Sickle Cell Disease and Gut Health: The Influence of Intestinal Parasites and the Microbiome on Angolan Children
Abstract
1. Introduction
2. Results
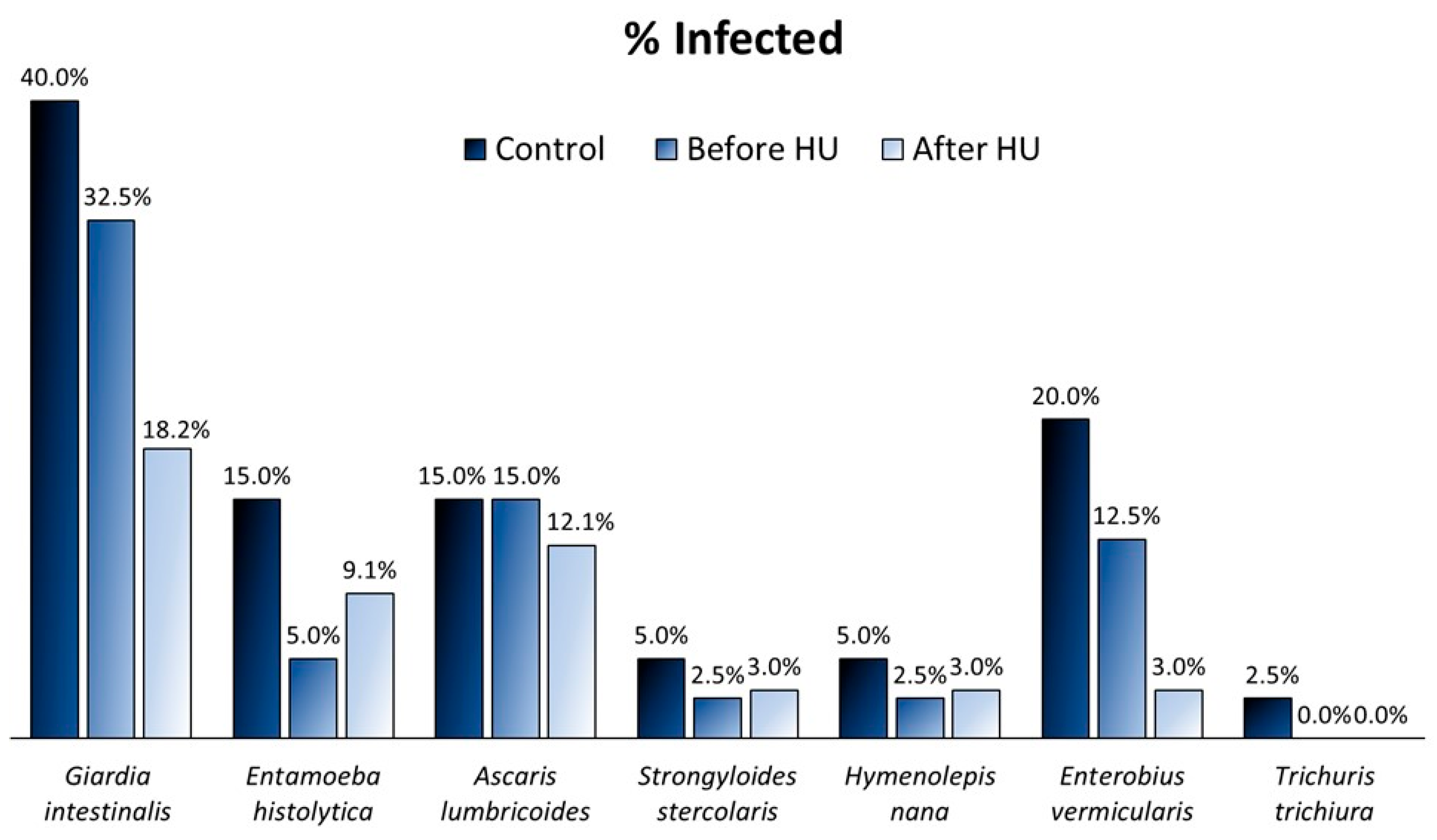
2.1. Prevalence of Parasitic Infections
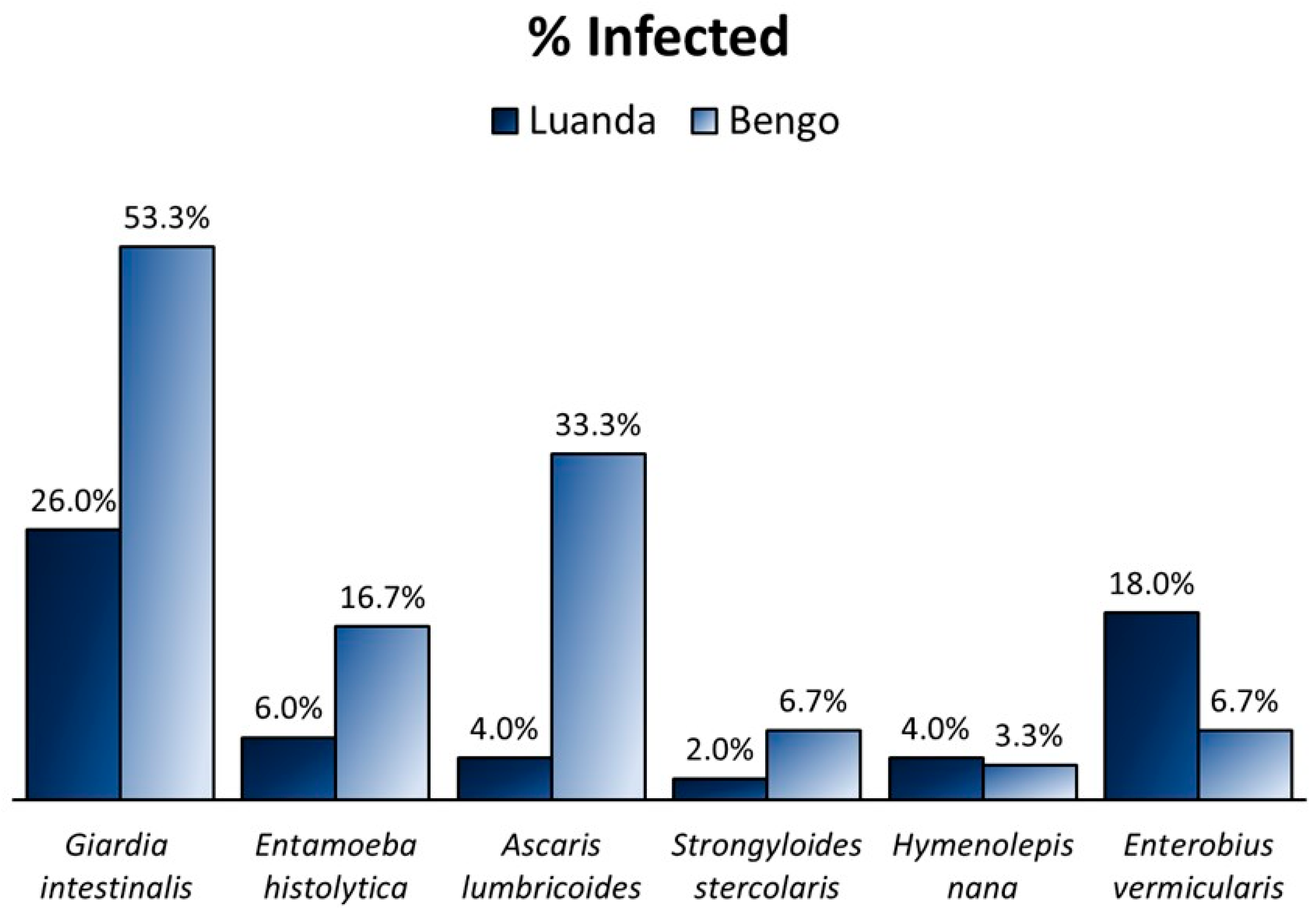
2.2. Parasite Prevalence according to Residency
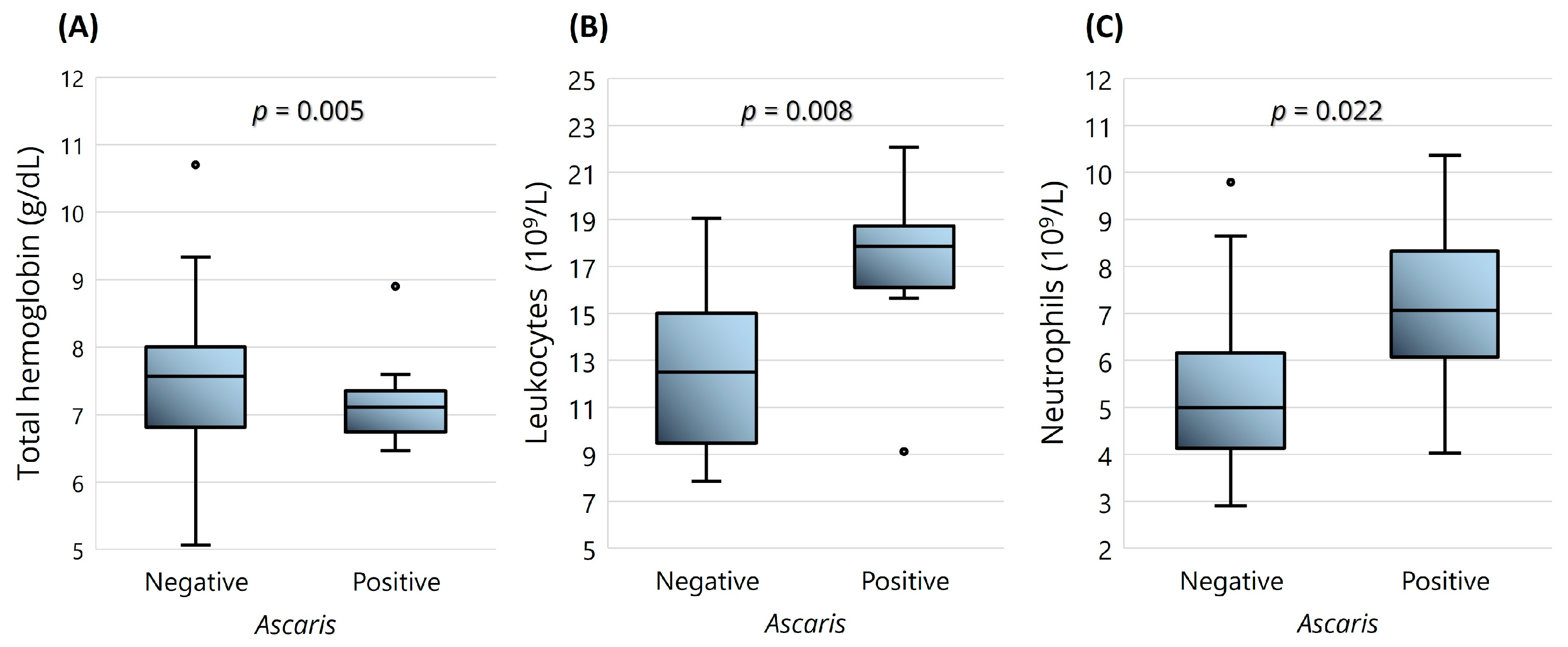
2.3. SCD Clinical Data and Parasitic Infections
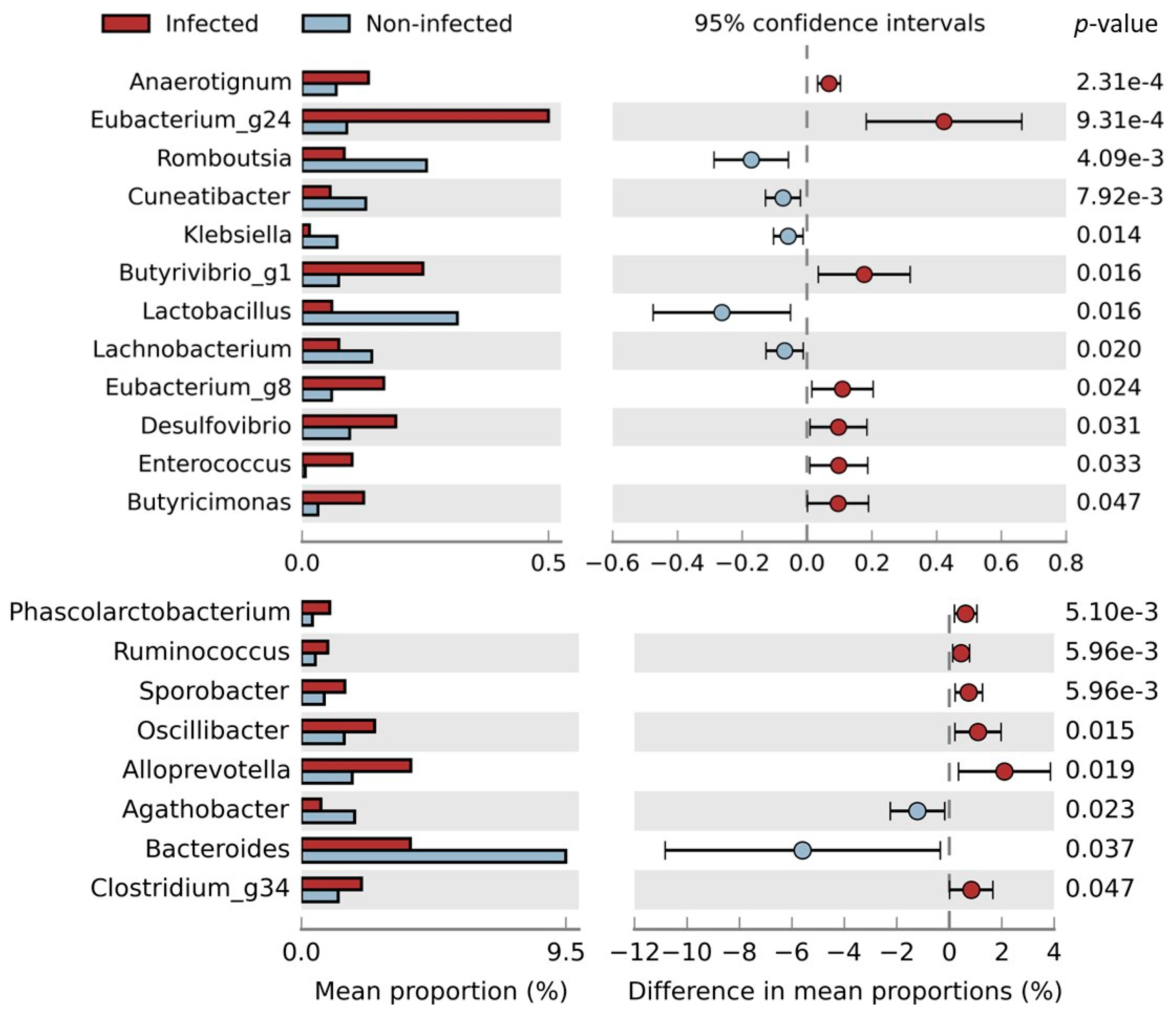
2.4. Impact of Parasites on Gut Microbiome
3. Discussion
4. Materials and Methods
4.1. Population and Sample Collection
4.2. Clinical Analysis
4.3. Molecular Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inusa, B.P.D.; Hsu, L.L.; Kohli, N.; Patel, A.; Ominu-Evbota, K.; Anie, K.A.; Atoyebi, W. Sickle cell disease—Genetics, pathophysiology, clinical presentation and treatment. Int. J. Neonatal. Screen. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. In Nature Reviews Disease Primers; Nature Publishing Group: New York, NY, USA, 2018; Volume 4. [Google Scholar]
- Chang, A.K.; Ginter Summarell, C.C.; Birdie, P.T.; Sheehan, V.A. Genetic modifiers of severity in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Jerrell, J.M.; Stallworth, J.R. Clinical complications in severe pediatric sickle cell disease and the impact of hydroxyurea. Pediatr. Blood Cancer 2011, 56, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H. Genetic etiologies for phenotypic diversity in sickle cell anemia. Sci. World J. 2009, 9, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.N. Sickle