Abstract
Foods enriched with insects can potentially prevent several health disorders, including cardiovascular diseases, by reducing inflammation and improving antioxidant status. In this study, Tenebrio molitor and Gryllus assimilis were selected to determine the effect on the development of atherosclerosis in ApoE/LDLR−/− mice. Animals were fed AIN-93G-based diets (control) with 10% Tenebrio molitor (TM) and 10% Gryllus assimilis (GA) for 8 weeks. The nutritional value as well as antioxidant activity of selected insects were determined. The lipid profile, liver enzyme activity, and the fatty acid composition of liver and adipose tissue of model mice were evaluated. Quantitative analysis of atherosclerotic lesions in the entire aorta was performed using the en face method, and for aortic roots, the cross-section method was used. The antioxidant status of the GA cricket was significantly higher compared to the TM larvae. The results showed that the area of atherosclerosis (en face method) was not significantly different between groups. Dietary GA reduced plaque formation in the aortic root; additionally, significant differences were observed in sections at 200 and 300 µm compared to other groups. Furthermore, liver enzyme ALT activity was lower in insect-fed groups compared to the control group. The finding suggests that a diet containing edible insect GA potentially prevents atherosclerotic plaque development in the aortic root, due to its high antioxidant activity.
1. Introduction
The prevalence of cardiovascular diseases (CVDs) is increasing worldwide, and it is becoming a widespread public health issue [1]. One of the leading causes of cardiovascular diseases is atherosclerosis, an inflammation of the arteries associated with lipid and other metabolic alterations.
Atherosclerosis is a progressive disease characterized by a gradual buildup of fatty deposits (plaque) within artery walls. This plaque narrows the arteries, hindering blood flow and potentially leading to serious complications like heart attack and stroke [2]. The development of atherosclerosis is linked to risk factors like high levels of low-density lipoprotein cholesterol (LDL) [3]. Furthermore, deficiencies in Apolipoprotein E (ApoE) and the LDL receptor (LDLR) are known to contribute to the disease. ApoE and LDLR play crucial roles in clearing LDL cholesterol from the bloodstream [4]. ApoE exerts anti-atherogenic effects through beneficial changes in plasma lipids and direct action on the artery. In ApoE/LDLR−/− mice (animal model of atherosclerosis), a high level of plasma cholesterol is observed, which contributes to promoting the development of atherosclerotic plaque [4,5,6]. It has been indicated that instead of drug therapy, changing one’s diet and adopting a healthy lifestyle could prevent cardiovascular diseases, since diet significantly influences cardiovascular outcomes [7,8].
Evidence shows that consuming insects can reduce cytokines and modulate particular transcription factors to restore antioxidant and inflammatory status [9]. Edible insects provide various nutrients essential for human health, such as vitamins, minerals, fiber, protein, and lipids. These properties could enable them to improve gastrointestinal health, boost immunological function, decrease the risk of bacterial infection, and decrease chronic inflammation [10,11]. Studies on insects revealed decreased pro-inflammatory cytokine levels such as TNF-α (Tumor Necrosis Factor-alpha) in animals and humans. TNF-α is a critical pro-inflammatory cytokine linked to different diseases’ pathological processes [9,12,13].
Insects are a good source of crude fiber, most predominately in the form of chitin. Chitin and its degraded products have been shown to exert antimicrobial, antioxidant, anti-inflammatory, anticancer, and immunostimulatory activity [14,15].
A study in rats found that carbohydrate glycosaminoglycan derived from crickets showed a significant anti-inflammatory effect against chronic arthritis by lowering C-reactive protein (CRP) and rheumatoid factor, and thus suppressing a variety of inflammatory biomarkers [16,17]. Furthermore, previous research has shown that phytochemicals in mealworms have anti-obesity properties in mice. Obesity is one of the leading causes of inflammation in the body [18]. Additionally, creating biostimulants known as protein hydrolysates, edible insects, and foods enriched with insects may potentially prevent several health problems, including diabetes, hypertension, and cardiac issues [19].
Around the world, edible insects have been used as a food source, and in recent years, demand has grown in Europe. Within the European Union (EU), insects and insect-derived products are classified as novel foods and the manufacturing of insect products is governed by the Novel Foods Regulation (EU) 2015/2283, 25 November 2015 [20,21]. Placing freeze-dried and powder forms of Tenebrio molitor (TM, yellow mealworms) larvae on the market has been authorized by the Regulations (EU) 2021/882 and 2022/169. Tenebrio molitor and Gryllus assimilis (GA, Jamaican field crickets) are on the worldwide list of recorded edible insects [22].
Yellow mealworm larvae and Jamaican field crickets are gaining popularity as a potential source of nutrients and functional compounds. Research emphasizes their richness in essential amino acids, protein, and a diverse range of beneficial fatty acids [23,24,25]. The nutritional value of insects depends on many factors including rearing temperature, feed composition, strain, developmental stage, or sex [26]. Regarding the model species selected for this study, TM, belonging among the most researched edible insects, may vary in lipids 6.1–58.2% DM, 2.8–8.1% fiber DM, and proteins 38.9–76.2% DM. TM larvae reared on diets with a higher protein content tend to exhibit increased crude protein levels [27]. GA contains 62.7–65.5% of proteins, 20.8–33.0% of lipids, and 1.4–8.4% fiber in DM. It is also known that palmitic, oleic, and linoleic acids are the major fatty acid in both species [28,29,30,31]. TM larvae and GA cricket contain significant amounts of both monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs). Wu et al. [32] reported the percentage of MUFAs (47.35 ± 1.62%) and PUFAs (31.66 ± 0.99%) in freeze-dried mealworm larvae. Oleic acid, a major MUFA, is a key component (43.77 ± 1.62%) of TM larvae oil and exhibits well-documented anti-inflammatory properties [32]. Oleic acid exerts anti-inflammatory effects by reducing pro-inflammatory cytokines and promoting the production of anti-inflammatory mediators [33]. Furthermore, freeze-dried GA is a good source of MUFAs, containing 31.10 g/100 g of fat. Blanched GA contains PUFA at 35.12 g/100 g of fat [25]. Consuming both MUFAs and PUFAs has been linked to a lower risk of cardiovascular disease [34].
