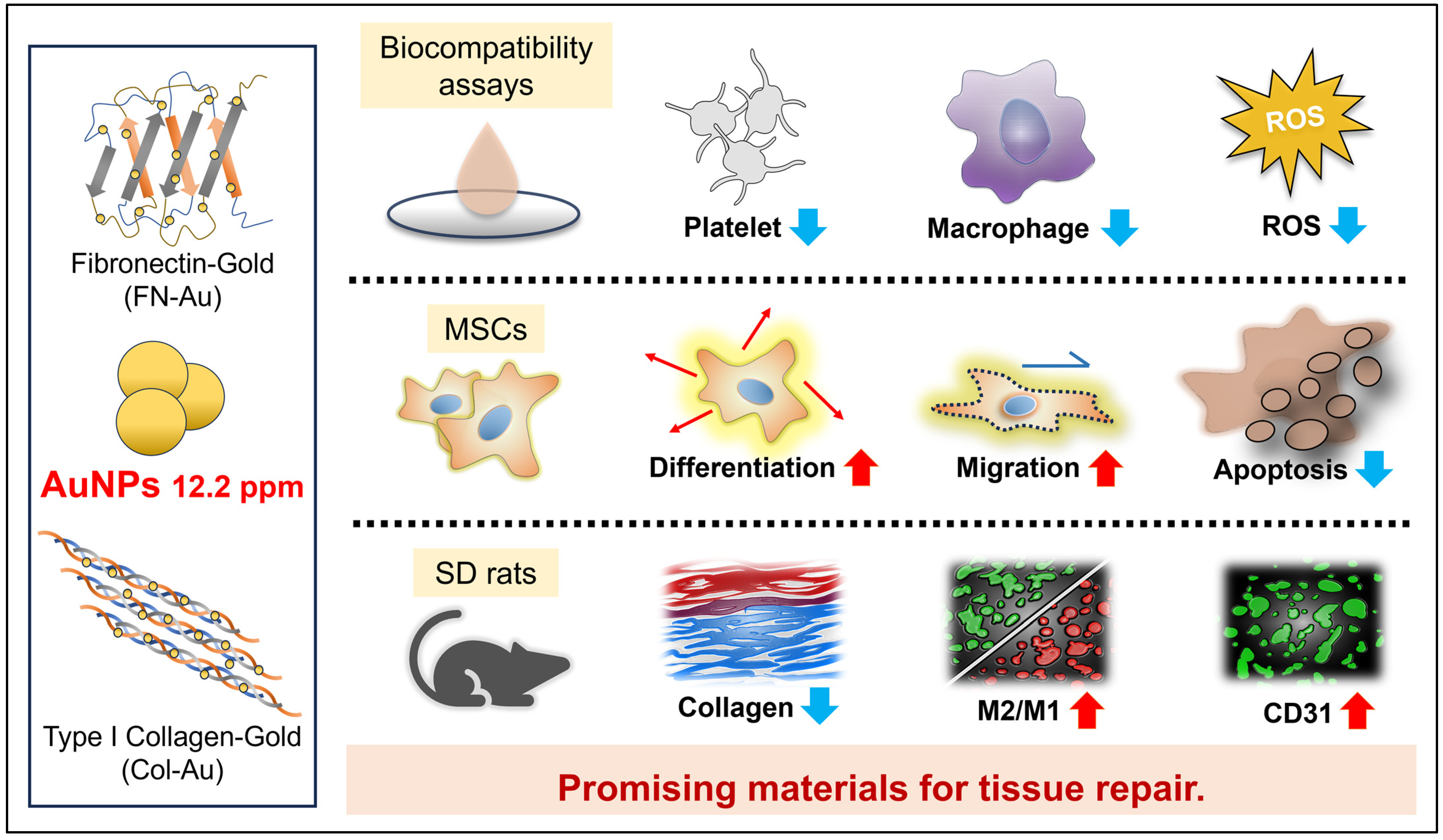
Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites
Abstract
:1. Introduction
2. Results
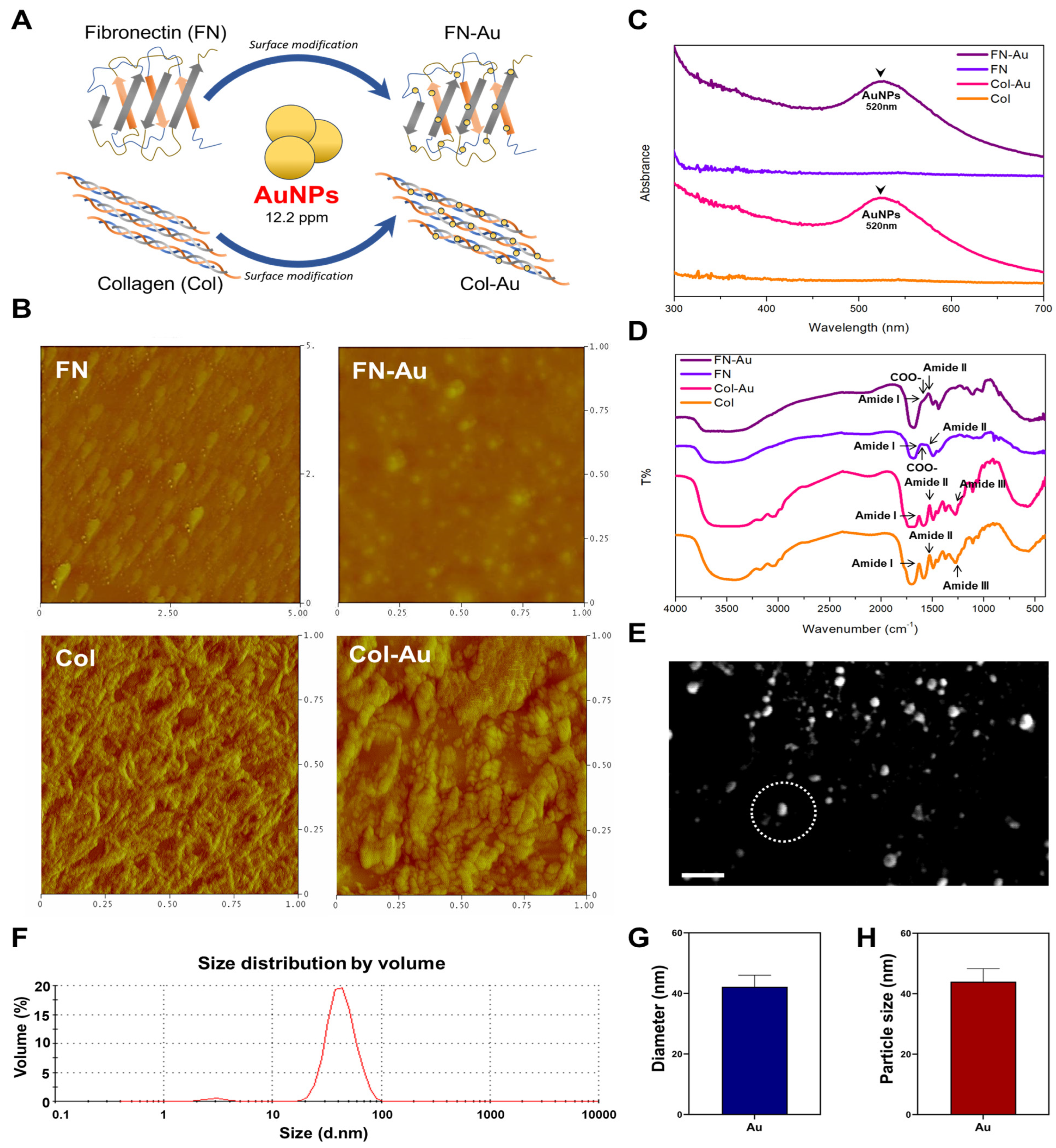
2.1. Characterization of the FN-Au and Col-Au Nanocomposites
2.2. Cell Morphology and Cytoskeleton Staining
2.3. Biocompatibility of FN-Au and Col-Au Nanocomposites Cultured with MSCs
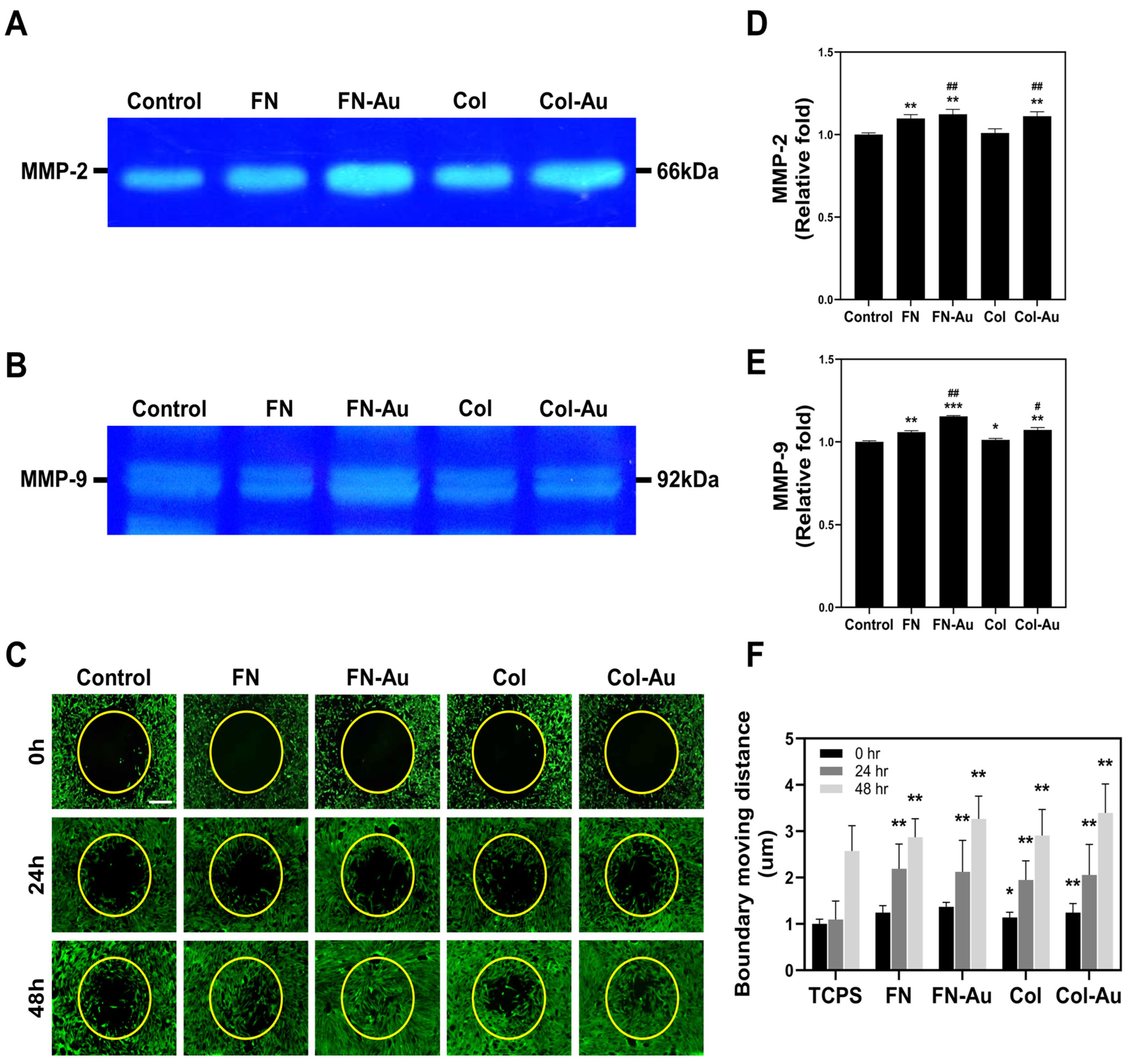
2.4. MMP2 and MMP9 Zymography
2.5. Assessment of MSC Migration Ability
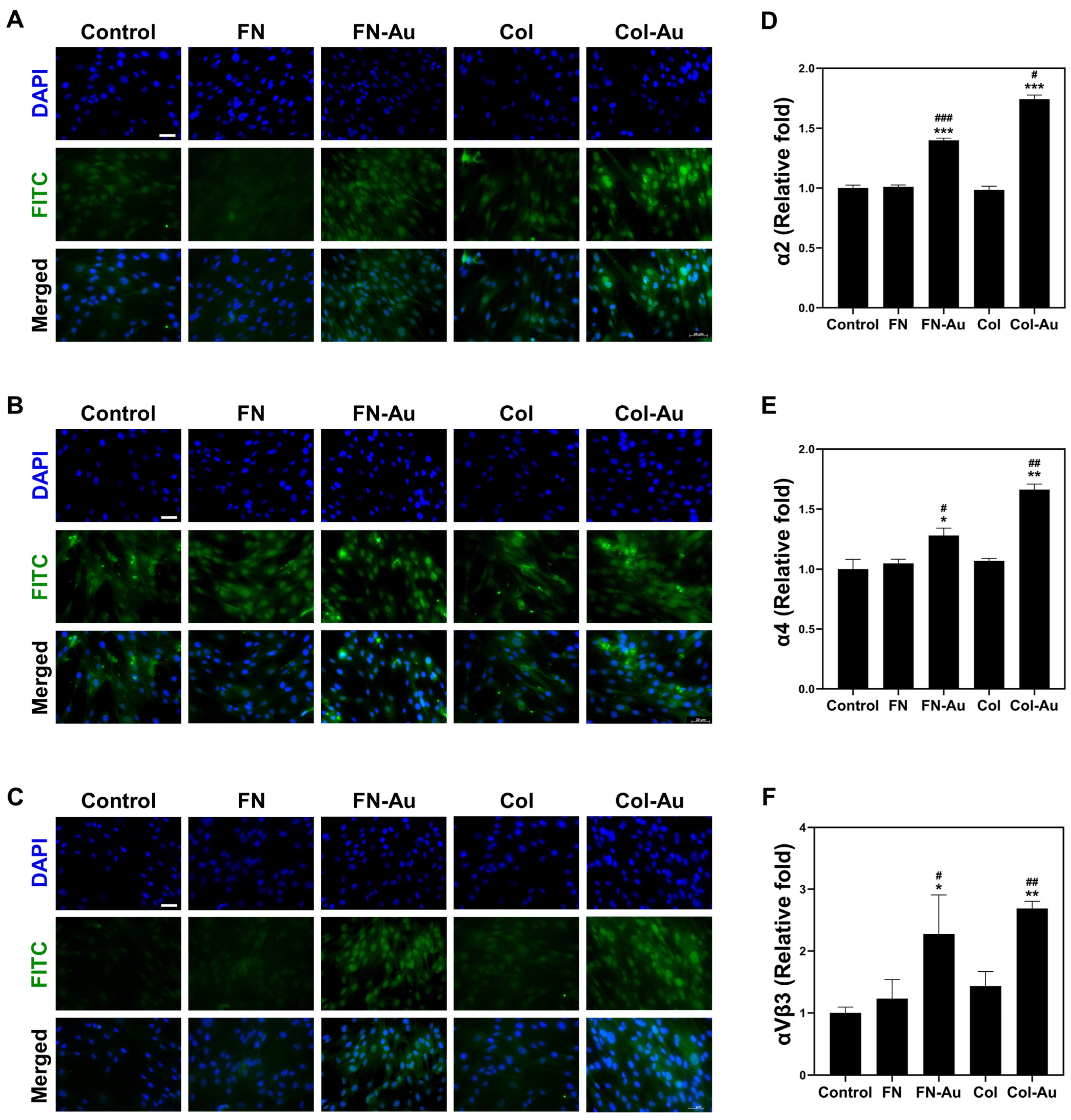
2.6. Integrin Expression: α2, α4, and αVβ3
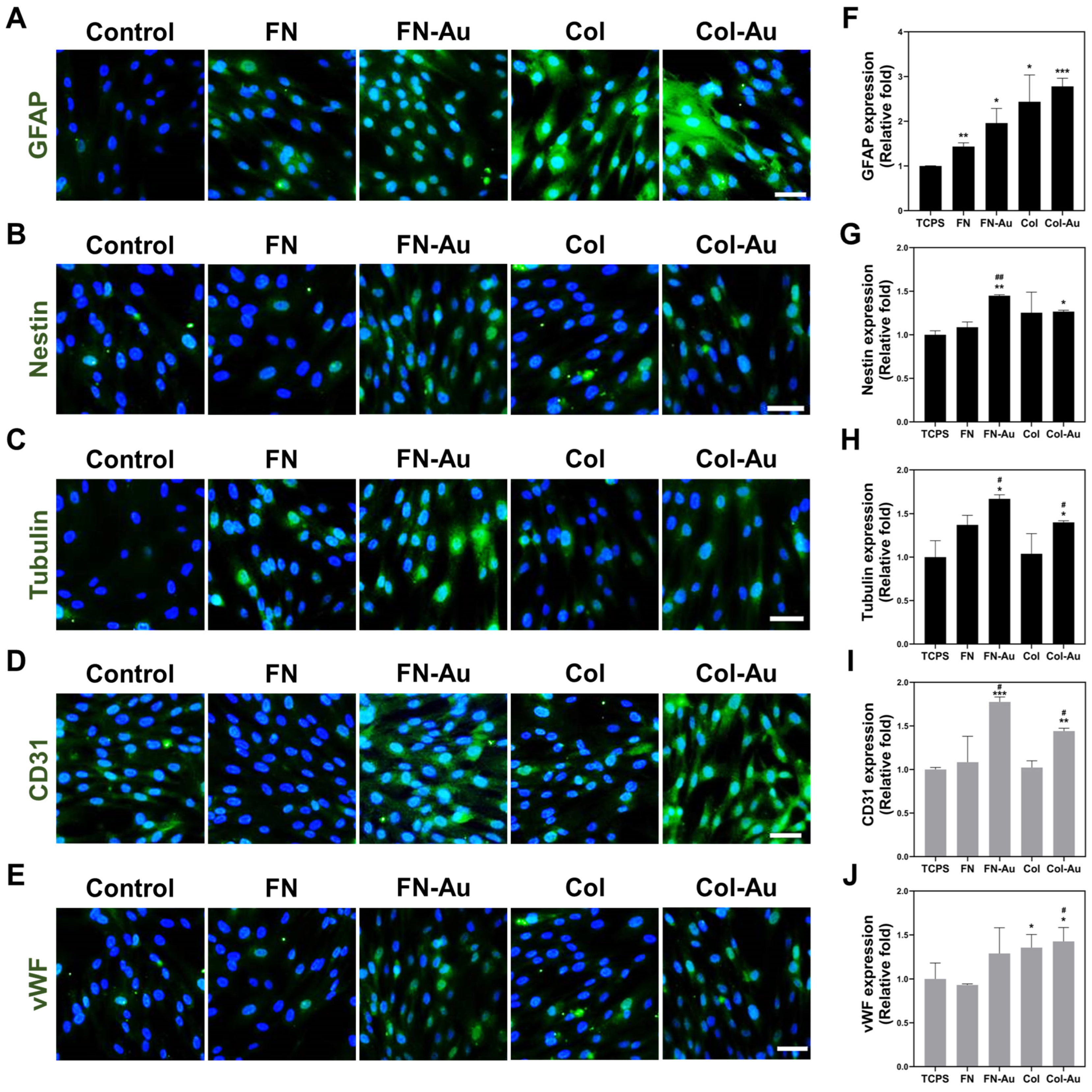
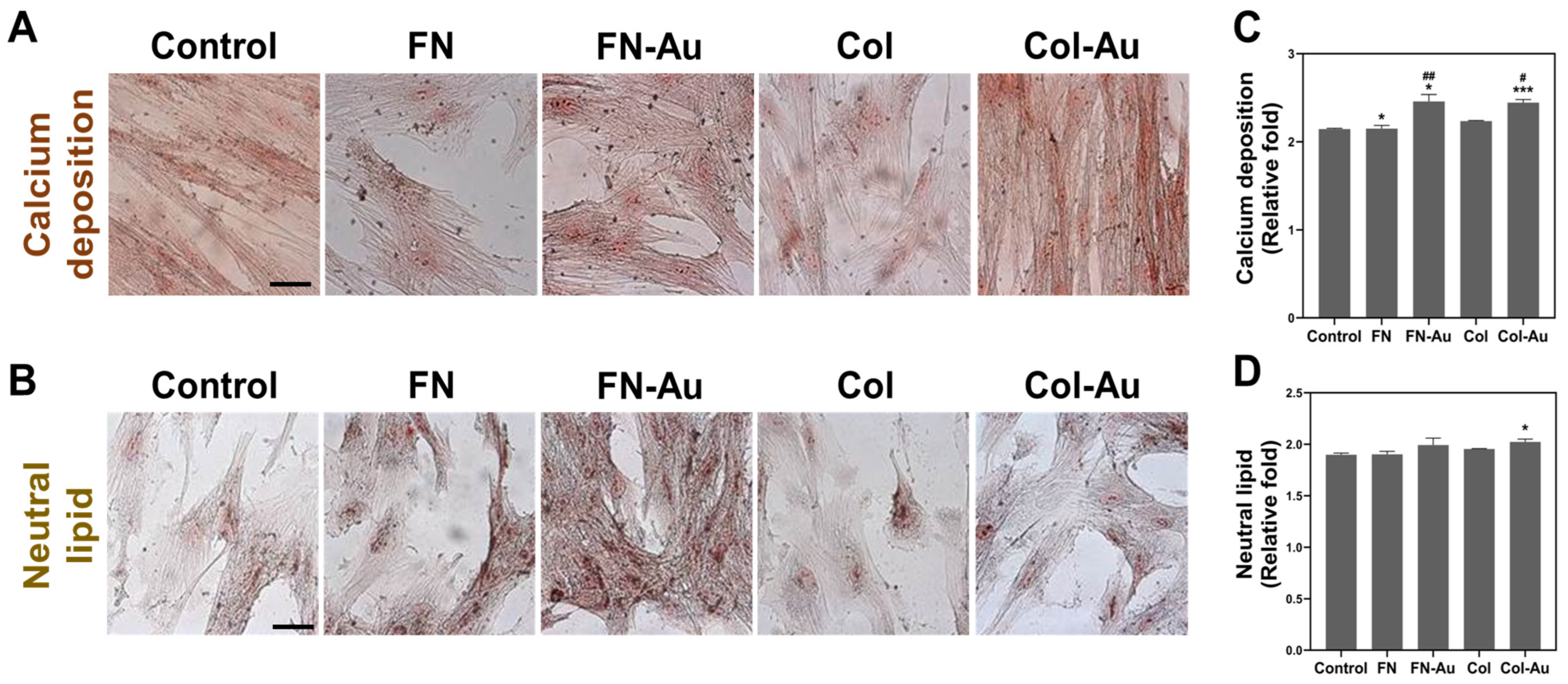
2.7. Assessments of the Multi-Differentiation Capacity of MSCs
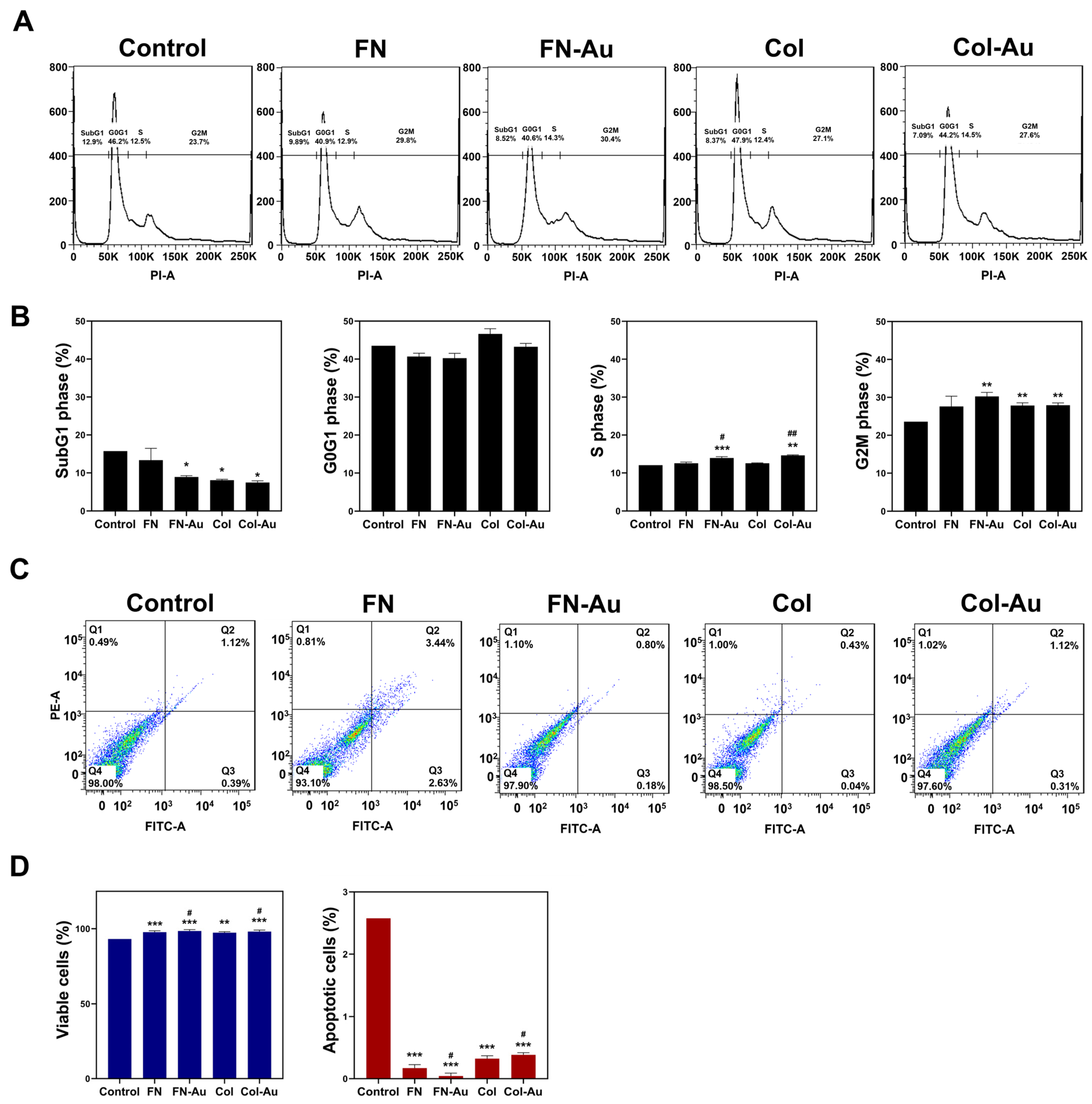
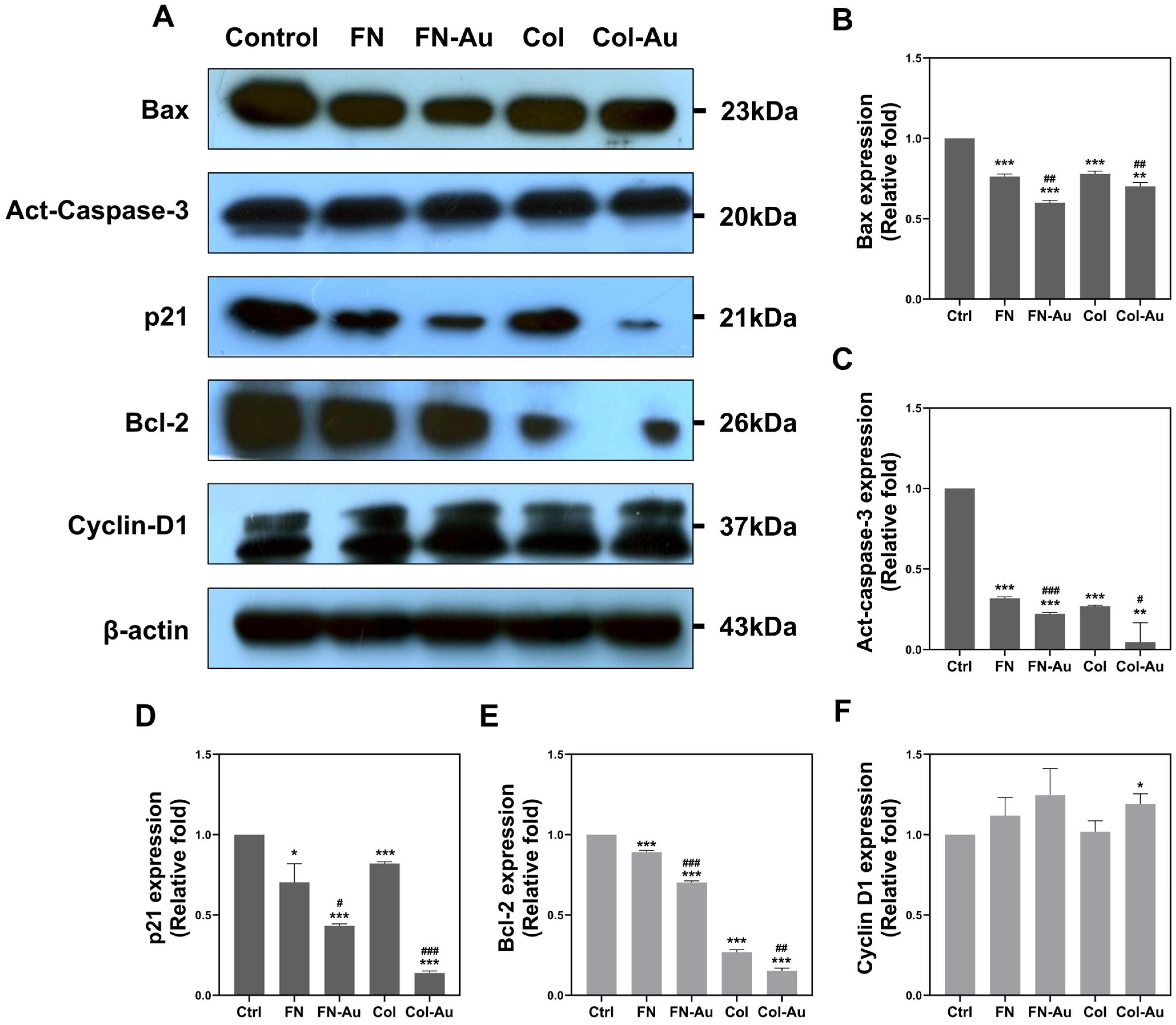
2.8. Cell Cycle Analysis and the Expression of Apoptotic-Related Proteins in MSCs
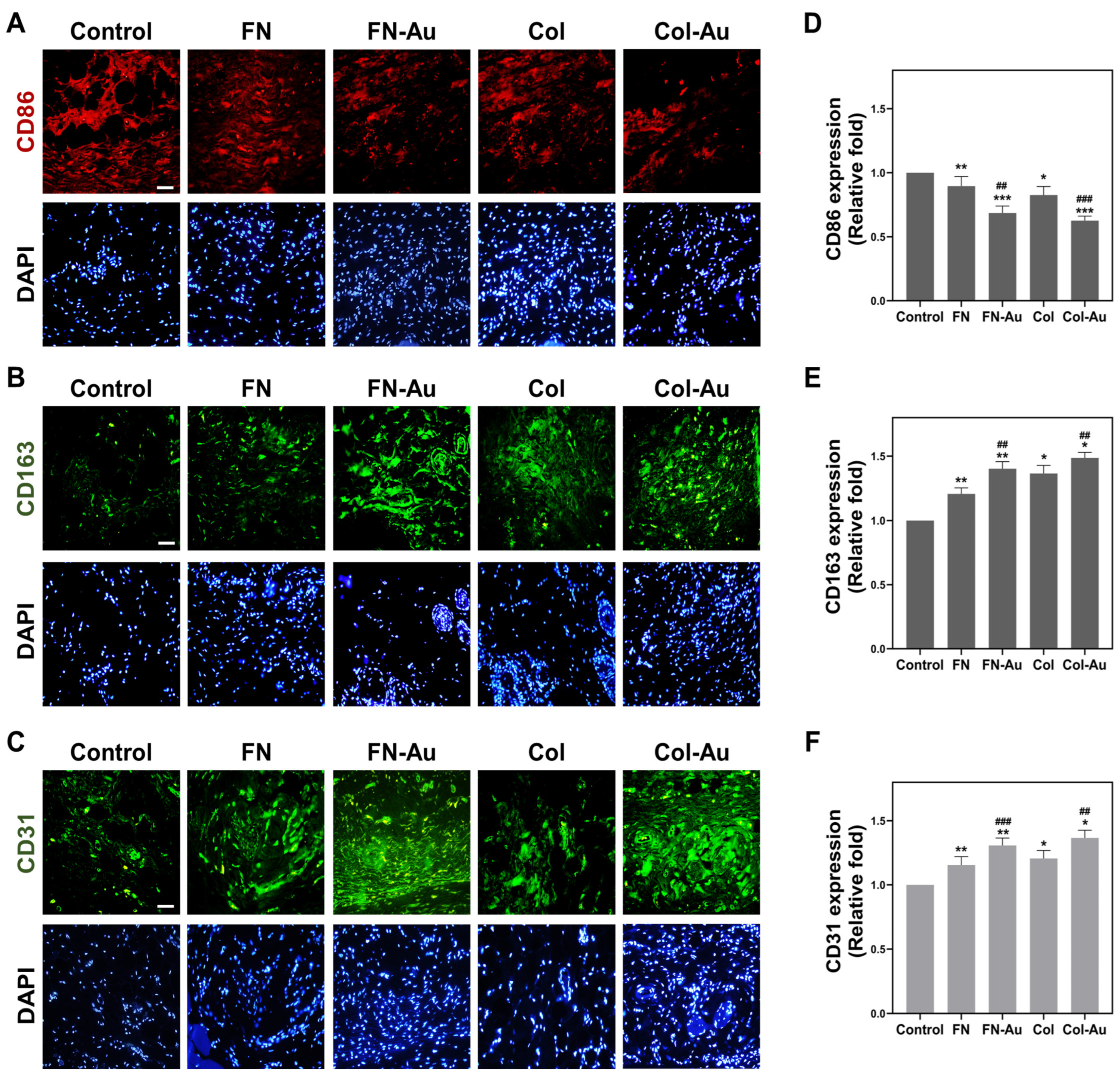
2.9. Assessment of In Vivo Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Materials Characterization
4.1.1. Preparation of FN-Au and Col-Au Nanocomposites
4.1.2. Atomic Force Microscopy (AFM)
4.1.3. Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
4.1.4. UV-Visible Spectrophotometry (UV-Vis)
4.1.5. Dynamic Light Scattering Assay (DLS)
4.2. Biocompatibility Assessment
4.2.1. Culturing of Human UCMSCs
4.2.2. MTT Assay
4.2.3. Platelet Activation Test
4.2.4. Monocyte Activation Test
4.2.5. Cell Morphology and Adhesion Ability
4.2.6. Measurement of Intracellular ROS
4.2.7. Fluorescent Staining of the Cytoskeleton
4.3. Biological Function Evaluation
4.3.1. Gelatin Zymography Analysis
4.3.2. Real-Time PCR
4.3.3. Western Blotting (WB)
4.3.4. Cell Migration Assay
4.3.5. IF Assay
4.3.6. Cell Cycle and Apoptosis Examination
4.4. Cell Differentiation Examination
4.4.1. Flow Cytometric and FACS Analysis
4.4.2. ARS Staining
4.4.3. ORO Staining
