Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy
Abstract
:1. Introduction
2. Results and Discussion
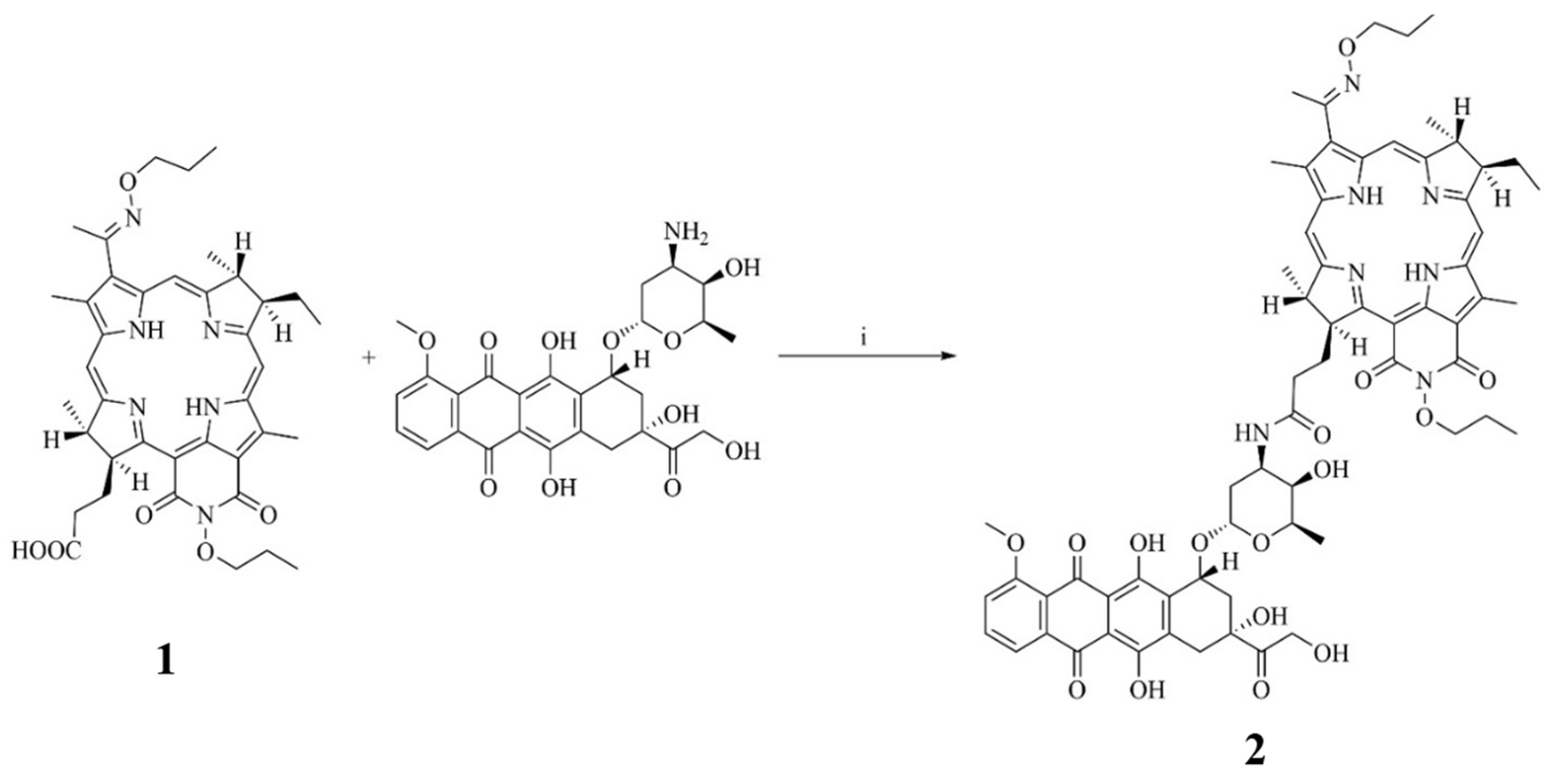
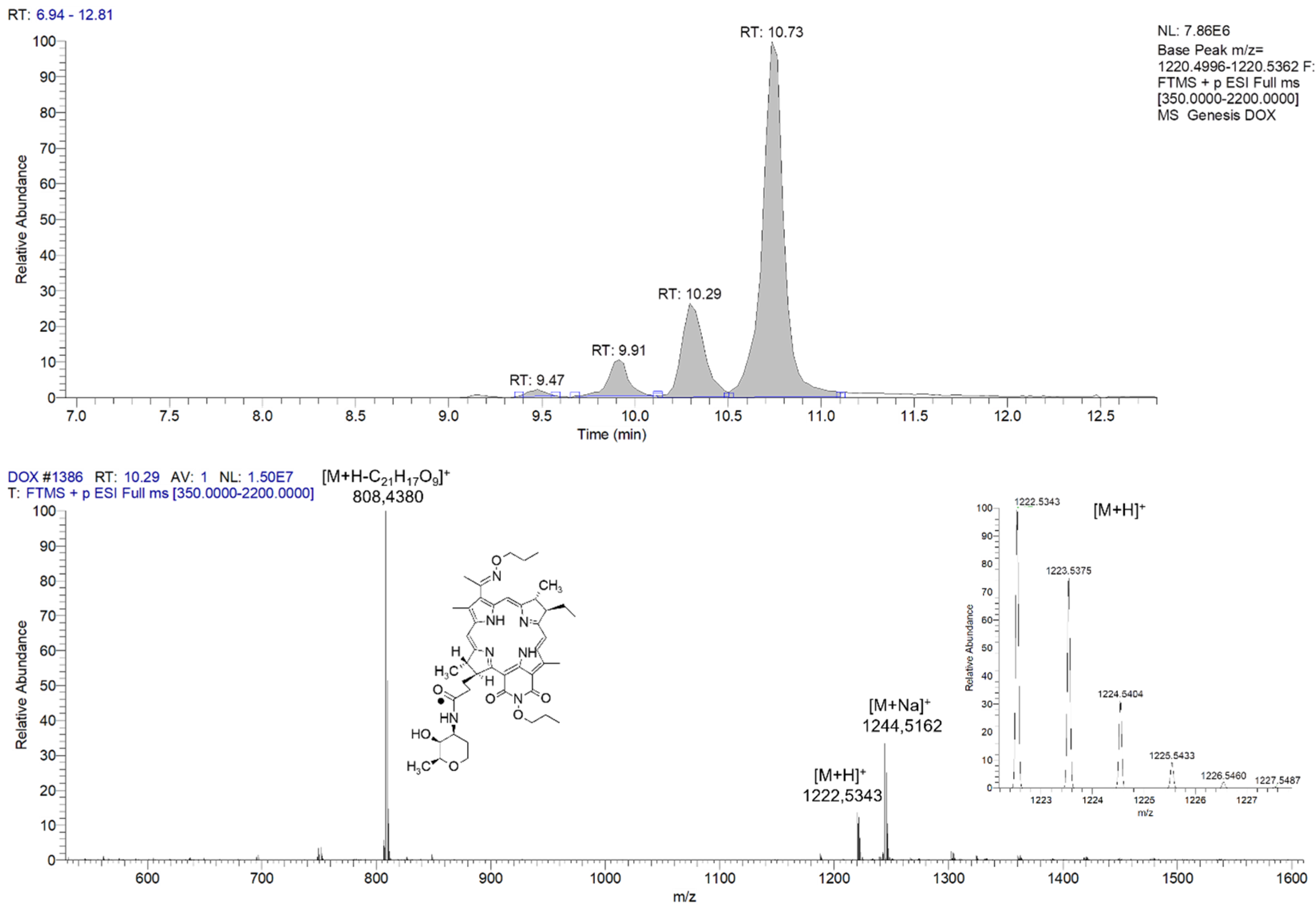
2.1. Synthesis and Physicochemical Properties of Compound 2 (PS+DOX)
2.2. In Silico Study of the Interaction of PS+DOX with Biological Targets
2.3. Determination of the Quantum Yield of Singlet Oxygen Generation by PS+DOX in Solution
2.4. Cellular Imaging of PS+DOX In Vitro
2.5. Photo- and Cytotoxicity In Vitro
2.6. Study of the Dynamics of PS, PS+DOX, and DOX Accumulation in a Tumor and in Healthy Tissue
2.7. Specific Activity In Vivo
3. Materials and Methods
3.1. Synthesis of The Bacteriochlorin Conjugate with Doxorubicin
3.2. Detection of The Quantum Yield of Singlet Oxygen Generation
3.3. Chromatography–Mass Spectrometry
3.4. In Silico Studies of The Resulting PS+DOX
3.5. In Vitro Study
3.5.1. Characteristics of in Vitro Test Systems
- MCF-7–human breast adenocarcinoma (collection of the Institute of Cytology of the Russian Academy of Sciences);
- 4T1–mouse mammary gland carcinoma (ATCC collection).
3.5.2. Cellular Imaging of the PS+DOX Studied In Vitro
3.5.3. Methodology for Studying Photoinduced and Cytotoxic Activity in an In Vitro System
3.6. In Vivo Study
3.6.1. Animals
3.6.2. Tumor Model
3.6.3. Biodistribution
3.6.4. Study of PDT Efficiency
3.6.5. Study of Chemotherapy Efficiency
3.6.6. Estimation of Antitumor Efficacy
3.6.7. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P. Combinations of Photodynamic Therapy with Other Minimally Invasive Therapeutic Technologies against Cancer and Microbial Infections. Int. J. Mol. Sci. 2023, 24, 10875. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The Physics, Biophysics and Technology of Photodynamic Therapy. Phys. Med. Biol. 2008, 53, 61–109. