Comparative Phenotypic and Transcriptomic Analyses Provide Novel Insights into the Molecular Mechanism of Seed Germination in Response to Low Temperature Stress in Alfalfa
Abstract
:1. Introduction
2. Results
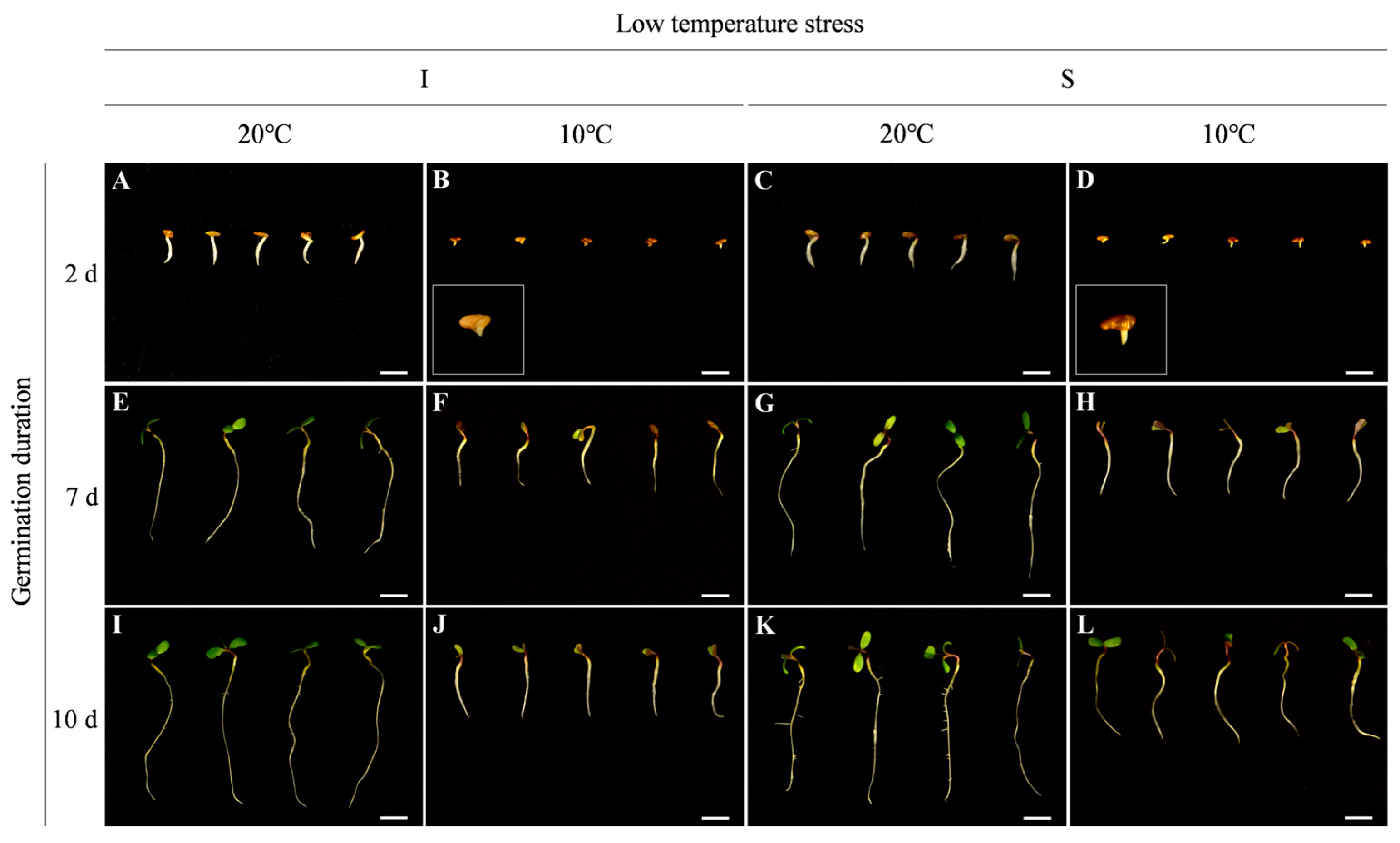
2.1. Morphological Differences in Seed Germination of Two Alfalfa Cultivars under Low Temperature Stress
2.2. Overview of Transcriptomic Analysis
2.3. Identification of DEGs in Response to Low Temperature Stress
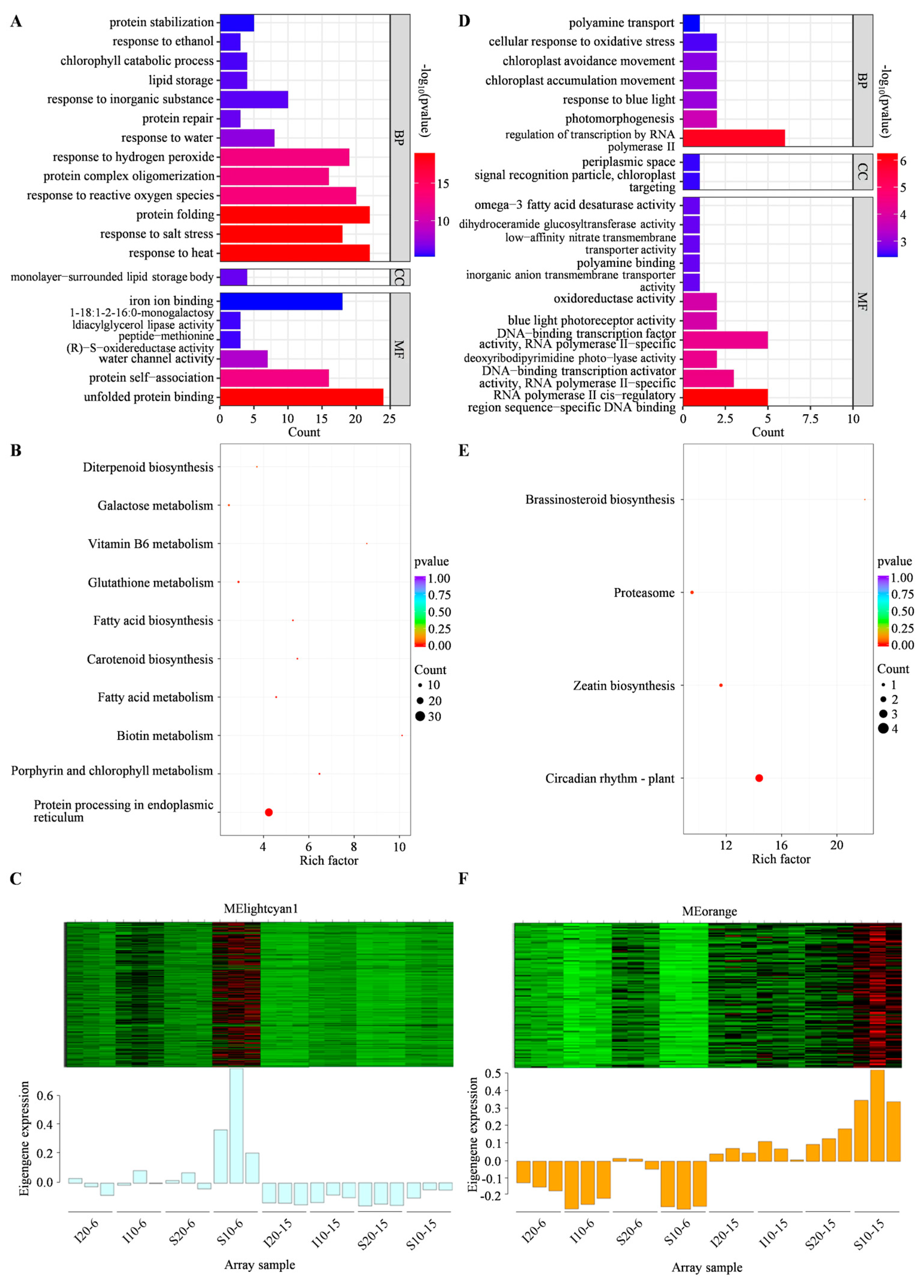
2.4. GO Functional Enrichment Analysis
2.5. KEGG Enrichment Analysis of DEGs
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
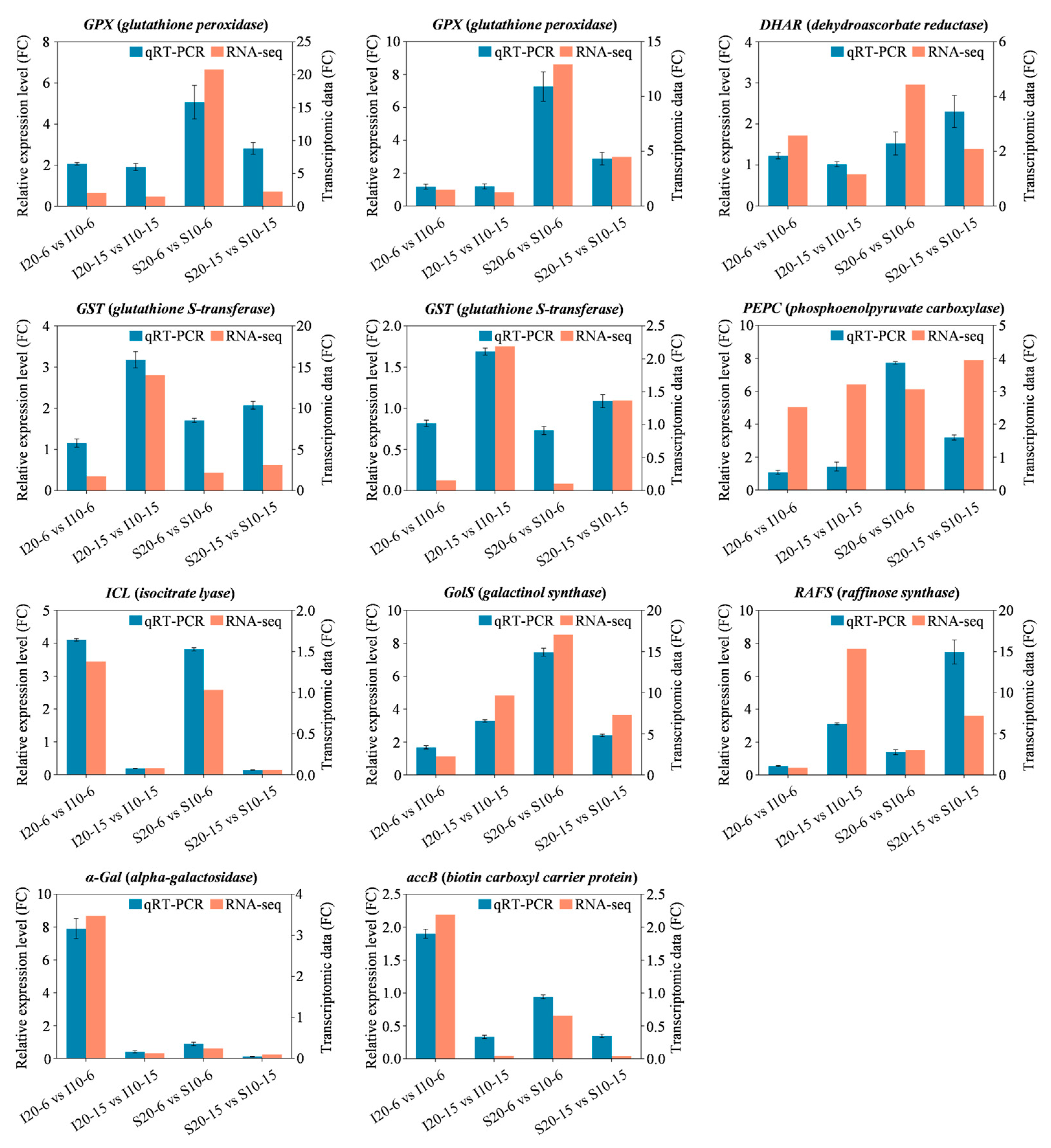
2.7. The qRT-PCR Validation of DEGs in Alfalfa Seed Germination under Low Temperature Stress
3. Discussion
3.1. Differences in Low Temperature Responses of Two Alfalfa Cultivars during Seed Germination
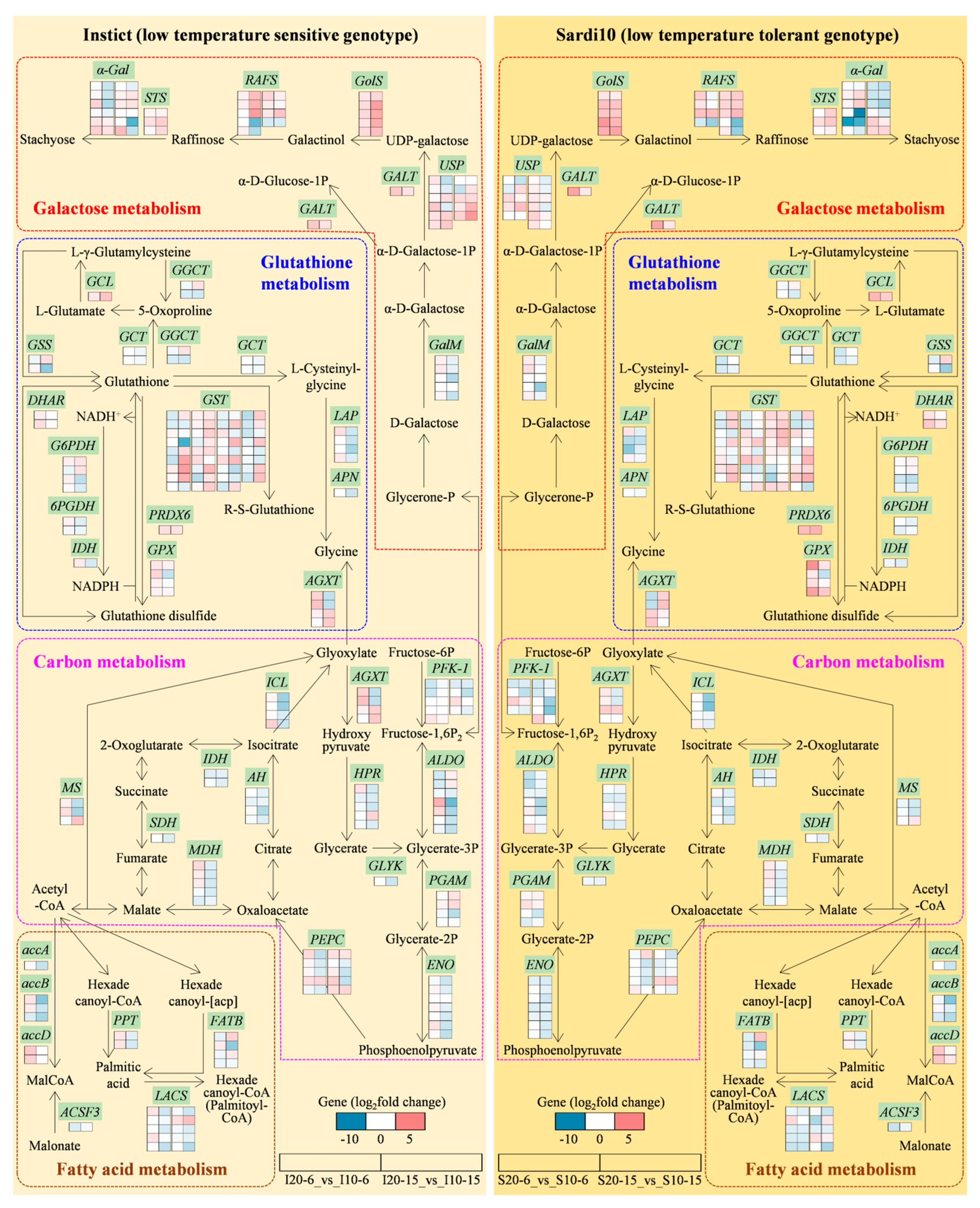
3.2. Galactose Metabolism Involved in Alfalfa Seed Germination in Response to Low Temperature Stress
3.3. Carbon Metabolism Involved in Alfalfa Seed Germination in Response to Low Temperature Stress
3.4. Fatty Acid Metabolism Involved in Alfalfa Seed Germination in Response to Low Temperature Stress
3.5. Glutathione Metabolism Involved in Alfalfa Seed Germination in Response to Low Temperature Stress
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination Characteristics under Low Temperature Stress
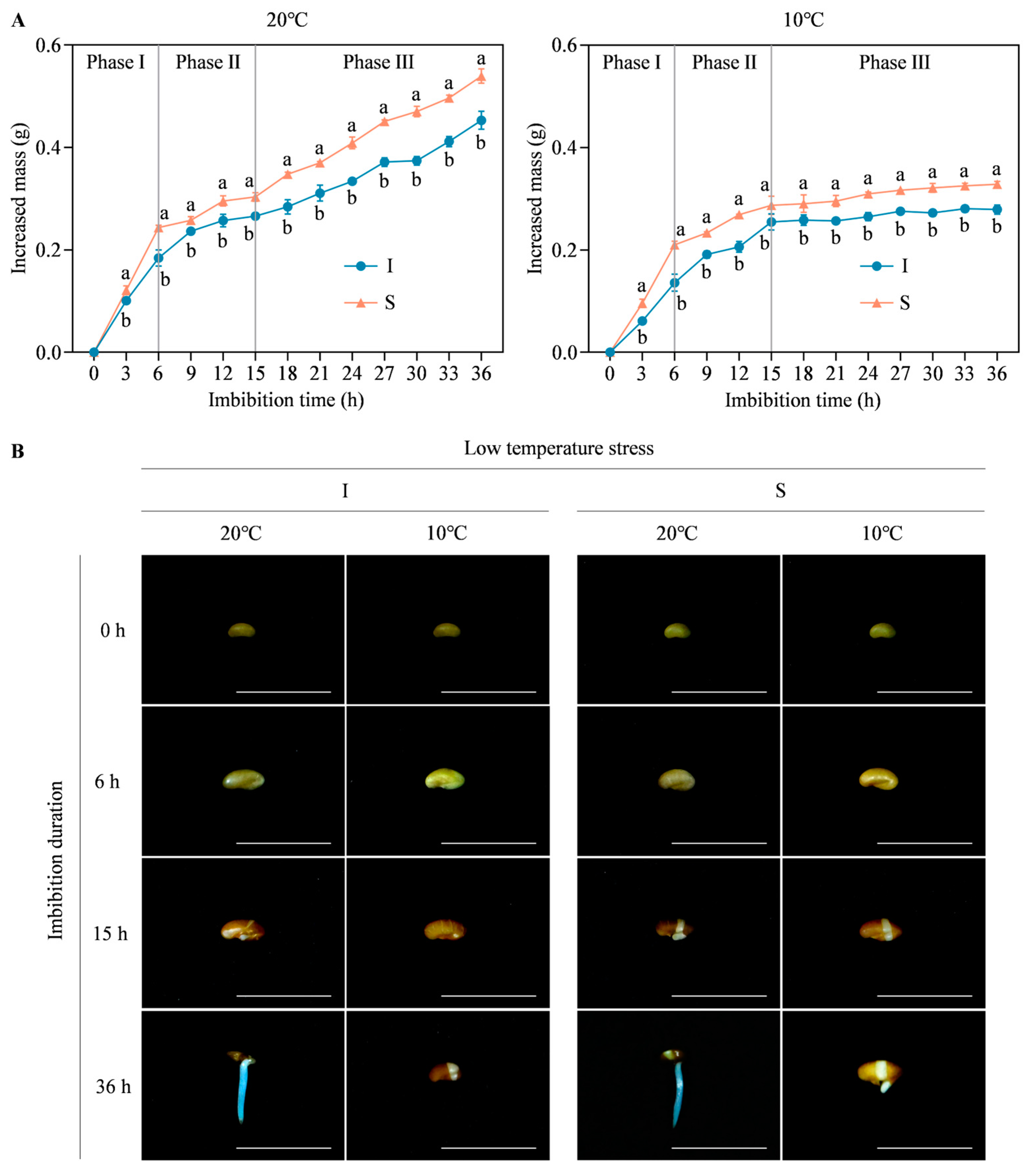
4.3. Establishment of Seed Germination Phase under Low Temperature Stress
4.4. Seed Sample Preparation
4.5. RNA Isolation, cDNA Library Construction, and Sequencing
4.6. The De Novo Assembly and Functional Annotation
4.7. Identification and Enrichment Analysis of Differentially Expressed Genes (DEGs)
4.8. WGCNA Analysis
4.9. Validation of Key Candidate DEGs by qRT-PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaafar, Y.Z.A.; Richert-Pöggeler, K.R.; Maaß, C.; Vetten, H.-J.; Ziebell, H. Characterisation of a novel nucleorhabdovirus infecting alfalfa (Medicago sativa). Virol. J. 2019, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, N.; Wang, X.; Zhang, Y.; Xie, Y. Alfalfa carbon and nitrogen sequestration patterns and effects of temperature and precipitation in three agro-pastoral ecotones of northern China. PLoS ONE 2012, 7, e50544. [Google Scholar] [CrossRef]
- Brouwer, D.J.; Duke, S.H.; Osborn, T.C. Mapping genetic factors associated with winter hardiness, fall growth, and freezing injury in autotetraploid alfalfa. Crop Sci. 2000, 40, 1387–1396. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J. Cold stress signaling networks in Arabidopsis. J. Plant Biol. 2013, 56, 69–76. [Google Scholar] [CrossRef]
