Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers
Abstract
:1. Introduction
2. Results
2.1. Poly L-lactic Acid Increases Intracellular Ca2+ Levels in Senescent Fibroblasts
2.2. Poly L-lactic Acid Enhances ERK1/2/AKT, CDK4, Cyclin D1, and Proliferation of Senescent Fibroblasts
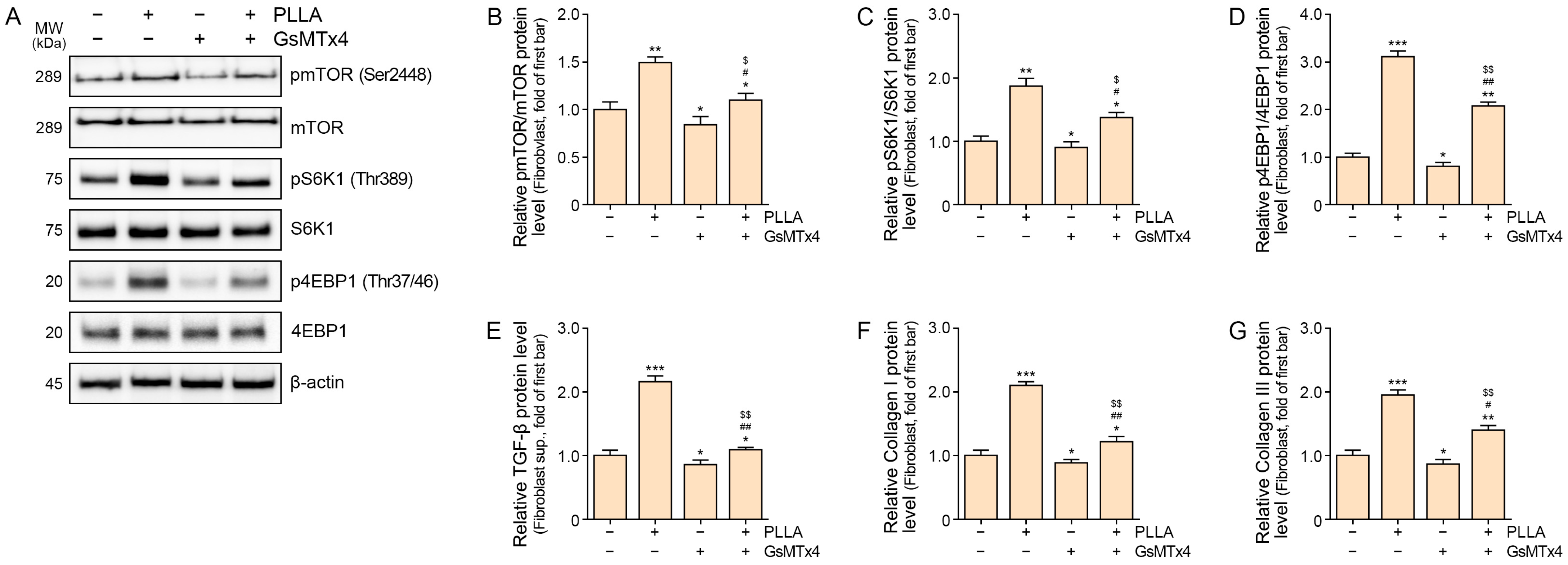
2.3. Poly L-lactic Acid Upregulates mTOR/S6K1/4EBP1, TGF-β, and COLLAGEN I/III Expression in Senescent Fibroblasts
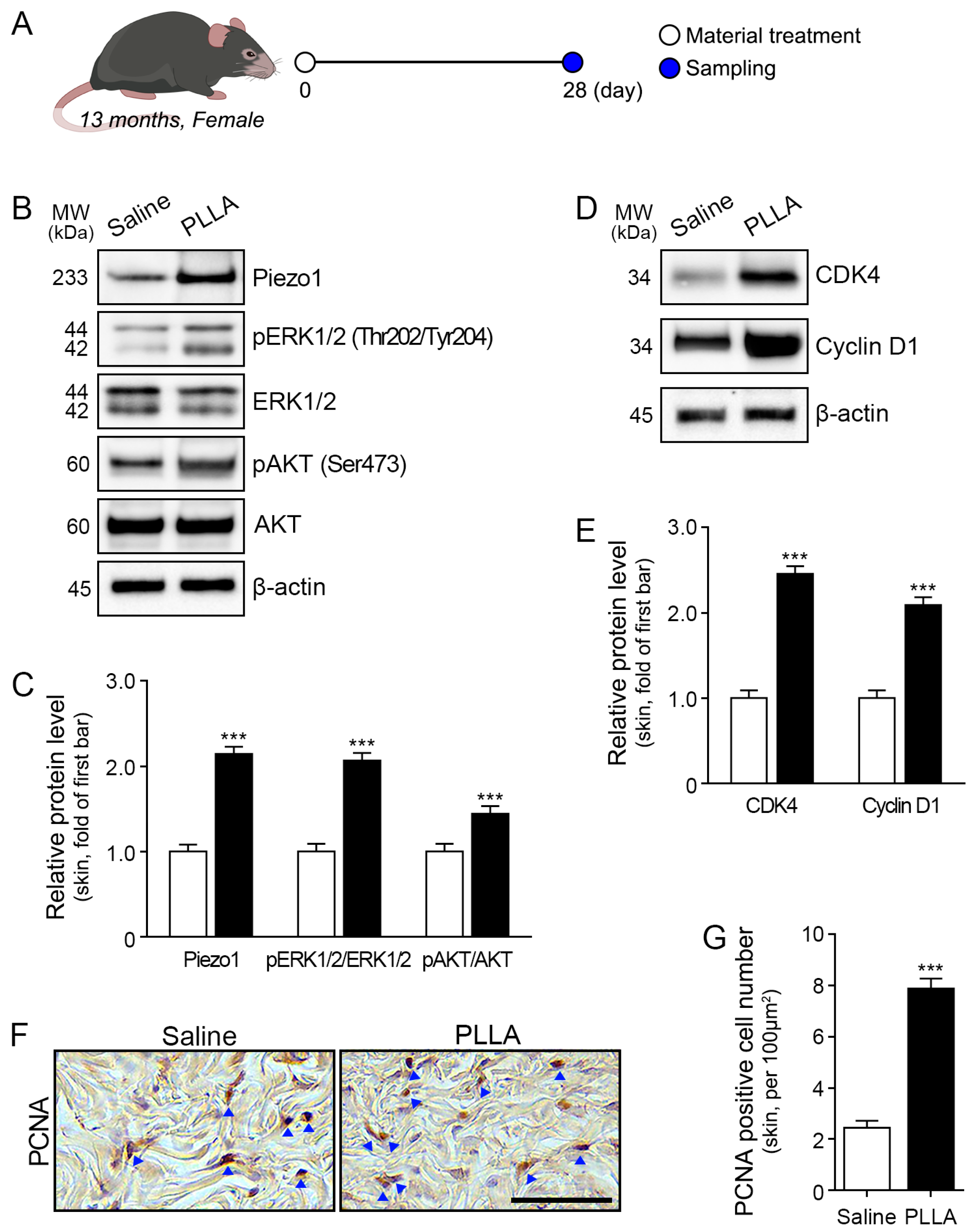
2.4. Poly L-lactic Acid Increases ERK1/2/AKT, CDK4, and Cyclin D1 Expression and Proliferation of Fibroblasts in Aged Skin
2.5. mTOR/S6K1/4EBP1, TGF-β, and Collagen I/III Expression Is Upregulated in Aged Skin
3. Discussion
4. Materials and Methods
4.1. Poly L-lactic Acid Preparation
4.2. In Vitro Experiments
4.2.1. Cell Culture
4.2.2. Experimental Design
4.3. In Vivo Experiments
4.3.1. Mouse Model and Maintenance
4.3.2. Experimental Design
4.4. Sample Preparation
4.4.1. Protein Isolation
4.4.2. Tissue Processing, Paraffin Embedding and Sectioning
4.5. Measurement of Intracellular Ca2+ Concentration
4.6. Western Blot Analysis
4.7. Enzyme-Linked Immunosorbent Assays
4.8. Staining
4.8.1. Immunohistochemistry
4.8.2. Masson Trichrome Staining
4.8.3. Herovici Staining
4.8.4. Quantitative Analysis and Procedure of Scanning and Image Selection
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, P.; Vitavska, O.; Kind, P.; Hoppe, W.; Wieczorek, H.; Schürer, N.Y. The biological basis for poly-L-lactic acid-induced augmentation. J. Dermatol. Sci. 2015, 78, 26–33. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.H.; Kim, H.M.; Batsukh, S.; Sung, M.J.; Lim, T.H.; Lee, M.H.; Son, K.H.; Byun, K. Poly-L-Lactic Acid Fillers Improved Dermal Collagen Synthesis by Modulating M2 Macrophage Polarization in Aged Animal Skin. Cells 2023, 12, 1320. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Graivier, M.H.; Kane, M.; Lorenc, Z.P.; Vleggaar, D.; Werschler, W.P.; Kenkel, J.M. Update on facial aging. Aesthet. Surg. J. 2010, 30, 11S–24S. [Google Scholar] [CrossRef]
- Junge, K.; Binnebösel, M.; von Trotha, K.T.; Rosch, R.; Klinge, U.; Neumann, U.P.; Lynen Jansen, P. Mesh biocompatibility: Effects of cellular inflammation and tissue remodelling. Langenbecks Arch. Surg. 2012, 397, 255–270. [Google Scholar] [CrossRef]