Studies have shown that insect hydrolysates and peptide fractions possess significant antioxidant activity, potentially reducing free radical levels and alleviating oxidative stress in the body. Previous work by Zielińska et al. [35] reported a particularly high antiradical activity against the free radical ABTS•+ in a hydrolysate derived from mealworms [35,36]. Additionally, TM larvae are a valuable source of bioactive compounds like phenolics, tocopherols, and chitosan. Protein and protein hydrolysates from mealworms have also been linked to anti-diabetic and anti-obesity effects [37,38]. A research study on GA identified a specific peptide within its hydrolysate that could potentially inhibit HMG-CoA reductase, an enzyme involved in cholesterol production, implying potential hypocholesterolemic properties. Crickets possess a high antioxidant potential, regardless of the analysis method used. This antioxidant activity might be attributed to the high tocopherol content found in cricket [39]. In addition to their antioxidant effects, edible insects exhibit anti-inflammatory properties. A review by D’Antonino et al. [9] highlights the ability of insect-derived compounds to reduce inflammatory cytokine production and modulate specific transcription factors, key contributors to inflammatory processes.
The nutritional composition of TM and GA can also be influenced by processing techniques [25,40,41,42,43,44]. Freeze-dried TM larvae, in particular, have been shown to possess higher levels of phenolic compounds and antioxidant activities compared to other methods like oven drying and microwaving [45]. Research by Khatun et al. [25] demonstrated that freeze-dried GA crickets contain higher protein (66.63 g/100 g DM) and lower fat (21.19 g/100 g DM) compared to oven-dried and blanched samples. Other studies, such as those by Adamkova et al. [27] and Jozefiak et al. [46], found lower protein (~56 g/100 g DM) and higher fat (23–32 g/100 g DM), attributed to differences in the feed and developmental stages.
While recent nutritional and environmental aspects of edible insects have been studied [47,48], data evaluating edible insects’ effects on the onset of atherosclerosis are limited. Therefore, the aim of the experiment was to describe the effect of Tenebrio molitor larvae (TM, yellow mealworms) and Gryllus assimilis (GA, Jamaican field crickets) on the development of atherosclerosis in apolipoprotein E/low-density lipoprotein receptor-deficient mice (ApoE/LDLR−/−) mice.
2. Results
2.1. Nutritive Value and Fatty Acid Composition of Insects
The basic composition, including dry matter, ash, crude protein, fat, and chitin content was shown in Table 1. These components provide a thorough understanding of the nutritional value and structural attributes of the insects’ powder. Dry matter was similar in both insects’ powders, e.g., TM and GA. The amounts of fat and chitin were significantly lower in GA. Additionally, a significantly higher amount of crude protein was observed in GA compared to TM.

Table 1.
Basic composition of insects’ powder.
The fatty acids profiles of insects’ powder are presented in Table 2. A higher level of SFAs, mainly 16:0 and 18:0, was observed in GA. Additionally, in GA, a higher amount of PUFAs, mainly octadecadienoic acid C18:2, was observed. However, cis-9-Octadecenoic acid (18:1) and finally MUFAs were lower compared to TM.

Table 2.
Fatty acids composition of insects’ powder (in relative % of total fatty acids).
2.2. Total Polyphenols Content and Antioxidant Activity of Insects’ Powder
The total polyphenols content of insects’ powder, including polyphenols in Gallic Acid Equivalents (GAE), is shown in Table 3. The antioxidant activity, expressed as ABTS•+ and DPPH• radical scavenging activity percentages, and the Trolox equivalent antioxidant capacity (TEAC) are presented in Table 3. These metrics provide a comprehensive overview of the significant antioxidant potential of the insects. In GA, a higher antioxidant activity expressed as TEAC DPPH• (μΜTrolox/1 g DM), as well as TEAC ABTS•+ (μΜTrolox/1 g DM), was observed.

Table 3.
Total polyphenols content and antioxidant status of insects’ powder.
2.3. Composition of Experimental Diets
The standard AIN-93G (American Institute of Nutrition) rodent diet [49] was modified by replacing 10% of its content with freeze-dried insect powder. The detailed composition of these experimental diets is provided in Table 4.

Table 4.
Composition of experimental diets (g/kg).
2.4. Effect of Edible Insects on Body and Liver Weight and Biochemical Parameters in ApoE/LDLR−/− Mice
There were no significant differences between experimental groups in body weight, fasting blood glucose, and plasma lipid profile after eight weeks of feeding (Table 5). The liver weight (g/100 g b.w.) was significantly higher in the GA group compared to the TM group. A significant decrease in liver enzyme activity ALT was also found in the group receiving GA and TM insects (Figure 1).

Table 5.
Body weight (b.w.), liver weight, blood glucose, and plasma lipid profile in ApoE/LDLR−/− mice fed control and diets with edible insects (TM and GA) (n = 10).
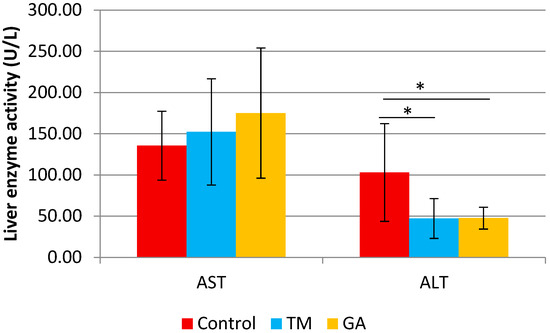
Figure 1.
The liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST) in ApoE/LDLR−/− mice fed control, TM, and GA diets (n = 10). The mean difference is significant at p < 0.05 and indicated by *.
2.5. Effect of Edible Insects on Fatty Acids Composition of Adipose Tissue and Liver in ApoE/LDLR−/− Mice
There were no changes in the proportion of saturated fatty acids (SFAs) between experimental groups in adipose tissue (Table 6). Mice fed a diet with TM had a significantly increased level of monounsaturated fatty acids (MUFAs) and decreased polyunsaturated fatty acids (PUFAs) compared to the control and GA groups. The palmitic acid (C16:0) level was significantly lower in the TM-fed group compared to the control. The linoleic acid (C18:2) level was significantly higher in the control and GA groups than in the TM-fed group.