4.5. Animal Models
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, N.; Mobarak, M.H.; Mimona, M.A.; Islam, M.; Hossain, A.; Zohur, F.; Chowdhury, M. Advances and significances of nanoparticles in semiconductor applications—A review. Results Eng. 2023, 19, 101347. [Google Scholar] [CrossRef]
- Ahmad, J.; Zhou, Z. Properties of concrete with addition carbon nanotubes: A review. Constr. Build. Mater. 2023, 393, 132066. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X. Study of the thermal, electrical and thermoelectric properties of metallic nanofilms. Int. J. Heat Mass Transf. 2013, 58, 639–651. [Google Scholar] [CrossRef]
- Foong, T.; Chan, K.; Hu, X. Structure and properties of nano-confined poly(3-hexylthiophene) in nano-array/polymer hybrid ordered-bulk heterojunction solar cells. Nanoscale 2011, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Cooke, J.; Taraballi, F. Biomimetic Nano drug delivery carriers for treating cardiovascular diseases. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102360. [Google Scholar] [CrossRef] [PubMed]
- Sknepnek, R.; Anderson, J.; Lamm, M.; Schmalian, J.; Travesset, A. Nanoparticle Ordering via Functionalized Block Copolymers in Solution. ACS Nano 2008, 2, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Sweeny, B.; Johnston-Peck, A.; Niu, W.; Graham, J.; DuChene, J.; Qiu, J.; Wang, Y.-C.; Engelhard, M.; Su, D.; et al. Surface Plasmon-Driven Water Reduction: Gold Nanoparticle Size Matters. J. Am. Chem. Soc. 2014, 136, 9842–9845. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of Engineered Nanoparticles for Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2012, 166, 182–194. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Cardoza, C.; Nagtode, V.; Pratap, A.; Mali, S.; Pratap, A. Emerging Applications of Nanotechnology in Cosmeceutical Health Science: Latest Updates. Health Sci. Rev. 2022, 4, 100051. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Du, W.; Ling, G.; Zhang, P. Functional gold nanoparticles for diagnosis, treatment and prevention of thrombus. J. Control. Release 2022, 345, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Chang, C.-H.; Chang, C.-J.; Tang, C.-M.; Kao, W.-C.; Lin, S.-Z.; Hsieh, H.-H.; Chu, M.-Y.; Sun, W.-S.; Hsu, S.-H. In Vitro Study of a Novel Nanogold-Collagen Composite to Enhance the Mesenchymal Stem Cell Behavior for Vascular Regeneration. PLoS ONE 2014, 9, e104019. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Chu, M.-Y.; Lin, C.-H.; Wu, C.-C.; Hsu, S.-H. Mediation of the migration of endothelial cells and fibroblasts on polyurethane nanocomposites by the activation of integrin-focal adhesion kinase signaling. J. Biomed. Mater. Res. Part A 2012, 100, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Tang, C.-M.; Lin, C.-H.; Lin, S.-Z.; Chu, M.-Y.; Sun, W.-S.; Kao, W.-C.; Hsien-Hsu, H.; Huang, C.-Y.; Hsu, S.-H. Biocompatibility and Favorable Response of Mesenchymal Stem Cells on Fibronectin-Gold Nanocomposites. PLoS ONE 2013, 8, e65738. [Google Scholar] [CrossRef] [PubMed]
- Naba, A. Ten Years of Extracellular Matrix Proteomics: Accomplishments, Challenges, and Future Perspectives. Mol. Cell. Proteom. 2023, 22, 100528. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Matias, G.; Junior, M.; Carreira, A.C.; Miglino, M. ECM proteins involved in cell migration and vessel formation compromise bovine cloned placentation. Theriogenology 2022, 188, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Du, M.-Z.; Wu, T.; Su, T.; Ai, L.-Y.; Jiang, D. The application of ECM-derived biomaterials in cartilage tissue engineering. Mechanobiol. Med. 2023, 1, 100007. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2020, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Hendel, A.; Granville, D. Granzyme B Cleavage of Fibronectin Disrupts Endothelial Cell Adhesion, Migration and Capillary Tube Formation. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 32, 14–22. [Google Scholar] [CrossRef]