Cell Disease in Sub-Saharan Africa. Hematol. Oncol. Clin. N. Am. 2016, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Pande, B.; Verma, H.K.; Bhaskar, L.V.K.S.; Sinha, M.; Sinha, R.; Rao, P.V. Infection and Potential Challenge of Childhood Mortality in Sickle Cell Disease: A Comprehensive Review of the Literature from a Global Perspective. Thalass. Rep. 2023, 13, 206–229. [Google Scholar] [CrossRef]
- Yeshitila, Y.G.; Zewde, H.; Mekene, T.; Manilal, A.; Lakew, S.; Teshome, A. Prevalence and Associated Risk Factors of Intestinal Parasites among Schoolchildren from Two Primary Schools in Rama Town, Northern Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5750891. [Google Scholar] [CrossRef]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. Human gastrointestinal nematode infections: Are new control methods required? Int. J. Exp. Pathol. 2006, 87, 325–341. [Google Scholar] [CrossRef]
- Tomlinson, M.; Adams, V.; Chopra, M.; Jooste, P.; Strydom, E.; Dhansay, A. Survey of iodine deficiency and intestinal parasitic infections in school-going children: Bie Province, Angola. Public Health Nutr. 2010, 13, 1314–1318. [Google Scholar] [CrossRef][Green Version]
- Dacal, E.; Saugar, J.M.; De Lucio, A.; Hernández-De-Mingo, M.; Robinson, E.; Köster, P.C.; Aznar-Ruiz-De-Alegría, M.L.; Espasa, M.; Ninda, A.; Gandasegui, J.; et al. Prevalence and molecular characterization of Strongyloides stercoralis, Giardia duodenalis, Cryptosporidium spp., and Blastocystis spp. isolates in school children in