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazanova, A.; Filonenko, E. Advantages of combined photodynamic therapy in the treatment of oncological diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Pantiushenko, I.; Rudakovskaya, P.; Starovoytova, A.; Mikhaylovskaya, A.; Abakumov, M.; Kaplan, M.; Tsygankov, A.; Majouga, A.; Grin, M.; Mironov, A. Development of bacteriochlorophyll a-based near-infrared photosensitizers conjugated to gold nanoparticles for photodynamic therapy of cancer. Biochemistry 2015, 80, 752–762. [Google Scholar] [CrossRef]
- Alam, A. Chemotherapy Treatment and Strategy Schemes: A Review. Open Access J. Toxicol. 2018, 2, 555–600. [Google Scholar] [CrossRef]
- Yang, S.S.; Guo, J.G.; Liu, J.N.; Liu, Z.Q.; Chen, E.N.; Chen, C.Y.; OuYang, P.Y.; Han, F.; Xie, F.Y. Effect of Induction Chemotherapy in Nasopharyngeal Carcinoma: An Updated Meta-Analysis. Front. Oncol. 2021, 10, 591205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, L.; Wang, S.; Pang, Q.; Wang, J. Addition of Induction or Consolidation Chemotherapy in Definitive Concurrent Chemoradiotherapy Versus Concurrent Chemoradiotherapy Alone for Patients With Unresectable Esophageal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 665231. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, X.; Sun, C.; Wu, M.; Xu, Z.; Zhou, S.; Yao, N.; Liu, S.; Qin, X.; Han, Z. Concurrent Chemoradiotherapy Followed by Adjuvant Chemotherapy versus Concurrent Chemoradiotherapy Alone in Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 997030. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Eugène, T.; Couec, M.L.; Strullu, M.; Frampas, E.; Campion, L.; Kraeber-Bodéré, F.; Bodet-Milin, C. Prognostic Value and Clinical Impact of 18 FDG-PET in the Management of Children with Burkitt Lymphoma after Induction Chemotherapy. Front. Med. 2014, 1, 54. [Google Scholar] [CrossRef]
- Bao, W.; Liu, R.; Xia, G.; Wang, F.; Chen, B. Applications of Daunorubicin-Loaded PLGA-PLL-PEG-Tf Nanoparticles in Hematologic Malignancies: An in Vitro and in Vivo Evaluation. Drug Des. Dev. Ther. 2019, 13, 1107–1115. [Google Scholar] [CrossRef]