Table 6.
Fatty acid profile (in relative % of total fatty acids) of adipose tissue in ApoE/LDLR−/− mice fed modified diets with edible insects.
The fatty acid profile of the liver showed a significantly higher share of SFAs in the GA group (Table 7). A significantly higher level of MUFAs was found in the TM group, whereas the level of PUFA was lower than in the other groups. There were no significant differences between the GA and the control group in relation to the proportions of MUFAs and PUFAs. Relatively higher levels of palmitoleic (C16:1), linoleic acid (C18:2), and α-linoleic (C18:3) acids were observed in the liver fatty acid profile of mice fed the control diet than the TM and GA groups. Furthermore, a significantly lower level of stearic acid (C18:0) was observed in the TM-fed group compared to the GA-fed group.

Table 7.
Fatty acids profile (in relative % of total fatty acids) in the liver in ApoE/LDLR−/− mice fed modified diets with edible insects.
2.6. Effect of Edible Insects on the Development of Atherosclerosis in ApoE/LDLR−/− Mice
After eight weeks of feeding ApoE/LDLR−/− mice, no significant difference in the mean area of the atherosclerotic lesion (%) in the entire aorta measured by the en face method were observed (Figure 2a). The addition of GA to the diet led to a slight decrease in plaque levels in the mice. However, this decrease was not statistically significant.
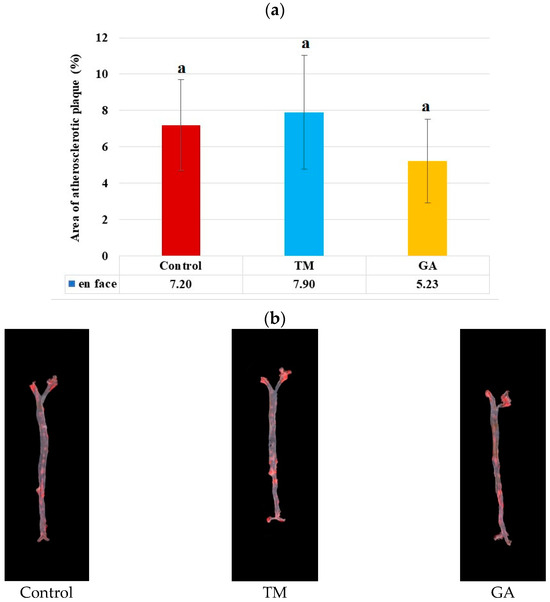
Figure 2.
(a) Area of atherosclerotic lesions (%) in the entire aorta of ApoE/LDLR−/− mice measured by the en face method (n = 10 for each group). The mean difference is significant at p < 0.05 and indicated by different letters. (b) Representative images of the aorta surface stained with Sudan IV. The red stained area indicates atherosclerosis plaques.
The addition of edible insects (TM and GA) to the diet of ApoE/LDLR−/− mice had no significant effect on the development of atherosclerosis counted as the total lesion area of the aortic root (Figure 3a). However, the analysis of individual cross-sections revealed that there was a significant (p < 0.05) difference in sections of the aortic root on the level of 200 and 300 µm far from the appearance of the first part of the aortic valve in the GA group compared to the control and TM groups (Figure 3c).
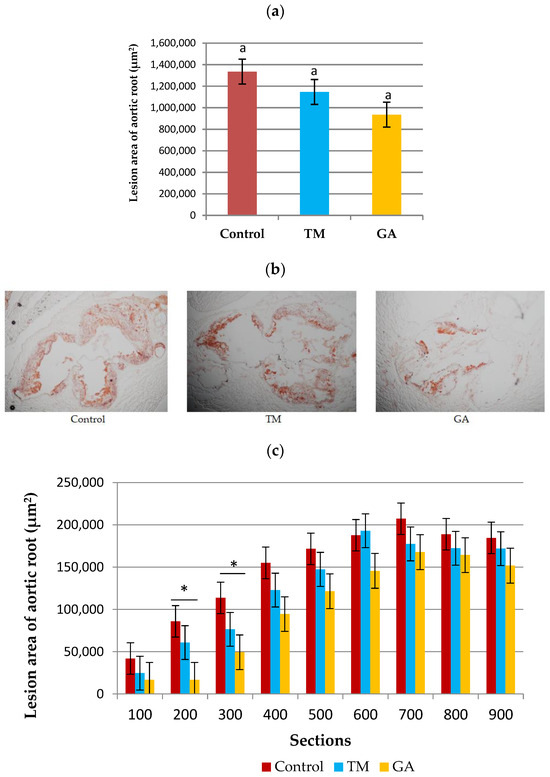
Figure 3.
(a) The development of atherosclerosis in ApoE/LDLR−/− mice (n = 10) measured by the cross-section method. (b) Representative images of cross-sections of aortic roots stained by ORO. (c) Sizes of the atherosclerotic lesion areas of nine individual cross-sections of the aortic root (n = 10 for each group). The mean difference is significant at p < 0.05 and indicated by different letters and *. Magnification of ×100.
3. Discussion
The effect of edible insects (TM and GA) on the area of atherosclerotic lesions in ApoE/LDLR−/− mice was studied. No significant changes in body weight or fasting blood glucose levels in mice fed diets containing edible insects (TM and GA) compared to the control group were observed. While previous research suggests that extracts from TM larvae can influence body weight, variations in insect type, origin, and diet could cause the observed differences [48,50].
Certain substances from insects, such as chitin from mealworms and glycosaminoglycans from crickets, have been shown to regulate lipid metabolism and blood lipid levels [16,47]. Also, it was shown that edible insects are a novel source of bioactive peptides. Gryllus assimilis was the only insect whose hydrolysis generated a peptide that was predicted to act as an inhibitor of the HMG-CoA reductase, thus suggesting a hypocholesterolemic property. In our study, it was observed that the amount of protein was significantly higher in GA compared to TM. Additionally, the cholesterol concentration as well as the LDL level tended to decrease in GA compared to TM. The present study did not observe changes in the plasma lipid profile between experimental groups; this represents a difference from previous findings on edible insects lowering blood cholesterol, triglycerides, and LDL levels [47,51].