- Peng, M.; Chang, Y.; Chu, G.; Wang, M. Low-temperature tolerance and transcriptome analyses during seed germination of Anabasis aphylla. J. Plant Interact. 2019, 14, 254–264. [Google Scholar] [CrossRef]
- Cui, G.; Chai, H.; Yin, H.; Yang, M.; Hu, G.; Guo, M.; Yi, R.; Zhang, P. Full-length transcriptome sequencing reveals the low-temperature-tolerance mechanism of Medicago falcata roots. BMC Plant Biol. 2019, 19, 575. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Wen, D.; Zhang, C. Dynamic changes in seed germination under low-temperature stress in maize. Int. J. Mol. Sci. 2022, 23, 5495. [Google Scholar] [CrossRef]
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, Q.; Yu, T.; Li, S.; Li, S.; Li, Y.; Sun, Y.; Ren, H.; Zhang, J.; Zhao, Y.; et al. ZmG6PDH1 in glucose-6-phosphate dehydrogenase family enhances cold stress tolerance in maize. Front. Plant Sci. 2023, 14, 1116237. [Google Scholar]
- Raju, S.K.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef]
- Seki, M.; Kamei, A.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Molecular responses to drought, salinity and frost: Common and different paths for plant protection. Curr. Opin. Biotechnol. 2003, 14, 194–199. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, Y.; Liu, J.; Li, Z.; Fu, D.; Wu, S.; Li, M.; Yang, Z.; Shi, Y.; Lai, J.; et al. Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat. Plants 2022, 8, 1176–1190. [Google Scholar] [CrossRef]
- Li, Z.; Fu, D.; Wang, X.; Zeng, R.; Zhang, X.; Tian, J.; Zhang, S.; Yang, X.; Tian, F.; Lai, J.; et al. The transcription factor bZIP68 negatively regulates cold tolerance in maize. Plant Cell 2022, 34, 2833–2851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat. Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Zhao, W.; Song, J.; Wang, M.; Chen, X.; Du, B.; An, Y.; Zhang, L.; Wang, D.; Guo, C. Alfalfa MsATG13 confers cold stress tolerance to plants by promoting autophagy. Int. J. Mol. Sci. 2023, 24, 12033. [Google Scholar] [CrossRef]
- Du, B.; Chen, N.; Song, L.; Wang, D.; Cai, H.; Yao, L.; Li, X.; Guo, C. Alfalfa (Medicago sativa L.) MsCML46 gene encoding calmodulin-like protein confers tolerance to abiotic stress in tobacco. Plant Cell Rep. 2021, 40, 1907–1922. [Google Scholar] [CrossRef]
- Yu, S.; Wu, J.; Sun, Y.; Zhu, H.; Sun, Q.; Zhao, P.; Huang, R.; Guo, Z. A calmodulin-like protein (CML10) interacts with cytosolic enzymes GSTU8 and FBA6 to regulate cold tolerance. Plant Physiol. 2022, 190, 1321–1333. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.M.; Wang, R.; Xia, J.Q.; Li, C.; Xu, Q.H.; Cang, J.; Wang, Y.Y.; Zhang, D. JA-induced TaMPK6 enhanced the freeze tolerance of Arabidopsis thaliana through regulation of ICE-CBF-COR module and antioxidant enzyme system. Plant Sci. 2023, 329, 111621. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Finch-Savage, W.E.; Grappin, P.; Job, D. Post-genomics dissection of seed dormancy and germination. Trends Plant Sci. 2008, 13, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, L.; Zeng, P.; He, Y.; Zhou, R.; Zhang, H.; Wang, Z. Identification of genes involved in rice seed priming in the early imbibition stage. Plant Biol. 2017, 19, 61–69. [Google Scholar] [CrossRef]