- Hu, W.; Lv, K.; Teng, R.; Chen, J.; Xu, C.; Jin, L.; Chen, Y.; Zhao, W. Pegylated Liposomal Doxorubicin Versus Epirubicin as Adjuvant Therapy for Stage I–III Breast Cancer. Front. Genet. 2021, 12, 746114. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Yang, B.; Chen, J.; Wang, C.; Jin, L.; Zhao, W.; Gao, X.; Wang, H.; Li, J.; Zhao, H.; et al. Efficacy and Safety of Mitoxantrone Hydrochloride Injection for Tracing Axillary Sentinel Nodes in Breast Cancer: A Self-Controlled Clinical Trial. Front. Oncol. 2022, 12, 914057. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, J.; Sun, D.; Li, J.; Yao, L.; Meng, S.; Li, Y.; Dang, Y.; Wang, K. The Mechanism of Dynamic Interaction between Doxorubicin and Calf Thymus DNA at the Single-Molecule Level Based on Confocal Raman Spectroscopy. Micromachines 2022, 13, 940. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, Y.; Xie, C.; Zhang, X.; You, H. Detecting the Formation Kinetics of Doxorubicin-DNA Interstrand Cross-Link at the Single-Molecule Level and Clinically Relevant Concentrations of Doxorubicin. Anal. Chem. 2020, 92, 4504–4511. [Google Scholar] [CrossRef]
- Yang, E.Y.; Howard, G.R.; Brock, A.; Yankeelov, T.E.; Lorenzo, G. Mathematical Characterization of Population Dynamics in Breast Cancer Cells Treated with Doxorubicin. Front. Mol. Biosci. 2022, 9, 972146. [Google Scholar] [CrossRef]
- Yi, R.; Lv, W.; Zheng, S.; Zhang, N.; Zhang, Y.; Yang, K.; Huang, T.; Yang, Y.; Chu, H.; Chen, J. IFN-γ/Doxorubicin Complex Nanoparticles for Enhancing Therapy in the Context of Human Ovarian Carcinoma. Front. Mater. 2022, 9, 944930. [Google Scholar] [CrossRef]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-Loaded Platelets as a Smart Drug Delivery System: An Improved Therapy for Lymphoma. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Chen, Y.; Wu, Z.; Luo, L.; Yirga, S.K.; Zhang, N.; Ye, F.; Chen, H.; Hu, J.; Chen, Y. Doxorubicin/Nucleophosmin Binding Protein-Conjugated Nanoparticle Enhances Anti-Leukemia Activity in Acute Lymphoblastic Leukemia Cells in Vitro and in Vivo. Front. Pharmacol. 2021, 12, 607755. [Google Scholar] [CrossRef] [PubMed]