- Brady, J.M.; Cutright, D.E.; Miller, R.A.; Barristone, G.C. Resorption rate, route, route of elimination, and ultrastructure of the implant site of polylactic acid in the abdominal wall of the rat. J. Biomed. Mater. Res. 1973, 7, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Cutright, D.E.; Hunsuck, E.E. Tissue reaction to the biodegradable polylactic acid suture. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue response and in vivo degradation of selected polyhydroxyacids: Polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic. Plast. Surg. 2003, 27, 354–367. [Google Scholar] [CrossRef]
- Song, E.; Ouyang, N.; Hörbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Coste, B.; Chadha, A.; Cook, B.; Patapoutian, A. The role of Drosophila Piezo in mechanical nociception. Nature 2012, 483, 209–212. [Google Scholar] [CrossRef]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Shin, K.C.; Park, H.J.; Kim, J.G.; Lee, I.H.; Cho, H.; Park, C.; Sung, T.S.; Koh, S.D.; Park, S.W.; Bae, Y.M. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep. 2019, 9, 6446. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.A.; Li, W.; Ma, Y.; Shen, J.; Wang, Y.; Shen, Y.; Chen, J.; Ji, Y.; Xie, Y.; et al. Piezo1-Mediated Mechanotransduction Promotes Cardiac Hypertrophy by Impairing Calcium Homeostasis to Activate Calpain/Calcineurin Signaling. Hypertension 2021, 78, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef] [PubMed]
- Yarishkin, O.; Phuong, T.T.T.; Baumann, J.M.; De Ieso, M.L.; Vazquez-Chona, F.; Rudzitis, C.N.; Sundberg, C.; Lakk, M.; Stamer, W.D.; Križaj, D. Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. J. Physiol. 2021, 599, 571–592. [Google Scholar] [CrossRef]
- Filser, M.; Giansily-Blaizot, M.; Grenier, M.; Monedero Alonso, D.; Bouyer, G.; Pérès, L.; Egée, S.; Aral, B.; Airaud, F.; Da Costa, L.; et al. Increased incidence of germline PIEZO1 mutations in individuals with idiopathic erythrocytosis. Blood 2021, 137, 1828–1832. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, Y.; Liang, B.; Xu, J.; Lin, Y.; Zhou, N.; Li, J.; Jiang, B.; Cheng, J.; Li, C.; et al. Mechanosensitive Piezo1 channels mediate renal fibrosis. JCI Insight 2022, 7, 152330. [Google Scholar] [CrossRef]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife 2019, 8, 47454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, L.; Wei, M.; Duan, W.; Wu, S.; Kasim, V. Mechanosensitive Ion Channel PIEZO1 Signaling in the Hall-Marks of Cancer: Structure and Functions. Cancers 2022, 14, 4955. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Lu, Y.; Zhong, W.; Wang, T.; Li, Y.; Ruan, X.; Chen, H.; Sun, L.; Guan, Z.; Li, G.; et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. 