Elevated serum ALT is associated with increased oxidative stress and systemic inflammation, critical components of atherosclerosis [52,53]. This study demonstrated that adding GA and TM into the diet of ApoE/LDLR−/− mice could significantly reduce the activity of liver enzyme ALT but did not cause any difference in the serum activities of AST. The reduced activity of liver enzyme ALT might be caused by nutrients, antioxidants, and bioactive compounds found in edible insects [9,16]. The study by [54] reported that the defatted mealworm fermentation extract supplementation improved relative liver weight and reduced serum AST and ALT levels in alcohol-induced rats. Their findings imply that protein hydrolysates may benefit liver function [55,56]. Administering glycosaminoglycans (GAGs) extracted from field crickets was found to reduce oxidative enzyme levels, including ALT and AST. GAGs played a role in improving the metabolic activity of hepatocytes [57]. Insects are consumed as rich sources of protein, vitamins, minerals, fiber, and unsaturated fatty acids in some species [10].
The growing body of evidence highlights the potential benefits of PUFAs, especially n−3 PUFAs, for human health [58]. In our study, the amount of total PUFAs in adipose tissue and liver was higher in the GA group compared to the TM group. Our study also demonstrated that linoleic acid was significantly higher in the control and GA groups than in the TM-fed group. According to [59], the contents of unsaturated fatty acids in the field cricket were high, especially the oleic, linoleic, and linolenic acids. The fatty acid profile in adipose tissue indicated an increase in C18:1 and C18:2 (n−6) levels in the TM group compared to the control and GA groups. C18:1 and C18:2 (n−6) were the major fatty components of TM larvae [60].
The result from fatty acid analysis in the liver showed that the concentrations of MUFAs and PUFAs were significantly higher in the TM group, whereas the level of SFAs was significantly higher in the GA group. No significant difference in liver weight in mice fed experimental diets compared to the control was observed. Nevertheless, a significantly higher liver weight was observed in the GA group compared to the TM group. The elevated liver weight in the GA group may be due to the difference in fatty acid profiles of the insects. GA had a significantly higher SFA content (36.64%) compared to TM (33.84%). Studies suggest excessive SFA intake can promote liver fat accumulation, potentially contributing to a higher liver weight, whereas PUFAs prevent liver fat accumulation [61,62]. Furthermore, the lower MUFA content (35.27%) in GA crickets compared to TM larvae (41.19%) could also play a role, as MUFAs are generally considered beneficial for liver condition [63]. This difference might be caused by variations in the nutritional profiles of TM and GA, influencing the fatty acid assimilation and metabolism in the liver.
The 16:0/18:2 fatty acid ratio was calculated to assess the balance between palmitic acid and linoleic acid in liver and adipose tissue samples. In the liver, the TM group exhibited a notably higher 16:0/18:2 ratio than the control group, indicating an increased presence of palmitic acid relative to linoleic acid. Palmitate has been found to promote neointima formation by inducing inflammatory phenotypes in smooth muscle cells [64]. A study found that palmitate and stearate are proapoptotic, while non-esterified fatty acids, such as palmitoleate, oleate, and linoleate, exhibit anti-apoptotic properties by inhibiting NFκB activation in endothelial cells [65]. Interestingly, the GA group demonstrated a significantly lower 16:0/18:2 ratio in adipose tissue than the TM group. This result suggests a relatively higher proportion of linoleic acid than palmitic acid in the GA group, potentially indicating a more favorable fatty acid composition regarding inflammation and cardiovascular health [60,66].
It is mentioned that antioxidants combat oxidative stress, a key player in atherosclerosis development [67]. Despite no significant changes in total atherosclerotic lesion area, the incorporation of Gryllus assimilis into the diet has shown promising results in reducing atherosclerotic plaque areas in specific sections of the aortic root at 200 and 300 µm levels compared to the control and TM groups. This effect may be attributed to the strong antioxidant activity of these crickets. Recent studies have suggested that the consumption of edible insects can modulate oxidative stress due to their antioxidant potential [68]. While specific research on the antioxidant profile of GA is limited, broader studies on edible insects like Acheta domesticus (house cricket) larvae demonstrate the presence of various antioxidants, including phenolics [69,70]. In our study, we analyzed the antioxidant activity using various assays and we found that the ABTS•+ and DPPH• expressed as Trolox equivalent antioxidant capacity (TEAC) assays showed the significantly higher values for Gryllus assmilis.
Antioxidants are known to combat oxidative stress by neutralizing free radicals, reducing inflammatory cytokine production, and improving endothelial function [47]. The presence of phenolic compounds and other antioxidants in crickets has been well-documented [48,49,50]. Our findings support this with the notable amount observed in GA, which aligns with the observed health-related effects in our in vivo study. The radical scavenging activity observed in GA in our study suggests a potential mechanism for the observed health benefits. The high levels of antioxidants found in these crickets could explain the reduction in atherosclerotic plaque formation. The radical scavenging activity of GA, as observed in our study (75.75% for DPPH•), was higher compared to other cricket species such as Acheta domesticus, which showed a DPPH• inhibition of 72%. This highlights the antioxidant properties of GA, further supporting their potential health benefits. The reduced oxidative stress might relate to the plaque reduction through several pathways. Antioxidants from GA may suppress inflammatory cytokine production and maintain healthy endothelial function; both crucial factors in preventing plaque buildup [71,72].
A research on nut bars fortified with cricket flour highlights the potential of edible insects as a source of antioxidants. This study found that cricket flour significantly increased the total phenolic content and tocopherol levels of the nut bars compared to standard bars [70]. Furthermore, another study reported reduced plasma TNF-α levels in healthy adults consuming cricket powder [12]. These findings suggest the potential anti-inflammatory effects of cricket’s antioxidants.