- Lykoshin, D.D.; Zaitsev, V.V.; Kostromina, M.A.; Esipov, R.S. New-Generation Osteoplastic Materials Based on Biological and Synthetic Matrices. Tonkie Khimicheskie Tekhnologii 2021, 16, 36–54. [Google Scholar] [CrossRef]
- Lomovskaya, N.; Otten, S.; Doi-Katayama, Y.; Fonstein, L.; Liu, X.; Takatsu, T.; Inventi-Solari, A.; Filippini, S.; Torti, F.; Colombo, A.; et al. Doxorubicin overproduction in Streptomyces peucetius: Cloning and characterization of the dnrU ketoreductase and dnrV genes and the doxA cytochrome P-450 hydroxylase gene. J. Bacteriol. 1999, 181, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, G.; Wang, S.; Huang, J. Bibliometric and Visual Analysis of Doxorubicin-Induced Cardiotoxicity. Front. Pharmacol. 2023, 14, 1255158. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, J.; Wei, Q. Combination of Photodynamic Therapy with Radiotherapy for Cancer Treatment. J. Nanomater. 2016, 2016, 8507924. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 00081. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic Therapy: A Clinical Reality in the Treatment of Cancer. Lancet Oncol. 2000, 1, 00166. [Google Scholar] [CrossRef]
- Choi, B.H.; Ryoo, I.G.; Kang, H.C.; Kwak, M.K. The Sensitivity of Cancer Cells to Pheophorbide A-Based Photodynamic Therapy Is Enhanced by NRF2 Silencing. PLoS ONE 2014, 9, 0107158. [Google Scholar] [CrossRef]
- Xing, Y.; Ding, T.; Wang, Z.; Wang, L.; Guan, H.; Tang, J.; Mo, D.; Zhang, J. Temporally Controlled Photothermal/Photodynamic and Combined Therapy for Overcoming Multidrug Resistance of Cancer by Polydopamine Nanoclustered Micelles. ACS Appl. Mater. Interfaces 2019, 11, 13945–13953. [Google Scholar] [CrossRef]