2023, 56, 13440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Xu, C.; Sun, B.; Zhong, F.J.; Cao, M.; Yang, L.Y. Piezo1 promoted hepatocellular carcinoma progression and EMT through activating TGF-β signaling by recruiting Rab5c. Cancer Cell Int. 2022, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, S.; Gong, M.; Zhang, W.; Zhang, W.; Zhu, Z.; Wu, S.; Yue, Y.; Qian, W.; Ma, Q.; et al. Mechanically activated ion channel Piezo1 contributes to melanoma malignant progression through AKT/mTOR signaling. Cancer Biol. Ther. 2022, 23, 336–347. [Google Scholar] [CrossRef]
- Maydan, M.; McDonald, P.C.; Sanghera, J.; Yan, J.; Rallis, C.; Pinchin, S.; Hannigan, G.E.; Foster, L.J.; Ish-Horowicz, D.; Walsh, M.P.; et al. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation. PLoS ONE 2010, 5, 12356. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef] [PubMed]
- Roderick, H.L.; Cook, S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019, 55, 629–644. [Google Scholar] [CrossRef]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Dev. Ther. 2019, 13, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Blythe, N.M.; Muraki, K.; Ludlow, M.J.; Stylianidis, V.; Gilbert, H.T.J.; Evans, E.L.; Cuthbertson, K.; Foster, R.; Swift, J.; Li, J.; et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 2019, 294, 17395–17408. [Google Scholar] [CrossRef] [PubMed]
- Jacques-Fricke, B.T.; Seow, Y.; Gottlieb, P.A.; Sachs, F.; Gomez, T.M. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J. Neurosci. 2006, 26, 5656–5664. [Google Scholar] [CrossRef] [PubMed]
- Herovici, C. Picropolychrome: Histological staining technic intended for the study of normal and pathological connective tissue. Rev. Fr. Etud. Clin. Biol. 1963, 8, 88–89. [Google Scholar] [PubMed]
- Anthony, P.P. Manual of Histological Demonstration Techniques. J. Clin. Pathol. 1975, 28, 339. [Google Scholar] [CrossRef]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting Fibrosis: The Bridge That Connects Pancreatitis and Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 4970. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wan, P.; Zhang, J.; Li, X.; Xing, J.; Zou, Y.; Wang, K.; Peng, H.; Zhu, Q.; Cao, L.; et al. Targeting Mechanosensitive Piezo1 Alleviated Renal Fibrosis through p38MAPK-YAP Pathway. Front. Cell Dev. Biol. 2021, 9, 741060. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cheng, X.; Fang, B.; Shan, S.; Li, Q. Mechanical stiffness promotes skin fibrosis via Piezo1-Wnt2/Wnt11-CCL24 positive feedback loop. Cell Death. Dis. 2024, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Braidotti, N.; Chen, S.N.; Long, C.S.; Cojoc, D.; Sbaizero, O. Piezo1 Channel as a Potential Target for Hindering Cardiac Fibrotic Remodeling. Int. J. Mol. Sci. 2022, 23, 8065. [Google Scholar] [CrossRef]
- Chen, J.; Rodriguez, M.; Miao, J.; Liao, J.; Jain, P.P.; Zhao, M.; Zhao, T.; Babicheva, A.; Wang, Z.; Parmisano, S.; et al. Mechanosensitive channel Piezo1 is required for pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L737–L760. [Google Scholar] [CrossRef]
- Hirano, K.; Tsuchiya, M.; Shiomi, A.; Takabayashi, S.; Suzuki, M.; Ishikawa, Y.; Kawano, Y.; Takabayashi, Y.; Nishikawa, K.; Nagao, K.; et al. The mechanosensitive ion channel PIEZO1 promotes satellite cell function in muscle regeneration. Life Sci. Alliance 2022, 6, e202201783. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guan, J.; Jin, Z.; Yin, C.; Wu, S.; Sun, W.; Zhang, H.; Yan, B. Mechanical force modulates macrophage proliferation via Piezo1-AKT-Cyclin D1 axis. FASEB. J. 2022, 36, e22423. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Azabdaftari, G.; Euscher, L.M.; Dai, S.; Karacosta, L.G.; Franke, T.F.; Edelman, A.M. Akt activation by Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J. Biol. Chem. 2017, 292, 14188–14204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Y.; Dong, X.; Liu, B.; Zhang, N.; Wang, X.; Liu, L.; Xu, C.; Huang, S.; Chen, L. Celastrol Attenuates Cadmium-Induced Neuronal Apoptosis via Inhibiting Ca2+ -CaMKII-Dependent Akt/mTOR Pathway. J. Cell. Physiol. 2017, 232, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Walker, N.M.; Belloli, E.A.; Stuckey, L.; Chan, K.M.; Lin, J.; Lynch, W.; Chang, A.; Mazzoni, S.M.; Fingar, D.C.; Lama, V.N. Mechanistic Target of Rapamycin Complex 1 (mTORC1) and mTORC2 as Key Signaling Intermediates in Mesenchymal Cell Activation. J. Biol. Chem. 2016, 291, 6262–6271. [Google Scholar] [CrossRef]
- Lim, J.; Munivez, E.; Jiang, M.M.; Song, I.W.; Gannon, F.; Keene, D.R.; Schweitzer, R.; Lee, B.H.; Joeng, K.S. mTORC1 Signaling is a Critical Regulator of Postnatal Tendon Development. Sci. Rep. 2017, 7, 17175. [Google Scholar] [CrossRef]