Studies have also shown that insect glycosaminoglycans and unsaturated fatty acids can suppress inflammatory markers linked to cardiovascular disease development. Glycosaminoglycans derived from crickets can modulate the production of inflammatory mediators like IL-6 and PGE2, potentially contributing to an anti-inflammatory effect [14,15,16,17].
Phenolic compounds and other antioxidants present in GA crickets may contribute to this effect by reducing oxidative stress and inflammation, thereby improving cardiovascular and liver health and contributing to the development of therapeutic foods to prevent atherosclerosis. This promising finding, potentially linked to the anti-inflammatory properties of certain insect biomolecules, warrants further exploration.
4. Materials and Methods
4.1. Insects
Both experimental species (Tenebrio molitor, Linnaeus, 1758, and Gryllus assimilis, Fabricuis, 1775) were obtained from the rearing facility of the Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences, Prague (FAFNR, CZU), and were maintained under conditions at 27 ± 1 °C and 40–50% relative humidity using a rack-system. The experimental crickets were kept in plastic rearing boxes (560 × 390 × 280 mm, SAMLA, IKEA, and Prague, Czech Republic) until their harvest at the age of 60 ± 1 days, when the majority of crickets were adults. The boxes were equipped with egg trays, two Petri dishes with feed and one Petri dish containing water gel (Oslavan, Náměšť nad Oslavou, Czech Republic). The mealworms were kept in plastic containers (280 × 140 × 390 mm, SAMLA, IKEA, Prague, Czech Republic) in feeding substrate when fresh sliced carrots were supplied as the only water source. TM were harvested using sieving when the first pupae occurred (the approximate age of harvested larvae was 90 days).
Regarding the insects’ diet, both species were provided with dry feed ad libitum. GA larvae were provided with chicken feed (77.9% wheat, 17.6% soybean meal, 1.8% rapeseed oil, 2.7% premix of minerals, macronutrients, and micronutrients; particle size < 1 mm) produced in collaboration with the experimental farm of the Demonstrational and Experimental Centre, FAFNR, CZU. TM larvae were fed by wheat bran mixed with chicken feed (4:1). Before harvest, the experimental insects were starving for 24 h. After that, they were freeze-killed, lyophilized (Trigon Plus lyophilizer, Čestlice, Czech Republic), homogenized using a laboratory mill (A10; IKA Werke GmbH & Co. KG, Staufen, Germany), and stored at −80 °C.
4.1.1. Nutritive Value and Fatty Acid Composition of Insects
Determination of basic composition of insect’s powder used as the feed component for experimental mice was carried out according to [73]. The dry matter (DM) was determined gravimetrically after drying the samples at 103 ± 2 °C (Memmert oven, Schwabach, Germany) to a constant weight. The samples were mineralized in a muffle furnace LAC (Verkon, Praha, Czech Republic) for ash content determination at 550 °C. The total fat content was determined using the Gerhardt Soxtherm SOX414 apparatus (C. Gerhardt GmbH and Co. KG, Königswinter, Germany). The crude protein content was evaluated with the Kjeltec 2400 analyzer (FOSS, Hilleroed, Denmark) using the nitrogen-to-protein conversion factor 6.25. Chitin was determined according to [36] after defatting and hydrolysis via 1M NaOH and 1M HCl.
Fatty acid composition was analyzed using GC-MS (Shimadzu GC-MS, Model QP 5050A) by the method described previously [74].
4.1.2. Determination of Total Polyphenols Content of Insects
The content of total phenolic compounds (TPC) of methanolic extracts was determined by the Folin–Ciocalteu (Sigma, St. Luis, MO, USA) colorimetric test developed by [69] with some modifications. The level of total polyphenolic compounds was determined spectrophotometrically at a wavelength of λ = 760 nm using a RayLeigh UV-1800 spectrophotometer (Beijing Beifen-Ruili Analytical Instrument, Beijing, China). The results were expressed as g of Gallic Acid Equivalents (GAE) per 100 g of extract using a standard curve of gallic acid.
4.1.3. Determination of Antioxidant Activity of Insects
ABTS•+ Radical Scavenging
Antioxidant activity was measured using the method of Re et al. with the ABTS•+ radical (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) [75]. The absorbance of the colored solution was measured using a RayLeigh UV-1800 spectrophotometer (Beijing Beifen-Ruili Analytical Instrument, Beijing, China) at a wavelength of 734 nm in the presence of 70% methanol used to prepare the extracts. The result was expressed in μM Trolox/g DM.
DPPH• Radical Scavenging
Antioxidant activity was determined by the method of Brand-Williams et al. using the free radical DPPH• [76]. To a 1.5 mL sample suitably diluted with methanol, 3 mL of the prepared DPPH• solution (diluted to an absorbance between 0.900 and 1.000) was added and the contents of the tube were mixed. The samples were incubated for 10 min and protected from light in room temperature. After this time, the absorbance was measured using a RayLeigh UV-1800 spectrophotometer at 515 nm against 99% pure undiluted methanol. The result was expressed in μM Trolox/g DM. The solution absorbance (Asample) was measured compared to the blank, in which the extract was replaced with methanol (Ablank).
The percentage of DPPH• inhibition was determined with the following equation:
% inhibition = ((Ablank − Asample)/Ablank) × 100
Ferric Reducing Antioxidant Power (FRAP)
Antioxidant activity was determined by the FRAP method according to Benzie and Strain [77]. The absorbance at 593 nm against 70% methanol was measured using a RayLeigh UV-1800 spectrophotometer (Beijing Beifen-Ruili Analytical Instrument Beijing, China). The results obtained were expressed in μM Trolox/g DM. The ABTS•+ radical scavenging activity was computed with the formula described in the section “DPPH• radical scavenging activity assay”.