- Abramova, O.; Kozlovtseva, E.; Drozhzhina, V.; Ostroverkhov, P.; Sivovolova, T.; Arkhipova, L.; Grin, M.; Ivanov, S.; Kaprin, A. Anti-Tumor Efficacy of Photodynamic Therapy of Solid Tumors in Laboratory Animals with Guanidine and Biguanidine Derivatives of Chlorine e6. Bull. Exp. Biol. Med. 2023, 174, 468–472, 05731. [Google Scholar] [CrossRef]
- Tikhonov, S.; Morozova, N.; Plutinskaya, A.; Plotnikova, E.; Pankratov, A.; Abramova, O.; Diachkova, E.; Vasil’ev, Y.; Grin, M. N-Heterocyclic Carbenes and Their Metal Complexes Based on Histidine and Histamine Derivatives of Bacteriopurpurinimide for the Combined Chemo- and Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2022, 23, 15776. [Google Scholar] [CrossRef]
- Koifman, O.I.; Ageeva, T.A.; Kuzmina, N.S.; Otvagin, V.F.; Nyuchev, A.V.; Fedorov, A.Y.; Belykh, D.V.; Lebedeva, N.S.; Yurina, E.S.; Syrbu, S.A.; et al. Synthesis Strategy of Tetrapyrrolic Photosensitizers for Their Practical Application in Photodynamic Therapy. Macroheterocycles 2022, 15, 207–304. [Google Scholar] [CrossRef]
- Abramova, O.; Kaplan, M.; Yuzhakov, V.; Drozhzhina, V.; Churikova, T.; Kozlovtseva, E.; Bandurko, L.; Yakovleva, N.; Sevankaeva, L.; Tsyganova, M.; et al. Efficiency of photosensitizer chlorin e6 dimethyl ether for photodynamic therapy of rat sarcoma M-1. Radiat. Risk 2022, 31, 151–161. [Google Scholar] [CrossRef]
- Mironov, A.F.; Ostroverkhov, P.V.; Tikhonov, S.I.; Pogorilyy, V.A.; Kirin, N.S.; Chudakova, O.O.; Tsygankov, A.; Grin, M.A. Amino Acid Derivatives of Natural Chlorins as a Platform for the Creation of Targeted Photosensitizers in Oncology. Tonkie Khimicheskie Tekhnologii 2020, 15, 16–33. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461, 21334. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Kang, J.S.; Kim, M.S.; Lee, K.S.; Lee, M.S. The Study of Doxorubicin and its Complex with DNA by SERS and UV-resonance Raman Spectroscopy. Bull. Korean Chem. Soc. 2004, 25, 1211–1216. [Google Scholar]
