Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival
Abstract
1. Introduction
2. Results
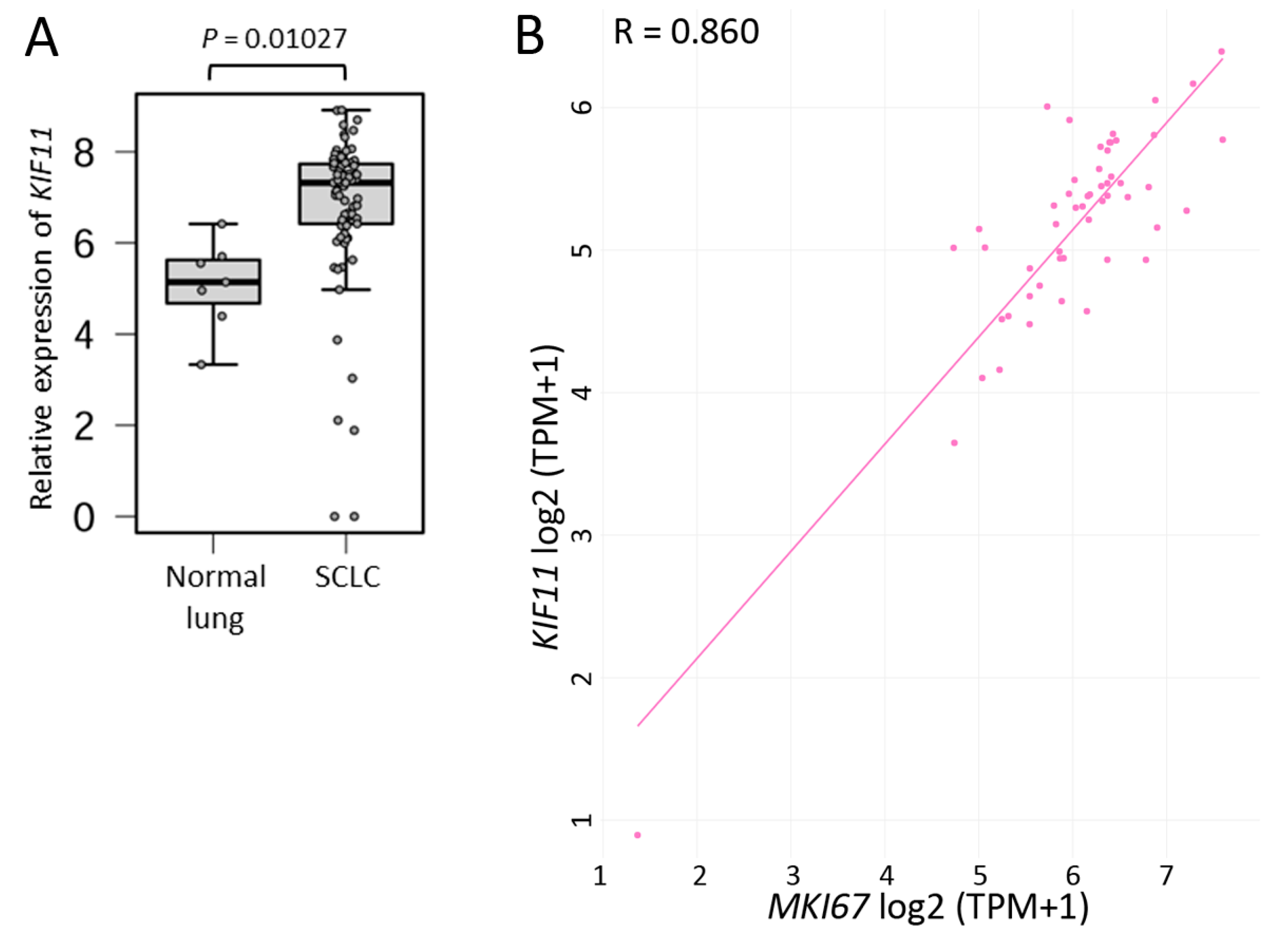
2.1. SCLC Tissues Highly Express KIF11 mRNA
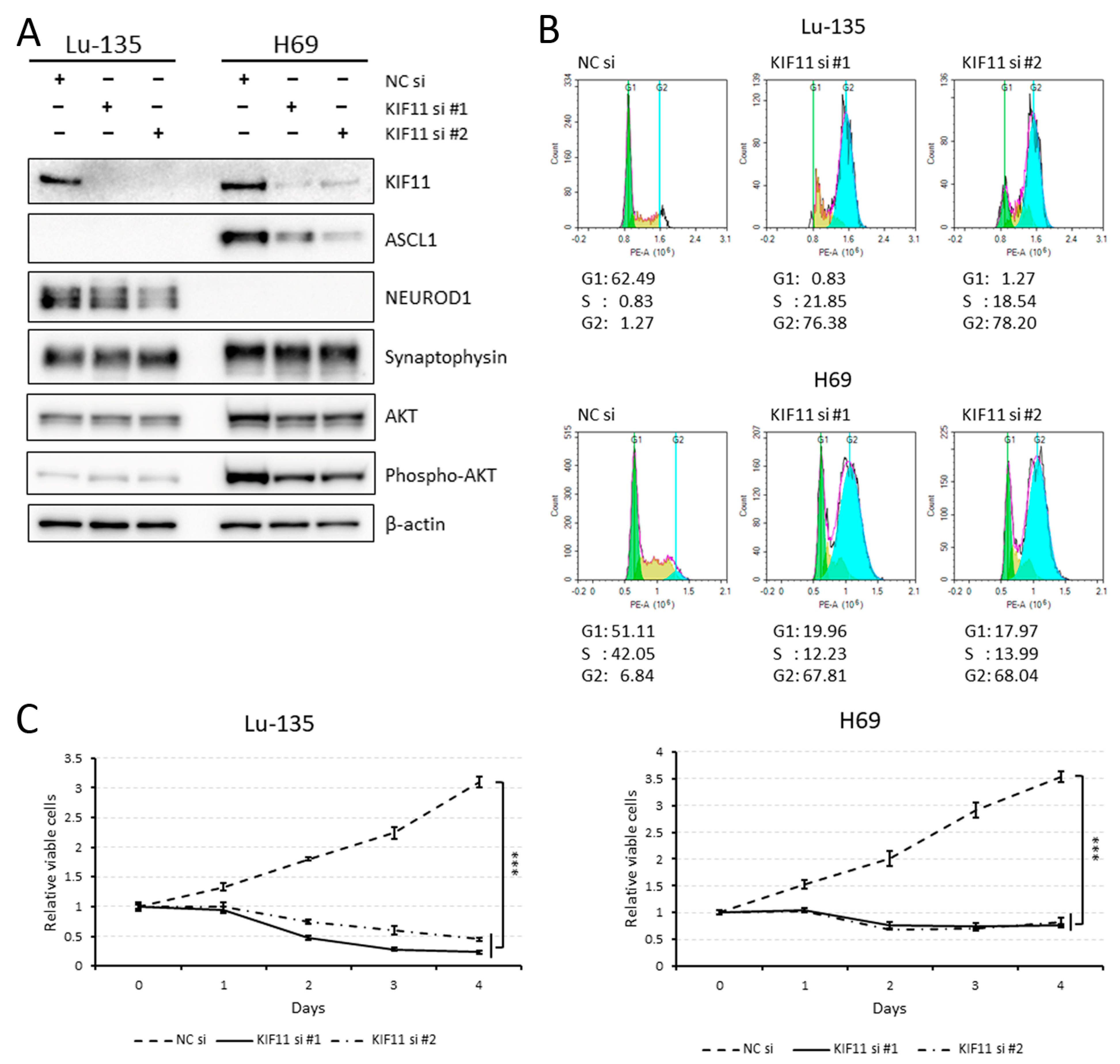
2.2. KIF11 Inhibition Causes Cell Cycle Arrest at the G2/M Phase in Lu-135 and H69 Cells
2.3. Another KIF11 Inhibitor, Ispinesib, Is More Effective against SCLC Cell Lines Expressing Higher KIF11 mRNA
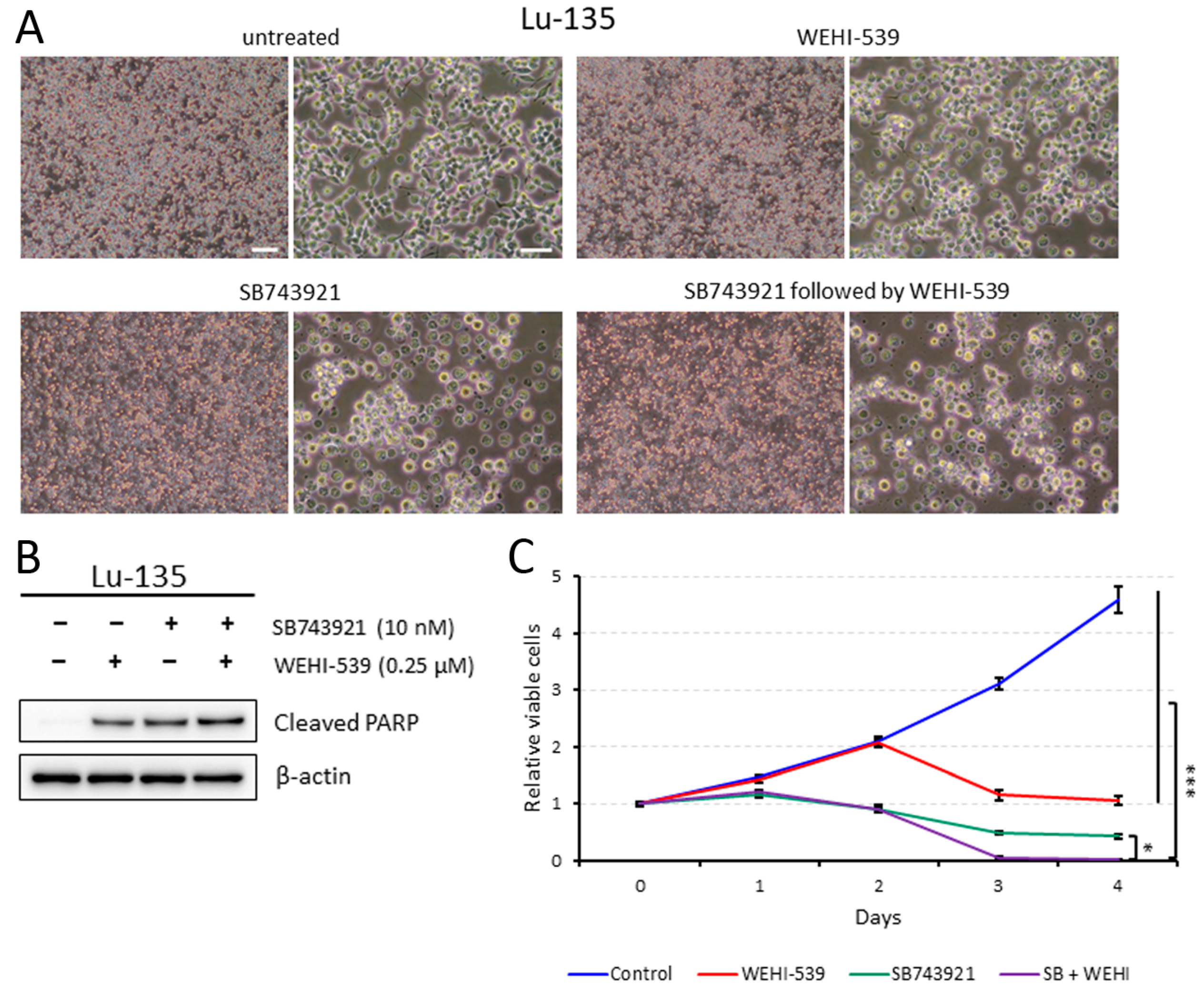
2.4. Lu-135 Cells Treated with SB743921 Are More Affected by BCL2L1 Inhibition
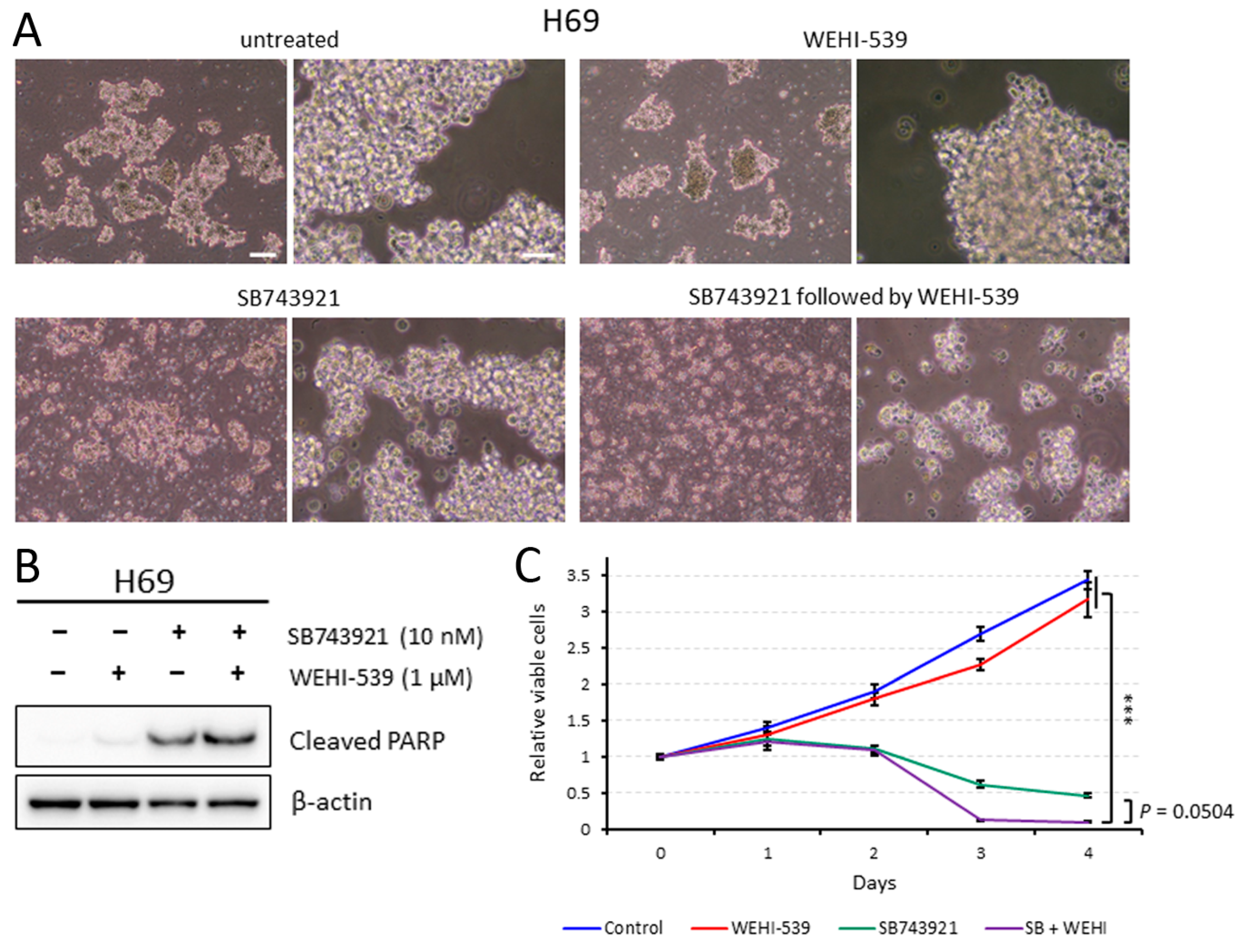
2.5. Sequentially Inhibiting KIF11 and BCL2L1 Is Also Effective against H69 Cells
2.6. Simultaneous Sirna-Mediated Knockdown of KIF11 and BCL2L1 Induces Significant Apoptosis in Lu-135 and H69 Cells
3. Discussion
4. Materials and Methods
4.1. Comparison of KIF11 mRNA Expression Levels in Normal Lung and SCLC Tissues
4.2. The Cancer Dependency Map
4.3. Cell Culture and Inhibitors