4.2. Animals and Housing
Animals (ApoE/LDLR−/− mice) were bred in the Department of Human Nutrition and Dietetics, University of Agriculture, in Krakow. ApoE/LDLR−/− mice are considered a suitable model to study the anti-atherosclerotic effect of treatments without an atherogenic diet since the animals develop hypercholesterolemia and atherosclerosis easily even when fed a standard chow diet [78,79]. This allows for observing the impact of dietary changes on a more established atherosclerotic process within a specific timeframe. ApoE plays a broader role in lipoprotein metabolism beyond just LDL uptake like LDLR [80]. According to [81], male animals appear to have more inflamed but smaller plaques than female animals.
Animals were housed in colony cages in constant environmental conditions (22–25 °C, 65–75% humidity) with a 12-h light/dark cycle and free access to diet and water. The body weight of mice was recorded weekly. All animal procedures were conducted according to the Guidelines for Animal Care and Treatment of the European Union and approved by the 1st Local Animal Ethics Commission in Krakow (Approval No. 562/2021, 28 October 2021).
4.3. Diets and Feeding
Mice were first fed a commercial cholesterol-free pelleted diet for two months (Sniff M-Z Spezialdiäten GmbH; Soest, Germany). Eight- to ten-week-old female mice (no signs of atherosclerosis) were used in the study. Animals were randomly assigned to three experimental groups (n = 10 in each group). The control group was fed the AIN-93G diet (American Institute of Nutrition) for the following eight weeks based on [49]. The second group was provided the AIN-93G diet supplemented with 10% of TM, and the third experimental group was fed the AIN-93G diet with 10% of GA for eight weeks.
4.4. Blood Sampling, Glucose, and Biochemical Analyses of Plasma
At the end of the nutritional experiment, mice were fasted for four hours and the blood glucose level from the tail vein was measured using Accu-Chek Active strips (Roche Diagnostics GmbH, Mannheim, Germany). Then, mice were injected intraperitoneally with heparin (1000 IU, Sanofi-Synthelabo; Paris, France) and after 10 min anaesthetised with ketamine/xylazine (20 μL, 100 mg/mL Biochemie; Vienna, Austria) and finally sacrificed by cervical translocation.
Blood was collected from the heart into tubes centrifuged at 14,000× g for 4 min to isolate plasma. The samples were kept at −80 °C for further analysis. ABX Pentra 400 biochemical analyzer (Horiba Medical, Japan) was used to perform the spectrophotometric method for determining the lipid profile, including total cholesterol level (TC), HDL and LDL cholesterol fractions and triacylglycerols (TAG), as well as the liver enzyme activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the blood plasma.
4.5. Fatty Acids Profile of Adipose Tissue and Liver
At the end of the experiment, abdominal adipose tissues and liver were dissected from the mice, weighted and snap-frozen at −80 °C. Fat from the liver was extracted using a Leco TFE 2000 fat analyzer (Leco, St. Joseph, MO, USA) with liquid carbon dioxide as a solvent. The fatty acid profile was analyzed using GC-MS (Shimadzu GC-MS, Model QP 5050A) by the method described previously [74].
4.6. Quantification of Atherosclerosis in Aorta (En Face Method)
In anesthetized mice, the thorax was longitudinally opened, the right atrium was incised, and the heart was perfused in situ with phosphate-buffered saline (PBS, pH 7.4) through the apex of the left ventricle. The entire aortas (from the aortic arch to the iliac bifurcation) were dissected and kept in 4% buffered formalin for further analysis. The aortas were longitudinally opened and pinned to silicone plates to expose the surface for quantitative analysis of atherosclerotic lesions. Afterwards, aortas were stained with Sudan IV (Sigma-Aldrich, St. Louis, MO, USA) to visualize the atherosclerotic lesions as described previously [82]. Finally, the aortas were imaged, and the diameters of the lesions were quantified using LSM Image Browser 4 software (Zeiss, Jena, Germany).
4.7. Quantification of Atherosclerosis in Aortic Roots (Cross-Section Method)
For quantitative analysis of atherosclerotic lesions in the aortic root, the heart was cut and embedded in the OCT (optimal cutting temperature) compound (CellPath, Oxford, UK), then frozen and cut into serial cryosections of 10 μm thickness using a Cryostat CM1950 (Leica Biosystems, Deer Park, IL, USA). Eight sections were consecutively taken at intervals of 100 μm, beginning at 100 μm from the appearance of the aortic valves, following the protocol described previously [83]. Each section was mounted on slides and stained with oil-red-O (ORO) (Sigma-Aldrich, St. Louis, MO, USA) and examined under an Olympus BX50 (Olympus, Tokyo, Japan) microscope. A quantitative analysis of atherosclerotic lesions was performed using the LSM Image Browser 4 software (Zeiss, Jena, Germany). For each animal, a mean lesion area was calculated from nine sections and each section individually, reflecting the cross-section area covered by atherosclerosis.
4.8. Statistical Analysis
Obtained data are presented as the mean ± standard deviation (SD). The significance levels for basic composition, total polyphenol content, and antioxidant status of insects’ powder were determined using Student’s t-test. The normality of the results of in vivo study was determined using the Shapiro–Wilk test. The significance levels were determined using either the non-parametric Kruskal–Wallis test or a parametric test (one-way analysis of variance ANOVA with Tukey’s test) with the level of significance set at p < 0.05. All statistical analyses were conducted using STATISTICA 14.0.0.15 (TIBCO Software Inc., Palo Alto, CA, USA).
5. Conclusions
The addition of Gryllus assimilis to the diet appears to significantly decrease the atherosclerotic plaque area in sections of the aortic root, potentially due to its high antioxidant activity. Interestingly, our study found that adding GA to the diet significantly decreased the atherosclerotic plaque area in specific sections of the aortic root (200 and 300 µm) compared to the control group. To our knowledge, this is the first study to determine the effects of Tenebrio molitor and Gryllus assimilis on atherosclerotic lesion development in ApoE/LDLR−/− mice.
The study does not include the composition of the atherosclerotic plaque, its stability, nor biomarkers of inflammation. It determined the effect of TM and GA only by quantifying the atherosclerotic plaque area. Further studies are needed to understand the underlying mechanisms, plaque composition, and stability. Additionally, clinical studies would be necessary in the future to prove the effect of insects on humans.