- Cho, J.; Mosher, D. Role of fibronectin assembly in platelet thrombus formation. J. Thromb. Haemost. JTH 2006, 4, 1461–1469. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Vascular Morphogenesis: An Integrin and Fibronectin Highway. Curr. Biol. 2017, 27, R158–R161. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Persikov, A. Molecular Structure of the Collagen Triple Helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ruan, C.; Niu, X. Collagen-based bioinks for regenerative medicine: Fabrication, application and prospective. Med. Nov. Technol. Devices 2023, 17, 100211. [Google Scholar] [CrossRef]
- Grant, S.; Zhu, V.; Gootee, J.; Snider, C.; Bellrichard, M.; Grant, D. Gold Nanoparticle-Collagen Gels for Soft Tissue Augmentation. Tissue Eng. Part A 2018, 24, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Weis, S. Evaluating Integrin Function in Models of Angiogenesis and Vascular Permeability. Methods Enzymol. 2007, 426, 505–528. [Google Scholar] [CrossRef] [PubMed]
- Bidone, T.; Odde, D. Multiscale models of integrins and cellular adhesions. Curr. Opin. Struct. Biol. 2023, 80, 102576. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernández, T.; Ballester, L.; Pérez-Temprano, N.; Rojas, E.; Sanz-San José, D.; Iglesias-Gaspar, M.; Moya, S.; González-Fernández, A.; Rey, M. Potential impact of metal oxide nanoparticles on the immune system: The role of integrins, L-selectin and the chemokine receptor CXCR4. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Qu, X.-J.; Gao, Z.-H. Arginine-Glycine-Aspartate–Binding Integrins as Therapeutic and Diagnostic Targets. Am. J. Ther. 2014, 23, e198–e207. [Google Scholar] [CrossRef]