4.4. RNA Interference (RNAi) Assay
4.5. Western Blot Analysis
4.6. Cell Cycle Analysis
4.7. Assessment of Cell Viability and Apoptosis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Paz-Ares, L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J. Clin. Oncol. 2022, 40, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Rath, O.; Kozielski, F. Kinesins and cancer. Nat. Rev. Cancer 2012, 12, 527–539. [Google Scholar] [CrossRef]
- Garcia-Saez, I.; Skoufias, D.A. Eg5 targeting agents: From new anti-mitotic based inhibitor discovery to cancer therapy and resistance. Biochem. Pharmacol. 2021, 184, 114364. [Google Scholar] [CrossRef]
- Sakuma, Y.; Hirai, S.; Sumi, T.; Niki, T.; Yamaguchi, M. Dual inhibition of KIF11 and BCL2L1 induces apoptosis in lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2023, 678, 84–89. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Anderson, M.A.; Czabotar, P.E.; Lessene, G.; Strasser, A.; Kelly, G.L. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 2022, 22, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Li, Z.W.; Sun, W.; Yang, X.; Zhou, L.X.; Huang, X.Z.; Jia, L.; Lin, D.M. Automated quantification of Ki-67 index associates with pathologic grade of pulmonary neuroendocrine tumors. Chin. Med. J. 2019, 132, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Higgs, B.W.; Hu, Z.; Xiao, Z.; Yao, X.; Conley, S.; Zhong, H.; Liu, Z.; Brohawn, P.; et al. Genomic Landscape Survey Identifies SRSF1 as a Key Oncodriver in Small Cell Lung Cancer. PLoS Genet. 2016, 12, e1005895. [Google Scholar] [CrossRef] [PubMed]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e16. [Google Scholar] [CrossRef] [PubMed]
- Polley, E.; Kunkel, M.; Evans, D.; Silvers, T.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Selby, M.; Connelly, J.; et al. Small Cell Lung Cancer Screen of Oncology Drugs, Investigational Agents, and Gene and microRNA Expression. J. Natl. Cancer Inst. 2016, 108, djw122. [Google Scholar] [CrossRef]