Cubal, Western Angola. Parasit Vectors 2018, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Otunu, O.; Damen, J. Prevalence of Intestinal Parasites in Sickle Cell Disease Patients Attending Jos University Teaching Hospital, Nigeria. J. Biosci. Med. 2019, 7, 94741. [Google Scholar] [CrossRef]
- Toro-Londono, M.A.; Bedoya-Urrego, K.; Garcia-Montoya, G.M.; Galvan-Diaz, A.L.; Alzate, J.F. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ 2019, 7, e6200. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Iorio, A.; Porcari, S.; Masucci, L.; Sanguinetti, M.; Perno, C.F.; Gasbarrini, A.; Putignani, L.; Cammarota, G. How the gut parasitome affects human health. Ther. Adv. Gastroenterol. 2022, 15, 17562848221091524. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Graham, A.L.; Knowles, S.C.L. Parasite-microbiota interactions with the vertebrate gut: Synthesis through an ecological lens. Front. Microbiol. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- von Huth, S.; Thingholm, L.B.; Kofoed, P.E.; Bang, C.; Rühlemann, M.C.; Franke, A.; Holmskov, U. Intestinal protozoan infections shape fecal bacterial microbiota in children from guinea-bissau. PLoS Negl. Trop. Dis. 2021, 15, e0009232. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.L.; Gilchrist, C.A.; Lynn, T.C.; Petri, W.A. Parasitic protozoa and interactions with the host intestinal microbiota. Infect. Immun. 2017, 85, 1110–1128. [Google Scholar] [CrossRef]
- Naveed, A.; Abdullah, S. Impact of parasitic infection on human gut ecology and immune regulations. Transl. Med. Commun. 2021, 6, 11. [Google Scholar] [CrossRef]