Author Contributions
Conceptualization, M.F.-Ż., R.B.K. and L.K.; methodology, H.H., R.B.K. and M.F.-Ż.; software, H.H. and M.F.-Ż.; validation, M.F.-Ż.; formal analysis, H.H., E.P. and M.F.-Ż.; investigation, K.P. and P.Š.; resources, H.H., M.F.-Ż., M.K. and L.K., data curation, T.T.; writing—original draft preparation, H.H. and M.F.-Ż.; writing—review and editing, M.F.-Ż., R.B.K. and L.K.; visualization, H.H. and M.F.-Ż.; supervision, R.B.K.; project administration, M.F.-Ż.; funding acquisition, T.T. and M.F.-Ż. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Education and Science through a subsidy for the University of Agriculture in Krakow. Additionally, support was provided by the Czech Science Foundation (GAČR) under Project Number 21–47159L (INPROFF), as well as by the METRO FOOD-CZ research infrastructure project (MEYS Grant No. LM2023064), which granted access to its facilities.
Institutional Review Board Statement
The animal study protocol was approved by the 1st Local Animal Ethics Committee in Krakow (protocol code 562/2021 and date of approval 28 October 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request from the corresponding authors via email: magdalena.franczyk-zarow@urk.edu.pl.
Acknowledgments
The authors would like to thank Barbara Czosnowska and Agata Strojewska for technical support and help in animal care.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- Roth, G.A.; Mensah, G.A.; Fuster, V. The global burden of cardiovascular diseases and risks: A compass for global action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis: A chronic inflammatory disease with an autoimmune component. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Greenow, K.; Pearce, N.J.; Ramji, D.P. The key role of apolipoprotein E in atherosclerosis. J. Mol. Med. 2005, 83, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; Maeda, N. Genetic modifiers of atherosclerosis in mice. Atheroscler. Thromb. Vasc. Biol. 2000, 20, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef]
- D’Antonio, V.; Battista, N.; Sacchetti, G.; Di Mattia, C.; Serafini, M. Functional properties of edible insects: A systematic review. Nutr. Res. Rev. 2023, 36, 98–119. [Google Scholar] [CrossRef]
- Payne, C.L.; Scarborough, P.; Rayner, M.; Nonaka, K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci. Technol. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.M. Tumor necrosis factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.U.; Petrusan, J.I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-derived chitin and chitosan: A still unexploited resource for the edible insect sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Hwang, J.S.; Kim, M.J.; Park, K.K. Antilipidemic effects and gene expression profiling of the glycosaminoglycans from cricket in rats on a high fat diet. Arch. Pharm. Res. 2016, 39, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Han, J.W.; Hwang, J.S.; Yun, E.Y.; Lee, B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. Part A J. Toxicol. Environ. Health A 2014, 77, 1332–1345. [Google Scholar] [CrossRef]
- Ferrante, A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Quah, Y.; Tong, S.R.; Bojarska, J.; Giller, K.; Tan, S.A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.T. Bioactive peptide discovery from edible insects for potential applications in human health and agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef]
- EFSA. European Parliament and Council. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. OJEU 2015. Available online: https://eurlex.europa.eu/eli/reg/2015/2283/oj (accessed on 11 May 2024).
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World (April 1, 2017)—WUR. Available online: https://www.wur.nl/en/research-results/chair-groups/plant-sciences/laboratory-of-entomology/edible-insects/worldwide-species-list.htm (accessed on 10 May 2024).
- Oliveira, L.A.; Pereira, S.M.S.; Dias, K.A.; da Silva Paes, S.; Grancieri, M.; Jimenez, L.G.S.; Della, L.C.M. Nutritional content, amino acid profile, and protein properties of edible insects (Tenebrio molitor and Gryllus assimilis) powders at different stages of development. J. Food Compos. Anal. 2024, 125, 105804. [Google Scholar] [CrossRef]
- Gonzalez-de la Rosa, T.; Montserrat-de la Paz, S.; Rivero-Pino, F. Production, characterisation, and biological properties of Tenebrio molitor-derived oligopeptides. Food Chem. 2024, 450, 139400. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Claes, J.; Smets, R.; De Winne, A.; Akhtaruzzaman, M.; Van Der Borght, M. Characterization of freeze-dried, oven-dried and blanched house crickets (Acheta domesticus) and Jamaican field crickets (Gryllus assimilis) by means of their physicochemical properties and volatile compounds. Eur. Food Res. Technol. 2021, 247, 1291–1305. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. JIFF 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Adámek, M.; Borkovcová, M.; Bednářová, M.; Hlobilová, V.; Knížková, I.; Juríková, T. Tenebrio molitor (Coleoptera: Tenebrionidae)—Optimization of rearing conditions to obtain desired nutritional values. J. Insect Sci. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Araújo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Santos, E.M. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Kulma, M.; Petříčková, D.; Kurečka, M.; Kotíková, Z.; Táborský, J.; Michlová, T.; Kouřimská, L. Effect of carrot supplementation on nutritional value of insects: A case study with Jamaican field cricket (Gryllus assimilis). JIFF 2022, 8, 621–629. [Google Scholar] [CrossRef]
- Syahrulawal, L.; Torske, M.O.; Sapkota, R.; Næss, G.; Khanal, P. Improving the nutritional values of yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) larvae as an animal feed ingredient: A review. J. Anim. Sci. Biotechnol. 2023, 14, 146. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on anti-inflammatory molecular mechanisms induced by oleic acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martin-Pelaez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Pino, F.R.; Gálvez, R.P.; Carpio, F.J.E.; Guadix, E.M. Evaluation of Tenebrio molitor protein as a source of peptides for modulating physiological processes. Food Funct. 2020, 11, 4376–4386. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former food stuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From farm to fork: Crickets as alternative source of protein, minerals, and vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef]