- He, D.; Han, C.; Yao, J.; Shen, S.; Yang, P. Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics 2011, 11, 2693–2713. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Sano, N.; Malabarba, J.; Chen, Z.; Gaillard, S.; Windels, D.; Verdier, J. Chromatin dynamics associated with seed desiccation tolerance/sensitivity at early germination in Medicago truncatula. Front. Plant Sci. 2022, 13, 1059493. [Google Scholar] [CrossRef]
- Smolikova, G.; Strygina, K.; Krylova, E.; Leonova, T.; Frolov, A.; Khlestkina, E.; Medvedev, S. Transition from seeds to seedlings: Hormonal and epigenetic aspects. Plants 2021, 10, 1884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, P.; Zou, C.; Chen, Z.; Yuan, G.; Gao, S.; Pan , G.; Shen , Y.; Ma , L. Comprehensive analysis of transcriptional data on seed germination of two maize inbred lines under low-temperature conditions. Plant Physiol. Biochem. 2023, 201, 107874. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, S.; Liu, S.; Chen, J.; Ma, H.; Cui, Z.; Zhang, X.; Ge, C.; Liu, R.; Li, Y.; et al. Comparative transcriptome analysis provides insights into the seed germination in cotton in response to chilling stress. Int. J. Mol. Sci. 2020, 21, 2067. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Jiang, G.; Zhao, J.; Wang, W.; Hong, X. Integrated analysis of transcriptome and metabolome reveals insights for low-temperature germination in hybrid rapeseeds (Brassica napus L.). J. Plant Physiol. 2023, 291, 154120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Miao, Y.; Zhang, L.J.; Zhao, Y.Y.; Wang, J. Mitochondrial structure and respiratory metabolism in cold resistance of alfalfa seedling root. Theor. Exp. Plant Phys. 2023, 35, 319–330. [Google Scholar] [CrossRef]
- Lee, K.-W.; Rahman, M.; Choi, G.; Kim, K.-Y.; Ji, H.; Kim, J.; Lee, S.-H. Identification of cold and heat-induced differentially expressed genes in alfalfa (Medicago sativa L.) leaves. J. Anim. Plant Sci. 2017, 27, 1231–1237. [Google Scholar]
- Jiang, G.; Wang, S.; Xie, J.; Tan, P.; Han, L. Discontinuous low temperature stress and plant growth regulators during the germination period promote roots growth in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2023, 197, 107624. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Hu, X.; Wang, G.; Du, X.; Li, L.; Wang, F.; Fu, J.; Wang, G.; Wang, J.; et al. Transcriptome analysis of near-isogenic lines provides novel insights into genes associated with seed low-temperature germination ability in maize (Zea mays L.). Plants 2022, 11, 887. [Google Scholar] [CrossRef]