- Galvez, B.G.; Matías-Román, S.; Yáñez-Mó, M.; Sánchez-Madrid, F.; Arroyo, A. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 2002, 159, 509–521. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Harvey, V.S.; Estrella, P.; Brown, L.F.; McDonagh, J.; Dvorak, A.M. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab. Investig. 1987, 57, 673–686. [Google Scholar] [PubMed]
- Ding, T.; Lu, W.; Zheng, Y.; Li, Z.; Pan, H.; Luo, Z. Rapid repair of rat sciatic nerve injury using a nanosilver-embedded collagen scaffold coated with laminin and fibronectin. Regen. Med. 2011, 6, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Ghuman, M.; Hughes, F. Akt- and Erk-mediated regulation of proliferation and differentiation during PDGFRβ-induced MSC self-renewal. J. Cell. Mol. Med. 2012, 16, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-K.; Kim, B.-S. Modulation of Stem Cell Differentiation with Biomaterials. Int. J. Stem Cells 2010, 3, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Greene, A.; Melero-Martin, J. Engraftment of human MSCs as perivascular cells of bioengineered microvessels enhances mesenchymal tissue formation. Cardiovasc. Pathol. 2013, 22, e48. [Google Scholar] [CrossRef]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R. Bone marrow derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Schechner, J.; Nath, A.; Zheng, L.; Kluger, M.; Hughes, C.; Honigmann, R.; Lorber, M.; Tellides, G.; Kashgarian, M.; Bothwell, A.; et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 9191–9196. [Google Scholar] [CrossRef]