- Khan, S.; Zhang, X.; Lv, D.; Zhang, Q.; He, Y.; Zhang, P.; Liu, X.; Thummuri, D.; Yuan, Y.; Wiegand, J.S.; et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019, 25, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Skwarska, A.; Konopleva, M. BCL-xL Targeting to Induce Apoptosis and to Eliminate Chemotherapy-Induced Senescent Tumor Cells: From Navitoclax to Platelet-Sparing BCL-xL PROTACs. Cancer Res. 2023, 83, 3501–3503. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wen, J.; Chen, J.; Wang, B.; Xu, X.; Zhang, Z.; Fan, M. A Comparative Analysis of the Gene Expression Profiles of Small Cell Esophageal Carcinoma, Small Cell Lung Cancer, and Esophageal Adeno/Squamous Carcinoma. Front. Surg. 2021, 8, 655159. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hirai, S.; Idogawa, M.; Sumi, T.; Uchida, H.; Fujitani, N.; Takahashi, M.; Sakuma, Y. Junctional adhesion molecule 3 is a potential therapeutic target for small cell lung carcinoma. Exp. Cell Res. 2023, 426, 113570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, Y.; Hirai, S.; Yamaguchi, M.; Idogawa, M. Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival. Int. J. Mol. Sci. 2024, 25, 7230. https://doi.org/10.3390/ijms25137230
Sakuma Y, Hirai S, Yamaguchi M, Idogawa M. Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival. International Journal of Molecular Sciences. 2024; 25(13):7230. https://doi.org/10.3390/ijms25137230
Chicago/Turabian StyleSakuma, Yuji, Sachie Hirai, Miki Yamaguchi, and Masashi Idogawa. 2024. "Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival" International Journal of Molecular Sciences 25, no. 13: 7230. https://doi.org/10.3390/ijms25137230
APA StyleSakuma, Y., Hirai, S., Yamaguchi, M., & Idogawa, M. (2024). Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival. International Journal of Molecular Sciences, 25(13), 7230. https://doi.org/10.3390/ijms25137230