- Thapa, R.; Tabien, R.E.; Johnson, C.D.; Septiningsih, E.M. Comparative transcriptomic analysis of germinating rice seedlings to individual and combined anaerobic and cold stress. BMC Genom. 2023, 24, 185. [Google Scholar] [CrossRef]
- Klos, K.L.E.; Brummer, E.C. Response of six alfalfa populations to selection under laboratory conditions for germination and seedling vigor at low temperatures. Crop Sci. 2000, 40, 959–964. [Google Scholar] [CrossRef]
- Ahmed, L.; Durand, J.-L.; Escobar-Gutiérrez, A. Genetic diversity of alfalfa (Medicago sativa) in response to temperature during germination. Seed Sci. Technol. 2019, 47, 351–356. [Google Scholar] [CrossRef]
- Cheshmi, M.; Khajeh-Hosseini, M. Effect of temperature on length of the lag period and its relationship with field performance of alfalfa (Medicago sativa) seeds. Seed Sci. Technol. 2018, 46, 317–326. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Zhao, G.; Gong, X.; Dang, K.; Yang, Q.; Feng, B. Transcriptome analysis reveals the mechanism associated with dynamic changes in fatty acid and phytosterol content in foxtail millet (Setaria italica) during seed development. Food Res. Int. 2021, 145, 110429. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- Ren, X.X.; Xue, J.Q.; Wang, S.L.; Xue, Y.Q.; Zhang, P.; Jiang, H.D.; Zhang, X.X. Proteomic analysis of tree peony (Paeonia ostii ‘Feng Dan’) seed germination affected by low temperature. J. Plant Physiol. 2018, 224–225, 56–67. [Google Scholar]
- Gangl, R.; Tenhaken, R. Raffinose family oligosaccharides act as galactose stores in seeds and are required for rapid germination of Arabidopsis in the dark. Front. Plant Sci. 2016, 7, 1115. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, M.; Althammer, M.; Foissner, I.; Tenhaken, R. Galactose induces formation of cell wall stubs and cell death in Arabidopsis roots. Planta 2022, 256, 26. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Liu, Y.; Li, X.; Hao, G.; Han, Q.; Dirk, L.M.A.; Downie, A.B.; Ruan, Y.L.; Wang, J.; et al. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. J. Biol. Chem. 2020, 295, 8064–8077. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, T.; Zhang, C.; Li, X.; Wang, C.; Zhang, Y.; Li, T.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 2019, 100, 128–142. [Google Scholar] [CrossRef]
- Han, Q.; Qi, J.; Hao, G.; Zhang, C.; Wang, C.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmDREB1A regulates raffinose synthase controlling raffinose accumulation and plant chilling stress tolerance in maize. Plant Cell Physiol. 2020, 61, 331–341. [Google Scholar] [CrossRef]