- Liamas, E.; Black, R.; Mulheran, P.; Tampé, R.; Wieneke, R.; Thomas, O.; Zhang, Z. Probing fibronectin adsorption on chemically defined surfaces by means of single molecule force microscopy. Sci. Rep. 2020, 10, 15662. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. BioMedical Eng. OnLine 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Costantino, F.; Minischetti, M.; Locci, P.; Becchetti, E.; Roncali, L.; Dammacco, F. Exogenous heparin induces fibronectin overexpression parallel to angiogenesis in the extracellular matrix of the chick embryo chorioallantoic membrane. Tissue Cell 1997, 29, 131–136. [Google Scholar] [CrossRef]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers 2017, 9, 154. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Tang, C.-M.; Tseng, H.-J. Biocompatibility of poly(ether)urethane-gold nanocomposites. J. Biomed. Mater. Res. Part A 2006, 79, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-W.; Hsu, S.-H.; Wang, P.-H. Biostability and biocompatibility of poly(ether)urethane containing gold or silver nanoparticles in a porcine model. J. Biomed. Mater. Res. Part A 2008, 84, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wu, L.; Santella, R.; Hei, T. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999, 59, 5922–5926. [Google Scholar] [PubMed]
- Hung, H.-S.; Hsu, S.-H. The response of endothelial cells to polymer surface composed of nanometric micelles. New Biotechnol. 2009, 25, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Avraamides, C.; Garmy-Susini, B.; Varner, J. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.; Cheresh, D. Role of integrins in cell invasion and migration. Nat. Rev. Cancer. 2002, 2, 91–100. [Google Scholar] [CrossRef]
- Fang, L.-J.; Guo, S.; Wang, Q.-X. Expression of MMP-2 during differentiation of vascular endothelial cell from bone marrow mesenchymal stem cell in porcine. Shanghai Kou Qiang Yi Xue = Shanghai J. Stomatol. 2010, 19, 270–274. [Google Scholar]
- Hattori, K.; Tashiro, Y. Role of MMP-9 in hematopoietic stem cell niche. Seikagaku J. Jpn. Biochem. Soc. 2010, 82, 979–984. [Google Scholar]