- Khalil, O.M.; Gedawy, E.M.; El-Malah, A.A.; Adly, M.E. Novel Nalidixic Acid Derivatives Targeting Topoisomerase II Enzyme; Design, Synthesis, Anticancer Activity and Effect on Cell Cycle Profile. Bioorg. Chem. 2019, 83, 262–276. [Google Scholar] [CrossRef]
- Blum, A.; Grossweiner, L. Singlet oxygen generation by hematoporphyrin IX, uroporphyrin I and hematoporphyrin derivative at 546 nm in phosphate buffer and in the presence of egg phosphatidylcholine liposomes. Photochem. Photobiol. 1985, 41, 27–32. [Google Scholar] [CrossRef]
- Gandin, E.; Lion, Y.; Van de Vorst, A. Quantum yield of singlet oxygen production by xanthene derivatives. Photochem. Photobiol. 1983, 37, 271–278. [Google Scholar] [CrossRef]
- Feofanov, A.; Grichine, A.; Karmakova, T.; Plyutinskaya, A.; Lebedeva, V.; Filyasova, A.; Yakubovskaya, R.; Mironov, A.; Egret-Charlier, M.; Vigny, P. Near-infrared photosensitizer based on a cycloimide derivative of chlorin p6: 13,15-N-(3′-hydroxypropyl)cycloimide chlorin p6. Photochem. Photobiol. 2002, 75, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Abramova, O.B.; Drozhzhina, V.V.; Beregovskaya, E.A.; Churikova, T.P.; Kaplan, M.A. Accumulation Dynamics of a Photosensitizer Liposomal Borated Chlorine e6 in Various Morphological Types of Experimental Tumors. Biol. Bull. Russ. Acad. Sci. 2022, 49, 2134–2142. [Google Scholar] [CrossRef]
- Kustov, A.V.; Berezin, D.B.; Zorin, V.P.; Morshnev, P.K.; Kukushkina, N.V.; Krestyaninov, M.A.; Kustova, T.V.; Strelnikov, A.I.; Lyalyakina, E.V.; Zorina, T.E.; et al. Monocationic Chlorin as a Promising Photosensitizer for Antitumor and Antimicrobial Photodynamic Therapy. Pharmaceutics 2023, 15, 61. [Google Scholar] [CrossRef]
- Mironov, A.N. Guidelines for Conducting Preclinical Studies of Drugs, 1st ed.; Grif and K: Moscow, Russia, 2012; pp. 657–671. [Google Scholar]
- Efremenko, A.; Dyakova, E.; Ostroverkhov, P.; Ignatova, A.; Grin, M.; Feofanov, A. Photodynamic properties of lysine and arginine derivatives of bacteriopurpurinimide. Future Med. Chem. 2022, 14, 1635–1647. [Google Scholar] [CrossRef]