- Jing, Y.; Lang, S.; Wang, D.; Xue, H.; Wang, X.F. Functional characterization of galactinol synthase and raffinose synthase in desiccation tolerance acquisition in developing Arabidopsis seeds. J. Plant Physiol. 2018, 230, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Wang, T.; Lu, S.; Zhao, Y.; Li, X.; Guo, Z. A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiol. Plant. 2013, 149, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Lugo, I.; Leopold, A.C. Changes in soluble carbohydrates during seed storage. Plant Physiol. 1992, 98, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, L.; Marchant, L.; Berninsone, P.; Hirschberg, C.B.; Silva, H.; Orellana, A. Transport of UDP-galactose in plants: Identification and functional characterization of AtUTr1, an arabidopsis thaliana UDP-galactose/UDP-glucose transporter. J. Biol. Chem. 2002, 277, 32923–32929. [Google Scholar] [CrossRef] [PubMed]
- Fekete, E.; Seiboth, B.; Kubicek, C.P.; Szentirmai, A.; Karaffa, L. Lack of aldose 1-epimerase in Hypocrea jecorina (anamorph Trichoderma reesei): A key to cellulase gene expression on lactose. Proc. Natl. Acad. Sci. USA 2008, 105, 7141–7146. [Google Scholar] [CrossRef]
- Althammer, M.; Regl, C.; Herburger, K.; Blöchl, C.; Voglas, E.; Huber, C.G.; Tenhaken, R. Overexpression of UDP-sugar pyrophosphorylase leads to higher sensitivity towards galactose, providing new insights into the mechanisms of galactose toxicity in plants. Plant J. 2022, 109, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Tak, Y.; Bhatia, S.; Asthir, B.; Lorenzo, J.M.; Amarowicz, R. Crosstalk during the carbon-nitrogen cycle that interlinks the biosynthesis, mobilization and accumulation of seed storage reserves. Int. J. Mol. Sci. 2021, 22, 12032. [Google Scholar] [CrossRef]
- Santos-Filho, P.R.; Saviani, E.E.; Salgado, I.; Oliveira, H.C. The effect of nitrate assimilation deficiency on the carbon and nitrogen status of Arabidopsis thaliana plants. Amino Acids 2014, 46, 1121–1129. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. The role of TCA cycle enzymes in plants. Adv. Biol. 2023, 7, 2200238. [Google Scholar] [CrossRef]
- Martins, S.J.; Medeiros, F.H.V.; Lakshmanan, V.; Bais, H.P. Impact of seed exudates on growth and biofilm formation of Bacillus amyloliquefaciens ALB629 in common bean. Front. Microbiol. 2017, 8, 2631. [Google Scholar] [CrossRef] [PubMed]
- Crecelius, F.; Streb, P.; Feierabend, J. Malate metabolism and reactions of oxidoreduction in cold-hardened winter rye (Secale cereale L.) leaves. J. Exp. Bot. 2003, 54, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Fedorin, D.N.; Igamberdiev, A.U. Light-dependent expression and promoter methylation of the genes encoding succinate dehydrogenase, fumarase, and NAD-malate dehydrogenase in maize (Zea mays L.) leaves. Int. J. Mol. Sci. 2023, 24, 10211. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Z.; Yang, H.; Hu, D.; Wu, Y.; Xue, J.; Guo, D.; Xu, S. Characterization of the isocitrate dehydrogenase gene family and their response to drought stress in maize. Plants 2023, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Wang, Y.; Cui, Y.; Liu, Z.; Hua, J. RNA interference of GhPEPC2 enhanced seed oil accumulation and salt tolerance in Upland cotton. Plant Sci. 2018, 271, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ballesta, I.; Baena, G.; Gandullo, J.; Wang, L.; She, Y.M.; Plaxton, W.C.; Echevarría, C. New insights into the post-translational modification of multiple phosphoenolpyruvate carboxylase isoenzymes by phosphorylation and monoubiquitination during sorghum seed development and germination. J. Exp. Bot. 2016, 67, 3523–3536. [Google Scholar] [CrossRef]
- Qian, B.; Li, X.; Liu, X.; Chen, P.; Ren, C.; Dai, C. Enhanced drought tolerance in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca2+. J. Plant Physiol. 2015, 175, 9–20. [Google Scholar] [CrossRef]
- de la Osa, C.; Pérez-López, J.; Feria, A.B.; Baena, G.; Marino, D.; Coleto, I.; Pérez-Montaño, F.; Gandullo, J.; Echevarría, C.; García-Mauriño, S.; et al. Knock-down of phosphoenolpyruvate carboxylase 3 negatively impacts growth, productivity, and responses to salt stress in sorghum (Sorghum bicolor L.). Plant J. 2022, 111, 231–249. [Google Scholar] [CrossRef]
- Pan, L.-J.; Zhang, J.; Chi, X.-Y.; Chen, N.; Chen, M.L.; Wang, M.; Wang, T.Y.; Yang, Z.; Zhang, Z.X.; Wan, Y.; et al. The antisense expression of AhPEPC1 increases seed oil production in peanuts (Arachis hypogaea L.). Grasas Y Aceites 2016, 67, 164. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Guo, X.; Jin, S.; Zhang, X. Metabolic engineering of cottonseed oil biosynthesis pathway via RNA interference. Sci. Rep. 2016, 6, 33342. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–78. [Google Scholar] [CrossRef]
- de los Reyes, B.G.; Myers, S.J.; McGrath, J.M. Differential induction of glyoxylate cycle enzymes by stress as a marker for seedling vigor in sugar beet (Beta vulgaris). Mol. Genet. Genom. 2003, 269, 692–698. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, W.; Li, D.; Jin, Y.; Rong, X.; Xu, Y.; Wang, R. Characterization of a salt-resistant isocitrate lyase gene from mangrove wetland using shotgun metagenomic sequencing. Lett. Appl. Microbiol. 2023, 76, ovad044. [Google Scholar] [CrossRef]
- Lee, C.-H.; Olson, P.; Evans, R.M. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Baud, S.; Guyon, V.; Kronenberger, J.; Wuillème, S.; Miquel, M.; Caboche, M.; Lepiniec, L.; Rochat, C. Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J. 2003, 33, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Waldrop, G.L. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 2002, 41, 407–435. [Google Scholar] [CrossRef]