- Bujor, A.M.; Pannu, J.; Bu, S.; Smith, E.A.; Muise-Helmericks, R.C.; Trojanowska, M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J. Investig. Dermatol. 2008, 128, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stefanovic, B. Akt mediated phosphorylation of LARP6; critical step in biosynthesis of type I collagen. Sci. Rep. 2016, 6, 22597. [Google Scholar] [CrossRef] [PubMed]
- Krepinsky, J.C.; Li, Y.; Chang, Y.; Liu, L.; Peng, F.; Wu, D.; Tang, D.; Scholey, J.; Ingram, A.J. Akt mediates mechanical strain-induced collagen production by mesangial cells. J. Am. Soc. Nephrol. 2005, 16, 1661–1672. [Google Scholar] [CrossRef]
- Runyan, C.E.; Schnaper, H.W.; Poncelet, A.C. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J. Biol. Chem. 2004, 279, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, 5183. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- O’Leary, E.M.; Tian, Y.; Nigdelioglu, R.; Witt, L.J.; Cetin-Atalay, R.; Meliton, A.Y.; Woods, P.S.; Kimmig, L.M.; Sun, K.A.; Gökalp, G.A.; et al. TGF-β Promotes Metabolic Reprogramming in Lung Fibroblasts via mTORC1-dependent ATF4 Activation. Am. J. Respir. Cell Mol. Biol. 2020, 63, 601–612. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Lee, D.Y.; White, E.S.; Cui, Z.; Larios, J.M.; Chacon, R.; Horowitz, J.C.; Day, R.M.; Thomas, P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003, 278, 12384–12389. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Thatcher, T.H.; Olsen, K.C.; Maggirwar, S.B.; Phipps, R.P.; Sime, P.J. PPAR-γ ligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: Implications for therapy of fibrosis. PLoS ONE 2011, 6, 15909. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Ray, S.; Ta, H.T. Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter. J. Funct. Biomater. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Shirokova, N.; Niggli, E. Cardiac phenotype of Duchenne Muscular Dystrophy: Insights from cellular studies. J. Mol. Cell Cardiol. 2013, 58, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef]
- Mulier, M.; Vriens, J.; Voets, T. TRP channel pores and local calcium signals. Cell Calcium 2017, 66, 19–24. [Google Scholar] [CrossRef]
- Runel, G.; Cario, M.; Lopez-Ramirez, N.; Malbouyres, M.; Ruggiero, F.; Bernard, L.; Puisieux, A.; Caramel, J.; Chlasta, J.; Masse, I. Stiffness measurement is a biomarker of skin ageing in vivo. Exp. Dermatol. 2020, 29, 1233–1237. [Google Scholar] [CrossRef]