- Mironov, A.F.; Grin, M.A. Bacteriochlorin series sensitizers: Prospects for use in photodynamic therapy. Fine Chem. Technol. 2006, 4, 5–28. [Google Scholar]

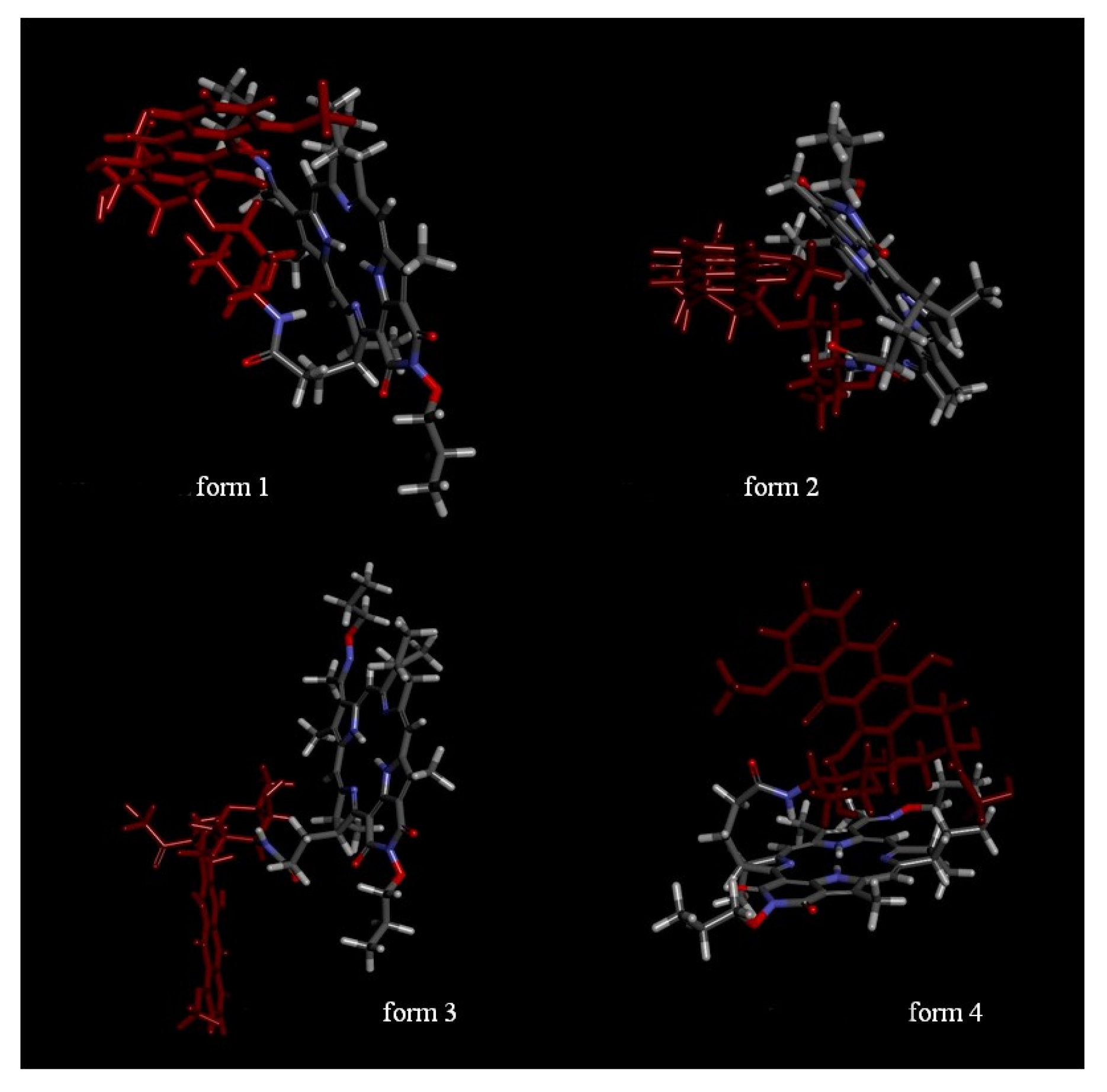
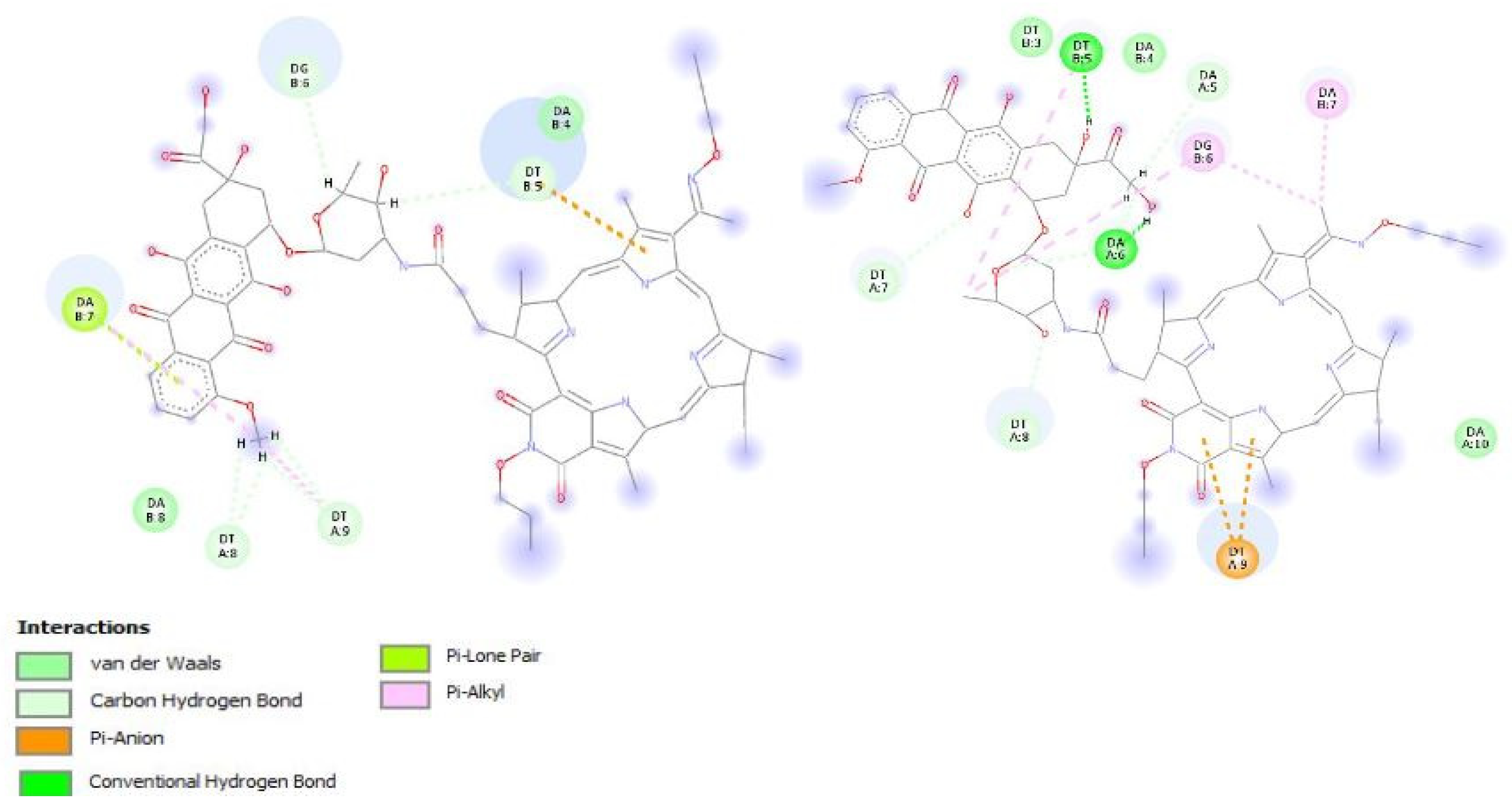
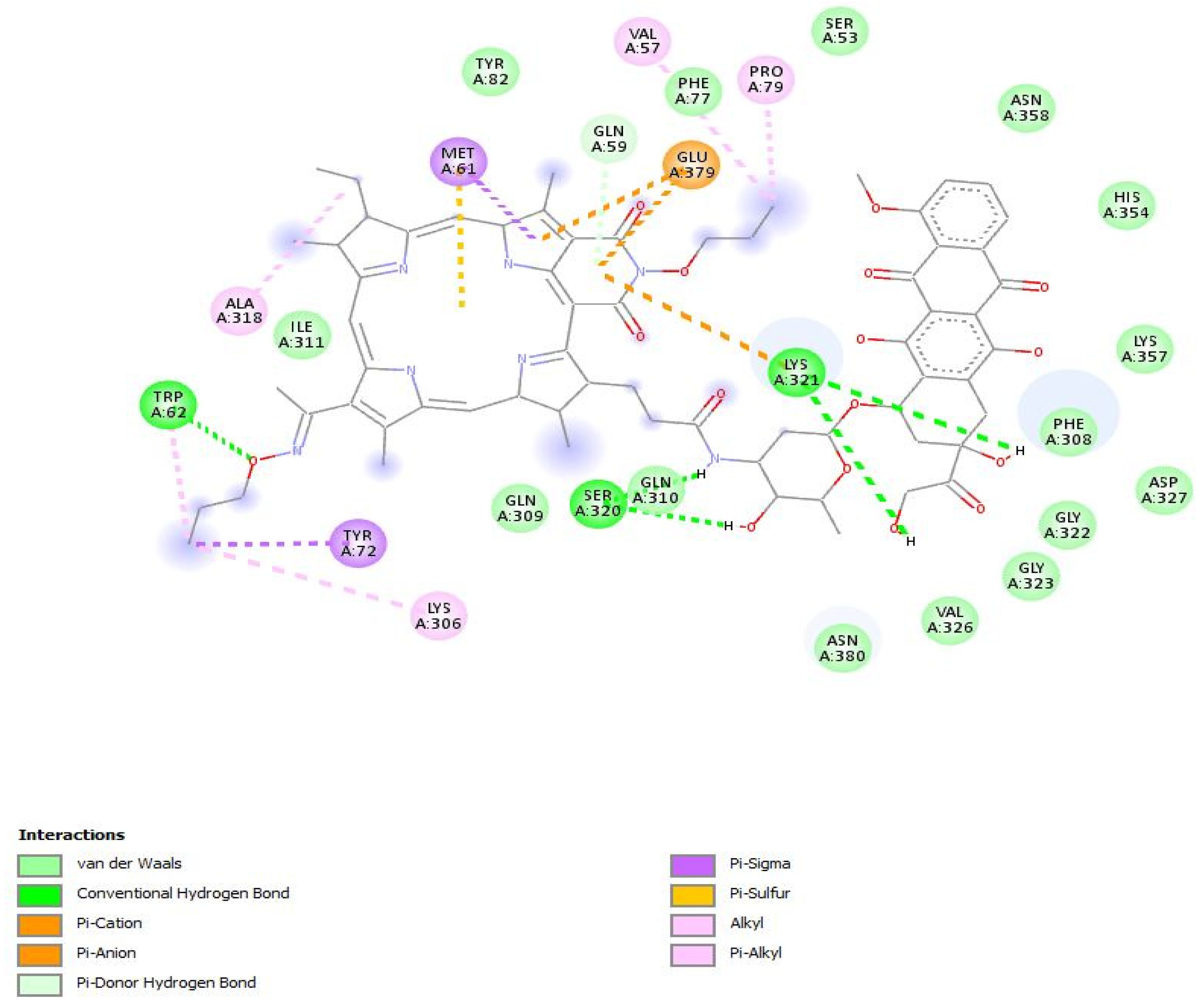
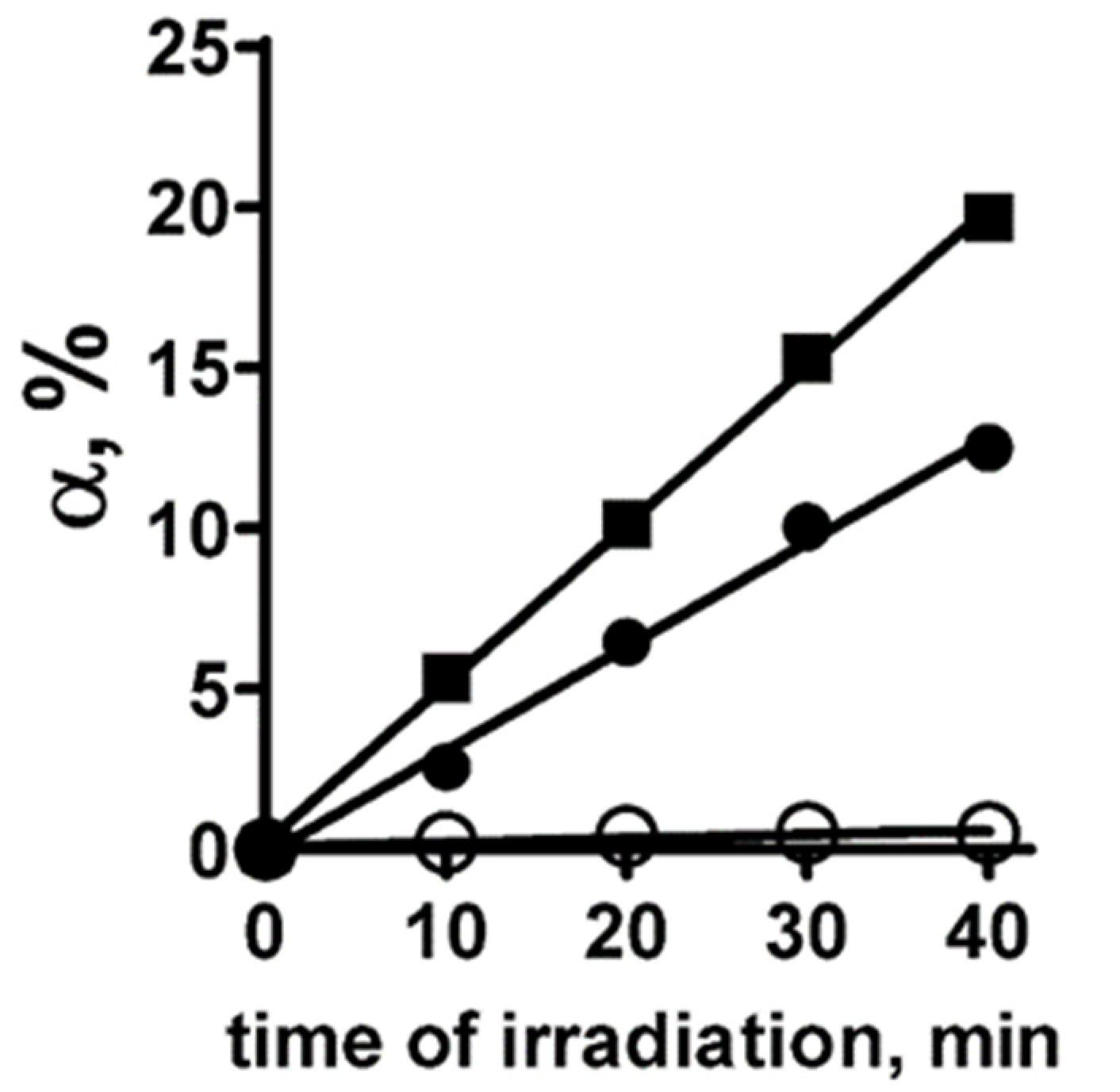


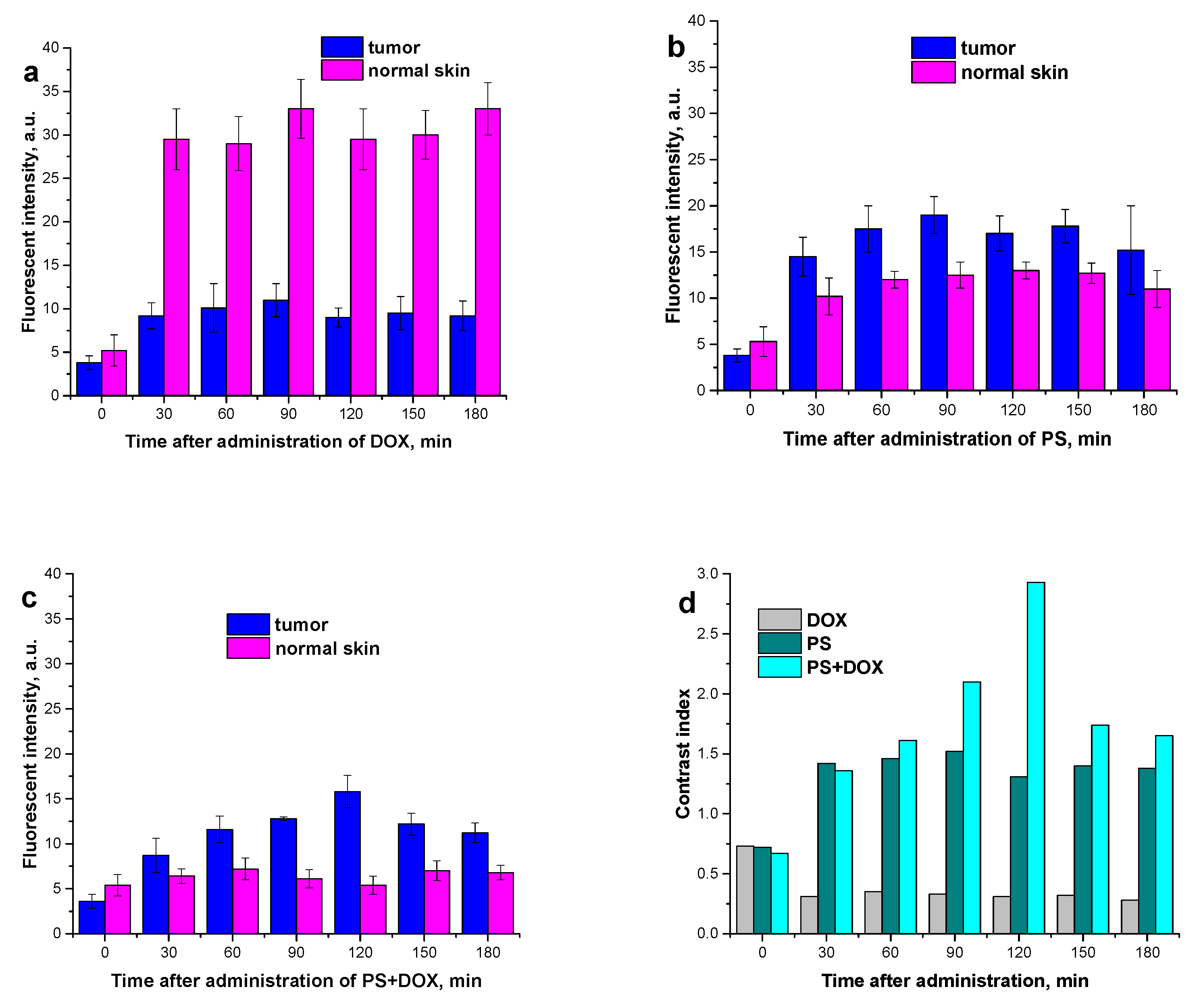


| Compound’s E, kJ/mol | Etotal | Eshape | H-Bonds |
|---|---|---|---|
| DOX | −303.00 | −319.80 | 6 |
| PS+DOX | −305.90 | −333.40 | 8 * |
| IC50, nM | |||
|---|---|---|---|
| Compound | Photoinduced Activity | Combined Activity | Cytotoxic Activity |
| 4 h PS Incubation with Cells → Irradiation at 10 J/cm2 → MTT after 24 h | 4 h PS Incubation with Cells → Irradiation at 10 J/cm2 → MTT after 72 h | 72 h Incubation of Compounds with Cells → MTT | |
| MCF-7—Human Breast Adenocarcinoma | |||
| PS | 707 ± 16 nM | 691 ± 14 nM | 10643 ± 68 nM |
| PS+DOX | 671 ± 14 nM | 442 ± 11 nM | 8672 ± 49 nM |
| DOX | – | – | 6237 ± 96 nM |
| 4T1—Mouse Breast Carcinoma | |||
| PS | 194 ± 10 nM | 188 ± 9 nM | 8056 ± 51 nM |
| PS+DOX | 188 ± 8 nM | 123 ± 7 nM | 295 ± 15 nM |
| DOX | – | – | 5525 ± 71 nM |
| Group No. | Agent Dose, mg/kg | MLE, Days | LEI, % | RN, % |
|---|---|---|---|---|
| Schedule I: monophotodynamic therapy with PS | ||||
| 1 | PS 2.76 | 50.2 ± 15.8 | 54.4 | 16.7 |
| Schedule II: monochemotherapy with DOX | ||||
| 2 | DOX 2.24 | 53.5 ± 22.6 | 64.6 | 33.2 |
| Schedule III: combined therapy with PS+DOX | ||||
| 3 | PS+DOX 5.00 (2.76 (PS) + 2.24 (DOX)) | 81.3 ± 17.0 | 150.3 | 83.3 |
| Schedule IV: PDT + chemotherapy with administration of PS and DOX | ||||
| 4 | 5.00 (2.76 (PS) and 2.24 (DOX)) | 65.2 ± 21.9 | 100.5 | 50.0 |
| 5 | Control | 32.5 ± 1.8 | – | 0 |
| Group No. | Agent | Dose, mg/kg | Administration Method | Irradiation Parameters | |
|---|---|---|---|---|---|
| E, J/cm2 | Ps, W/cm2 | ||||