- Kopecká, A.; Kouřimská, L.; Škvorová, P.; Kurečka, M.; Kulma, M. Effect of Temperature on the Nutritional Quality and Growth Parameters of Yellow Mealworm (Tenebrio molitor L.): A Preliminary Study. Appl. Sci. 2024, 14, 2610. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.A.; Kwon, Y.S.; Hwang, J.S.; Goo, T.W.; Jun, M.; Yun, E.Y. Effects of processing methods on nutritional composition and antioxidant activity of mealworm (Tenebrio molitor) larvae. Entomol. Res. 2019, 49, 284–293. [Google Scholar] [CrossRef]
- Jozefiak, A.; Engberg, R.M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The potential of insects as food sources–a review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Rothman, J.M. Nutritional ecology of entomophagy in humans and other primates. Annu. Rev. Entomol. 2013, 58, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Goo, T.W.; Chung, M.Y.; Baek, M.; Hwang, J.S.; Kim, M.A.; Yun, E.Y. Tenebrio molitor larvae inhibit adipogenesis through AMPK and MAPKs signaling in 3T3-L1 adipocytes and obesity in high-fat diet-induced obese mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Weru, J.; Chege, P.; Kinyuru, J. Nutritional potential of edible insects: A systematic review of published data. Int. J. Trop. Insect Sci. 2021, 41, 2015–2037. [Google Scholar] [CrossRef]
- Escobar-Ortiz, A.; Hernández-Saavedra, D.; Lizardi-Mendoza, J.; Pérez-Ramírez, I.F.; Mora-Izaguirre, O.; Ramos-Gómez, M.; Reynoso-Camacho, R. Consumption of cricket (Acheta domesticus) flour decreases insulin resistance and fat accumulation in rats fed with high-fat and-fructose diet. J. Food Biochem. 2022, 46, e14269. [Google Scholar] [CrossRef]
- Choi, R.Y.; Ham, J.R.; Ryu, H.S.; Lee, S.S.; Miguel, M.A.; Paik, M.J.; Ji, M.; Park, K.W.; Kang, K.Y.; Lee, H.I.; et al. Defatted Tenebrio molitor larva fermentation extract modifies steatosis, inflammation and intestinal microflora in chronic alcohol-fed rats. Nutrients 2020, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Tomiyama, H.; Yambe, M.; Koji, Y.; Motobe, K.; Shiina, K.; Yamamoto, Y.; Yamashina, A. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis 2006, 189, 198–205. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kim, B.J.; Kim, H.J.; Jin, J.M.; Yoon, H.J.; Hwang, J.S.; Lee, B.M. Anti-diabetic activity of field cricket glycosaminoglycan by ameliorating oxidative stress. BMC Complement. Med. Ther. 2020, 20, 232. [Google Scholar] [CrossRef]
- Glick, N.R.; Fischer, M.H. The role of essential fatty acids in human health. Evid. Based Complement. Altern. Med. 2013, 18, 268–289. [Google Scholar] [CrossRef]
- Wang, D.; Bai, Y.Y.; Li, J.H.; Zhang, C.X. Nutritional value of the field cricket (Gryllus testaceus Walker). J. Insect Sci. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Green, C.J.; Hodson, L. The influence of dietary fat on liver fat accumulation. Nutrients 2014, 6, 5018–5033. [Google Scholar] [CrossRef]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: A randomized trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Ullah, R.; Rauf, N.; Nabi, G.; Ullah, H.; Shen, Y.; Zhou, Y.D.; Fu, J. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: Recent updates. Int. J. Biol. Sci. 2019, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Eguchi, K.; Kono, N.; Fujiu, K.; Matsumoto, S.; Shibata, M.; Oishi-Tanaka, Y.; Komuro, I.; Arai, H.; Nagai, R.; et al. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2596–2607. [Google Scholar] [CrossRef]
- Staiger, K.; Staiger, H.; Weigert, C.; Haas, C.; Häring, H.U.; Kellerer, M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-κB activation. Diabetes 2006, 55, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Kinyuru, J.N.; Mogendi, J.B.; Riwa, C.A.; Ndung’u, N.W. Edible insects—A novel source of essential nutrients for human diet: Learning from traditional knowledge. Anim. Front. 2015, 5, 14–19. [Google Scholar]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. J. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- D’Antonio, V.; Serafini, M.; Battista, N. Dietary modulation of oxidative stress from edible insects: A mini-review. Front. Nutr. 2021, 8, 642551. [Google Scholar] [CrossRef]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on microbial quality, protein yield, and antioxidant properties of some frozen edible insects. Int. J. Food Sci. Technol. 2021, 2021, 5580976. [Google Scholar] [CrossRef]
- Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive compounds and antioxidant composition of nut bars with addition of various edible insect flours. Molecules 2023, 28, 3556. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Sewell, R.D.; Rafieian-Kopaei, M. Antioxidants and atherosclerosis: Mechanistic aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef]
- Franczyk-Żarów, M.; Szymczyk, B.; Kostogrys, R.B. Effects of dietary conjugated linoleic acid and selected vegetable oils or vitamin E on fatty acid composition of hen egg yolks. Ann. Anim. Sci. 2019, 19, 173–188. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bonthu, S.; Heistad, D.D.; Chappell, D.A.; Lamping, K.G.; Faraci, F.M. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Jawien, J.; Nastalek, P.; Korbut, R. Mouse models of experimental atherosclerosis. J. Physiol. Pharmacol. 2004, 55, 503–517. [Google Scholar] [PubMed]
- Yamada, N.; Shimano, H.; Yazaki, Y. Role of apolipoprotein E in lipoprotein metabolism and in the process of atherosclerosis. J. Atheroscler. Thromb. 1995, 2 (Suppl. S1), S29–S33. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a biological variable in atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef]
- Franczyk-Żarów, M.; Kostogrys, R.B.; Szymczyk, B.; Jawień, J.; Gajda, M.; Cichocki, T.; Wojnar, L.; Chlopicki, S.; Pisulewski, P.M. Functional effects of eggs, naturally enriched with conjugated linoleic acid, on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice (ApoE/LDLR−/−). Br. J. Nutr. 2008, 99, 49–58. [Google Scholar] [CrossRef]
- Centa, M.; Ketelhuth, D.F.; Malin, S.; Gisterå, A. Quantification of atherosclerosis in mice. J. Vis. Exp. 2019, 148, e59828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).