- Gordon, C.A.; Krause, L.; McManus, D.P.; Morrison, M.; Weerakoon, K.G.; Connor, M.C.; Olveda, R.M.; Ross, A.G.; Gobert, G.N. Helminths, polyparasitism, and the gut microbiome in the Philippines. Int. J. Parasitol. 2020, 50, 217–225. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.L.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth Colonization Is Associated with Increased Diversity of the Gut Microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Sardinha-Silva, A.; Alves-Ferreira, E.V.C.; Grigg, M.E. Intestinal immune responses to commensal and pathogenic protozoa. Front. Immunol. 2022, 13, 963723. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Fast, L.; Morris, A. Sickle cell vaso-occlusive crisis: It’s a gut feeling. J. Transl. Med. 2016, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Morris, A.; Li, K.; Fitch, A.C.; Fast, L.; Goldberg, L.; Quesenberry, M.; Sprinz, P.; Methé, B. Intestinal microbiome analysis revealed dysbiosis in sickle cell disease. Am. J. Hematol. 2018, 93, E91–E93. [Google Scholar] [CrossRef]
- Dutta, D.; Methe, B.; Amar, S.; Morris, A.; Lim, S.H. Intestinal injury and gut permeability in sickle cell disease. J. Transl. Med. 2019, 17, 8–11. [Google Scholar] [CrossRef]
- Delgadinho, M.; Ginete, C.; Santos, B.; Mendes, J.; Miranda, A.; Vasconcelos, J.; Brito, M. Microbial gut evaluation in an angolan paediatric population with sickle cell disease. J. Cell Mol. Med. 2022, 26, 5360–5368. [Google Scholar] [CrossRef]