| Treatment Schedule I: Monophotodynamic Therapy with PS, Latus Laser, λ = 810 nm | |||||
| 1 | PS | 2.76 | Intravenously | 150 | 0.48 |
| Treatment schedule II: monochemotherapy with DOX | |||||
| 2 | DOX | 2.24 | Intravenously | – | – |
| Treatment schedule III: combined therapy with PS+DOX, Latus laser, λ = 810 nm | |||||
| 3 | PS+DOX | 5.0 (2.76 (PS) + 2.24 (DOX)) | Intravenously | 150 | 0.48 |
| Treatment schedule IV: PDT + chemotherapy with administration of PS and DOX, Latus laser, λ = 810 nm | |||||
| 4 | PS and DOX | 5.0 (2.76 (PS) and 2.24 (DOX)) | Intravenously | 150 | 0.48 |
| 5 Control | 0.9% NaCl | 5.0 | Intravenously | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikova, E.; Abramova, O.; Ostroverkhov, P.; Vinokurova, A.; Medvedev, D.; Tihonov, S.; Usachev, M.; Shelyagina, A.; Efremenko, A.; Feofanov, A.; et al. Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7210. https://doi.org/10.3390/ijms25137210
Plotnikova E, Abramova O, Ostroverkhov P, Vinokurova A, Medvedev D, Tihonov S, Usachev M, Shelyagina A, Efremenko A, Feofanov A, et al. Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy. International Journal of Molecular Sciences. 2024; 25(13):7210. https://doi.org/10.3390/ijms25137210
Chicago/Turabian StylePlotnikova, Ekaterina, Olga Abramova, Petr Ostroverkhov, Aleksandra Vinokurova, Dmitry Medvedev, Sergei Tihonov, Maksim Usachev, Anastasia Shelyagina, Anastasija Efremenko, Alexey Feofanov, and et al. 2024. "Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy" International Journal of Molecular Sciences 25, no. 13: 7210. https://doi.org/10.3390/ijms25137210
APA StylePlotnikova, E., Abramova, O., Ostroverkhov, P., Vinokurova, A., Medvedev, D., Tihonov, S., Usachev, M., Shelyagina, A., Efremenko, A., Feofanov, A., Pankratov, A., Shegay, P., Grin, M., & Kaprin, A. (2024). Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy. International Journal of Molecular Sciences, 25(13), 7210. https://doi.org/10.3390/ijms25137210