- Megha, S.; Wang, Z.; Kav, N.N.V.; Rahman, H. Genome-wide identification of biotin carboxyl carrier subunits of acetyl-CoA carboxylase in Brassica and their role in stress tolerance in oilseed Brassica napus. BMC Genom. 2022, 23, 707. [Google Scholar] [CrossRef]
- Greene, J.G.; Greenamyre, J.T. Characterization of the excitotoxic potential of the reversible succinate dehydrogenase inhibitor malonate. J. Neurochem. 1995, 64, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.M.; Fulda, M.S.; Browse, J.A. Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 2002, 129, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Tan, W.J.; Yang, Y.C.; Tan, Y.F.; Zhou, Y.; Zhou, D.M.; Xiao, S.; Chen, Q.F. Long-chain acyl-CoA synthetase LACS2 contributes to submergence tolerance by modulating cuticle permeability in Arabidopsis. Plants 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Zhang, Y.L.; Hu, X.; Xiao, X.; Wang, G.L.; You, C.X.; Li, Y.Y.; Hao, Y.J. An apple long-chain acyl-CoA synthetase, MdLACS4, induces early flowering and enhances abiotic stress resistance in Arabidopsis. Plant Sci. 2020, 297, 110529. [Google Scholar] [CrossRef] [PubMed]
- Asthir, B.; Kaur, G.; Kaur, B. Convergence of pathways towards ascorbate–glutathione for stress mitigation. J. Plant Biol. 2020, 63, 243–257. [Google Scholar] [CrossRef]
- Pasternak, T.; Asard, H.; Potters, G.; Jansen, M.A.K. The thiol compounds glutathione and homoglutathione differentially affect cell development in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2014, 74, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Frendo, P.; Jiménez, M.J.; Mathieu, C.; Duret, L.; Gallesi, D.; Van de Sype, G.; Hérouart, D.; Puppo, A. A Medicago truncatula homoglutathione synthetase is derived from glutathione synthetase by gene duplication. Plant Physiol. 2001, 126, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Ohkama-Ohtsu, N.; Oikawa, A.; Zhao, P.; Xiang, C.; Saito, K.; Oliver, D.J. A gamma-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiol. 2008, 148, 1603–1613. [Google Scholar] [CrossRef]
- Leterrier, M.; Barroso, J.B.; Valderrama, R.; Palma, J.M.; Corpas, F.J. NADP-dependent isocitrate dehydrogenase from Arabidopsis roots contributes in the mechanism of defence against the nitro-oxidative stress induced by salinity. Sci. World J. 2012, 2012, 694740. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; An, Y.C.; Zhang, H.; Meng, D.; Jin, Y.; Huo, H.; Yu, L.; Zhang, J. Identification of glutathione peroxidase gene family in Ricinus communis and functional characterization of RcGPX4 in cold tolerance. Front. Plant Sci. 2021, 12, 707127. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, M.; Teng, Y.; Jia, S.; Yu, D.; Wei, T.; Chen, C.; Song, W. Overexpression of the glutathione peroxidase 5 (RcGPX5) gene from Rhodiola crenulata increases drought tolerance in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 1950. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Nie, Y.; Cui, K.; Sun, J. Integrative transcriptome and metabolome profiles reveal common and unique pathways involved in seed initial imbibition under artificial and natural salt stresses during germination of halophyte quinoa. Front. Plant Sci. 2022, 13, 853326. [Google Scholar] [CrossRef] [PubMed]
- Hernández Estévez, I.; Rodríguez Hernández, M. Plant glutathione S-transferases: An overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Duan, X.; Yu, X.; Wang, Y.; Fu, W.; Cao, R.; Yang, L.; Ye, X. Genome-wide identification and expression analysis of glutathione S-transferase gene family to reveal their role in cold stress response in cucumber. Front. Genet. 2022, 13, 1009883. [Google Scholar] [CrossRef] [PubMed]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2020. [Google Scholar]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The chromosome-level genome sequence of the autotetraploid alfalfa and resequencing of core germplasms provide genomic resources for alfalfa research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Wu, S.; Zhu, Y.; Chen, Y.; He, F. Integrated nr database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–72. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Saito, R.; Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Lotia, S.; Pico, A.R.; Bader, G.D.; Ideker, T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lv, Y.; Sun, Q.; Yao, X.; Yan, H. Comparative Phenotypic and Transcriptomic Analyses Provide Novel Insights into the Molecular Mechanism of Seed Germination in Response to Low Temperature Stress in Alfalfa. Int. J. Mol. Sci. 2024, 25, 7244. https://doi.org/10.3390/ijms25137244
Zhang Z, Lv Y, Sun Q, Yao X, Yan H. Comparative Phenotypic and Transcriptomic Analyses Provide Novel Insights into the Molecular Mechanism of Seed Germination in Response to Low Temperature Stress in Alfalfa. International Journal of Molecular Sciences. 2024; 25(13):7244. https://doi.org/10.3390/ijms25137244
Chicago/Turabian StyleZhang, Zhao, Yanzhen Lv, Qingying Sun, Xingjie Yao, and Huifang Yan. 2024. "Comparative Phenotypic and Transcriptomic Analyses Provide Novel Insights into the Molecular Mechanism of Seed Germination in Response to Low Temperature Stress in Alfalfa" International Journal of Molecular Sciences 25, no. 13: 7244. https://doi.org/10.3390/ijms25137244
APA StyleZhang, Z., Lv, Y., Sun, Q., Yao, X., & Yan, H. (2024). Comparative Phenotypic and Transcriptomic Analyses Provide Novel Insights into the Molecular Mechanism of Seed Germination in Response to Low Temperature Stress in Alfalfa. International Journal of Molecular Sciences, 25(13), 7244. https://doi.org/10.3390/ijms25137244




