 deletion causing ornithine transcarbamylase deficiency. JIMD Rep. 2019, 45, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Tsuda, J.; Mouri, Y.; Arai, J.; Arinami, T.; Noguchi, E. Contiguous Xp11.4 gene deletion leading to ornithine transcarbamylase deficiency detected by high-density single-nucleotide array. Clin. Pediatr. Endocrinol. 2010, 19, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jain-Ghai, S.; Hartley, J.; Fox, S.; Buhas, D.; Rockman-Greenberg, C.; Chan, A. Contiguous gene deletion of chromosome Xp in three families encompassing OTC, RPGR and TSPAN7 Genes. J. Rare Dis. Diagn. Ther. 2015, 1, 3. [Google Scholar]
- Gallant, N.M.; Gui, D.; Lassman, C.R.; Yong, W.H.; Teitell, M.; Mandelker, D.; Lorey, F.; Martinez-Agosto, J.A.; Quintero-Rivera, F. Novel liver findings in ornithine transcarbamylase deficiency due to Xp11.4-p21.1 microdeletion. Gene 2015, 556, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.; Patel, T.P.; Altman, E.K.; Tugarinov, N.; Trivellin, G.; Yanovski, J.A. Characterization of the adiponectin promoter + Cre recombinase insertion in the Tg(Adipoq-cre)1Evdr mouse by targeted locus amplification and droplet digital PCR. Adipocyte 2021, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, C.M.; Shril, S.; Hildebrandt, F. The genetics and pathogenesis of CAKUT. Nat. Rev. Nephrol. 2023, 19, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Capone, V.P.; Morello, W.; Taroni, F.; Montini, G. Genetics of Congenital Anomalies of the Kidney and Urinary Tract: The Current State of Play. Int. J. Mol. Sci. 2017, 18, 796. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Nakajima, Y.; Imamura, K.; Yoshida, T. Influence of testosterone on hydronephrosis in the inbred mouse strain DDD. Lab. Anim. 1985, 19, 85–88. [Google Scholar] [CrossRef]
- Metcalfe, P.D.; Leslie, J.A.; Campbell, M.T.; Meldrum, D.R.; Hile, K.L.; Meldrum, K.K. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E435–E443. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Rudduck, C.; Bennetts, B.; Peters, G.; Wilcken, B.; Ellaway, C. Contiguous gene deletion syndrome in a female with ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2010, 99, 34–41. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Gaddipati, H.; Kaplan, P.; Sanchez-Lara, P.A.; Sondheimer, N.; Spinner, N.B.; Hakonarson, H.; Ficicioglu, C.; Ganesh, J.; Markello, T.; et al. Complex management of a patient with a contiguous Xp11.4 gene deletion involving ornithine transcarbamylase: A role for detailed molecular analysis in complex presentations of classical diseases. Mol. Genet. Metab. 2008, 94, 498–502. [Google Scholar] [CrossRef]
- Bi, S.; Dai, L.; Jiang, L.; Wang, L.; Teng, M.; Liu, G.; Teng, R.J. Chronic granulomatous disease associated with Duchenne muscular dystrophy caused by Xp21.1 contiguous gene deletion syndrome: Case report and literature review. Front. Genet. 2022, 13, 970204. [Google Scholar] [CrossRef]
- Gassner, C.; Brönnimann, C.; Merki, Y.; Mattle-Greminger, M.P.; Sigurdardottir, S.; Meyer, E.; Engström, C.; O’Sullivan, J.D.; Jung, H.H.; Frey, B.M. Stepwise partitioning of Xp21: A profiling method for XK deletions causative of the McLeod syndrome. Transfusion 2017, 57, 2125–2135. [Google Scholar] [CrossRef]
- Bassani, S.; Cingolani, L.A.; Valnegri, P.; Folci, A.; Zapata, J.; Gianfelice, A.; Sala, C.; Goda, Y.; Passafaro, M. The X-linked intellectual disability protein TSPAN7 regulates excitatory synapse development and AMPAR trafficking. Neuron 2012, 73, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Murru, L.; Ponzoni, L.; Longatti, A.; Mazzoleni, S.; Giansante, G.; Bassani, S.; Sala, M.; Passafaro, M. Lateral habenula dysfunctions in Tm4sf2(−/y) mice model for neurodevelopmental disorder. Neurobiol. Dis. 2021, 148, 105189. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Louie, R.J.; Toutain, A.; Skinner, C.; Friez, M.J.; Stevenson, R.E. X-Linked intellectual disability update 2022. Am. J. Med. Genet. A 2023, 191, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Leitão, E.; Schröder, C.; Parenti, I.; Dalle, C.; Rastetter, A.; Kühnel, T.; Kuechler, A.; Kaya, S.; Gérard, B.; Schaefer, E.; et al. Systematic analysis and prediction of genes associated with monogenic disorders on human chromosome X. Nat. Commun. 2022, 13, 6570. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Nagali, S. Hyperammonemia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]


| Kidney | Large | Normal | ||||||
| BW% | ≥1.5907 | <1.5907 | ||||||
| N | 59 | 421 | ||||||
| %/All | 12.3 | 87.7 | ||||||
| Liver | Small | Normal | Small | Normal | ||||
| BW% | ≤4.902 | >4.902 | ≤4.902 | >4.902 | ||||
| N | 31 | 28 | 89 | 332 | ||||
| %/All | 6.5% | 5.8% | 18.5% | 69.2% | ||||
| %/Kidney | 52.5% | 47.5% | 21.1% | 78.9% | ||||
| NH3 | High | Normal | High | Normal | High | Normal | High | Normal |
| μg/mL | ≥41.7 | <41.7 | ≥41.7 | <41.7 | ≥41.7 | <41.7 | ≥41.7 | <41.7 |
| N | 29 | 2 | 2 | 25 | 42 | 47 | 54 | 275 |
| %/All | 6.0% | 0.4% | 0.4% | 5.2% | 8.8% | 9.8% | 11.3% | 57.3% |
| %/Kidney | 49.2% | 3.4% | 3.4% | 42.4% | 10.0% | 11.2% | 12.8% | 65.3% |
| %/Liver | 93.5% | 6.5% | 7.1% | 89.3% | 47.2% | 52.8% | 16.3% | 82.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, S.; Uchida, K.; Ohno, H. Implications of Genetic Factors Underlying Mouse Hydronephrosis: Cautionary Considerations on Phenotypic Interpretation in Genetically Engineered Mice. Int. J. Mol. Sci. 2024, 25, 7203. https://doi.org/10.3390/ijms25137203
Nemoto S, Uchida K, Ohno H. Implications of Genetic Factors Underlying Mouse Hydronephrosis: Cautionary Considerations on Phenotypic Interpretation in Genetically Engineered Mice. International Journal of Molecular Sciences. 2024; 25(13):7203. https://doi.org/10.3390/ijms25137203
Chicago/Turabian StyleNemoto, Shino, Kazuyo Uchida, and Hiroshi Ohno. 2024. "Implications of Genetic Factors Underlying Mouse Hydronephrosis: Cautionary Considerations on Phenotypic Interpretation in Genetically Engineered Mice" International Journal of Molecular Sciences 25, no. 13: 7203. https://doi.org/10.3390/ijms25137203