- Tanasie, G.; Schuszler, L.; Şereş, M.; Igna, C. Techniques for Dog Bone Marrow Stromal Cells Sampling, Culturing, Differentiation and Loading Scaffolds. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2008, 65, 177–181. [Google Scholar]
- Pittenger, M.; Mackay, A.; Beck, S.; Jaiswal, R.; Douglas, R.; Mosca, J.; Moorman, M.; Simonetti, D.; Craig, S.; Marshak, D. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Binarova, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Ziman, M. Nestin structure and predicted function in cellular cytoskeletal organisation. ECU Publ. 2005, 20, 665–671. [Google Scholar] [CrossRef]
- Parikka, V.; Väänänen, A.; Risteli, J.; Salo, T.; Sorsa, T.; Väänänen, K.; Lehenkari, P. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 438–447. [Google Scholar] [CrossRef]
- Son, D.; Choi, T.; Yeo, H.; Kim, J.; Han, K. The Effect of Centrifugation Condition on Mature Adipocytes and Adipose Stem Cell Viability. Ann. Plast. Surg. 2013, 72, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.; Kuo, C.; Taboas, J.; Tuan, R. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J. Cell. Biochem. 2009, 107, 714–722. [Google Scholar] [CrossRef]
- Alhosseini, S.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar] [CrossRef]
- Hanna, H.; Mir, L.; Andre, F. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Flynn, L.; Woodhouse, K. Adipose tissue engineering with cells in engineered matrices. Organogenesis 2008, 4, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal Stem Cells Can Be Differentiated Into Endothelial Cells In Vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]




| A. Size (μm) | |||
| Materials | 8 h | 24 h | 48 h |
| Control (TCPS) | 10.7 ± 1.2 | 12.7 ± 0.6 | 15.3 ± 0.6 |
| FN | 15.3 ± 1.5 * | 17.0 ± 1.0 * | 17.7 ± 1.5 |
| FN-Au | 21.0 ± 1.0 **,## | 22.3 + 2.1 **,# | 23.7 ± 1.5 **,## |