- Delgadinho, M.; Ginete, C.; Fernandes, C.; Silva, C.; Vasconcelos, J.; Brito, M. How Hydroxyurea Alters the Gut Microbiome: A Longitudinal Study involving Angolan Children with Sickle Cell Anemia. Int. J. Mol. Sci. 2022, 23, 9061. [Google Scholar] [CrossRef]
- Ochocinski, D.; Dalal, M.; Black, L.V.; Carr, S.; Lew, J.; Sullivan, K.; Kissoon, N. Life-Threatening Infectious Complications in Sickle Cell Disease: A Concise Narrative Review. Front. Pediatr. 2020, 8, 38. [Google Scholar] [CrossRef]
- Onoh, E.; Manyike, P.C.; Muoneke, U.V.; Okike, C.O.; Ikegwuonu, C.; Ibe, B.C. Intestinal Helminthic Infection among Children with Sickle Cell Anaemia in in Abakaliki, Ebonyi State: Prevalence and Predictors for its Development. Niger. J. Med. 2020, 29, 217–223. [Google Scholar]
- Ahmed, S.G.; Uraka, J. Impact of intestinal parasites on haematological parameters of sickle-cell anaemia patients in Nigeria. EMHJ 2011, 17, 710–713. [Google Scholar] [CrossRef]
- De Alegria, M.L.A.R.; Colmenares, K.; Espasa, M.; Amor, A.; Lopez, I.; Nindia, A.; Kanjala, J.; Guilherme, D.; Sulleiro, E.; Barriga, B.; et al. Prevalence of strongyloides stercoralis and other intestinal parasite infections in school children in a rural area of Angola: A cross-sectional study. Am. J. Trop. Med. Hyg. 2017, 97, 1226–1231. [Google Scholar] [CrossRef]