- Agache, P.G.; Monneur, C.; Leveque, J.L.; De Rigal, J. Mechanical properties and Young’s modulus of human skin in vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Skin aging as a mechanical phenomenon: The main weak links. Nutr. Healthy Aging 2018, 4, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Jardini, A.L.; Rubens Filho, M. Synthesis and Characterizations of Poly (Lactic Acid) by Ring-Opening Polymerization for Biomedical Applications. Chem. Eng. Trans. 2014, 38, 331–336. [Google Scholar]
- Pholharn, D.; Srithep, Y.; Morris, J. Ring opening polymerization of poly(Llactide) by macroinitiator. AIP Conf. Proc. 2019, 2065, 030016. [Google Scholar]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Pholharn, D.; Srithep, Y.; Morris, J. Effect of initiators on synthesis of poly(L-lactide) by ring opening polymerization. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012022. [Google Scholar] [CrossRef]
- Mammadov, R.; Cinar, G.; Gunduz, N.; Goktas, M.; Kayhan, H.; Tohumeken, S.; Topal, A.E.; Orujalipoor, I.; Delibasi, T.; Dana, A.; et al. Virus-like nanostructures for tuning immune response. Sci. Rep. 2015, 5, 16728. [Google Scholar] [CrossRef]
- Acheva, A.; Kärki, T.; Schaible, N.; Krishnan, R.; Tojkander, S. Adipokine Leptin Co-operates With Mechanosensitive Ca2+-Channels and Triggers Actomyosin-Mediated Motility of Breast Epithelial Cells. Front. Cell Dev. Biol. 2021, 8, 607038. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, H.Y.; Jang, J.T.; Hong, S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules 2023, 28, 8099. [Google Scholar] [CrossRef]
- Umair, Z.; Baek, M.O.; Song, J.; An, S.; Chon, S.J.; Yoon, M.S. MicroRNA-4516 in Urinary Exosomes as a Biomarker of Premature Ovarian Insufficiency. Cells 2022, 11, 2797. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Lee, J.H.; Lee, S.Y.; Oh, S.; Batsukh, S.; Cheon, G.-w.; Lee, D.; Hong, J.H.; Son, K.H.; Byun, K. Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers. Int. J. Mol. Sci. 2024, 25, 7232. https://doi.org/10.3390/ijms25137232
Byun K-A, Lee JH, Lee SY, Oh S, Batsukh S, Cheon G-w, Lee D, Hong JH, Son KH, Byun K. Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers. International Journal of Molecular Sciences. 2024; 25(13):7232. https://doi.org/10.3390/ijms25137232
Chicago/Turabian StyleByun, Kyung-A, Je Hyuk Lee, So Young Lee, Seyeon Oh, Sosorburam Batsukh, Gwahn-woo Cheon, Dongun Lee, Jeong Hee Hong, Kuk Hui Son, and Kyunghee Byun. 2024. "Piezo1 Activation Drives Enhanced Collagen Synthesis in Aged Animal Skin Induced by Poly L-Lactic Acid Fillers" International Journal of Molecular Sciences 25, no. 13: 7232. https://doi.org/10.3390/ijms25137232