| Col | 14.3 ± 1.5 ** | 15.7 ± 0.6 * | 16.7 ± 0.6 * |
| Col-Au | 18.0 + 2.0 * | 17.3 ± 0.6 **,# | 22.0 ± 2.0 **,# |
| B. Area (μm2) | |||
| Materials | 8 h | 24 h | 48 h |
| Control (TCPS) | 22.0 ± 1.0 | 24.7 ± 0.6 | 28.3 ± 0.6 |
| FN | 23.7 ± 1.5 | 25.7 ± 1.5 | 30.7 ± 0.6 ** |
| FN-Au | 26.3 ± 1.2 *,## | 28.7 ± 0.6 * | 34.3 ± 1.5 *,# |
| Col | 23.7 ± 0.6 | 25.7 ± 1.2 | 28.0 ± 1.0 |
| Col-Au | 25.3 ± 0.6 *,# | 28.3 ± 1.2 *,# | 32.3 ± 1.5 *,# |
| Materials | The Number of Monocytes (× 104) | The Number of Macrophages (× 104) | Conversion (%) |
|---|---|---|---|
| Control (TCPS) | 52 ± 4.00 | 11.3 ± 2.08 | 17.9 ± 0.19 |
| FN | 46 ± 4.50 | 4.3 ± 1.52 | 8.54 ± 0.15 ** |
| FN-Au | 53 ± 8.50 | 2.3 ± 0.57 | 4.16 ± 0.01 ***,## |
| Col | 51 ± 8.00 | 4.0 ± 1.00 | 7.27 ± 0.09 *** |
| Col-Au | 55 ± 5.68 | 2.3 ± 1.15 | 4.01 ± 0.07 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-S.; Shen, C.-C.; Wu, J.-T.; Yueh, C.-Y.; Yang, M.-Y.; Yang, Y.-C.; Cheng, W.-Y. Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites. Int. J. Mol. Sci. 2024, 25, 7241. https://doi.org/10.3390/ijms25137241
Hung H-S, Shen C-C, Wu J-T, Yueh C-Y, Yang M-Y, Yang Y-C, Cheng W-Y. Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites. International Journal of Molecular Sciences. 2024; 25(13):7241. https://doi.org/10.3390/ijms25137241
Chicago/Turabian StyleHung, Huey-Shan, Chiung-Chyi Shen, Jyun-Ting Wu, Chun-Yu Yueh, Meng-Yin Yang, Yi-Chin Yang, and Wen-Yu Cheng. 2024. "Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites" International Journal of Molecular Sciences 25, no. 13: 7241. https://doi.org/10.3390/ijms25137241
APA StyleHung, H.-S., Shen, C.-C., Wu, J.-T., Yueh, C.-Y., Yang, M.-Y., Yang, Y.-C., & Cheng, W.-Y. (2024). Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites. International Journal of Molecular Sciences, 25(13), 7241. https://doi.org/10.3390/ijms25137241