- Sackey, M.E.; Weigel, M.M.; Armijos, R.X. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J. Trop. Pediatr. 2003, 49, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Reaume, C.; Moore, B.; Hernández, P.; Ruzzini, A.; Chlebus, M.; Wasserman, M.; Yee, J. Evaluation of drugs and stationary growth on the cell cycle of Giardia intestinalis. Mol. Biochem. Parasitol. 2013, 187, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Munera López, J.; Ganuza, A.; Bogado, S.S.; Muñoz, D.; Ruiz, D.M.; Sullivan, W.J.; Vanagas, L.; Angel, S.O. Evaluation of ATM Kinase Inhibitor KU-55933 as Potential Anti-Toxoplasma gondii Agent. Front. Cell. Infect. Microbiol. 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Stephen Adeyemi, O.; Nadwa, E.; Rashwan, E.; Yokoyama, N.; Igarashi, I. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS ONE 2020, 15, e0228996. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Zhao, Z.; Englund, P.T. Effect of hydroxyurea on procyclic Trypanosoma brucei: An unconventional mechanism for achieving synchronous growth. Eukaryot. Cell 2008, 7, 425–428. [Google Scholar] [CrossRef]
- Odame, I. HU for SCA in Africa: Associated malaria benefit. Blood 2023, 141, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.; Taoufiq, Z.; Brun, M.; Tefit, M.; Franetich, J.F.; Ciceron, L.; Krishnamoorthy, R.; Mazier, D. Effects of hydroxyurea on malaria, parasite growth and adhesion in experimental models. Parasite Immunol. 2006, 28, 675–680. [Google Scholar] [CrossRef]
- Hoyne, G.F.; Boreham, P.F.L.; Parsons, P.G.; Ward, C.; Biggs, B. The effect of drugs on the cell cycle of Giardia intestinalis. Parasitology 1989, 99, 333–339. [Google Scholar] [CrossRef]
- Beyhan, Y.E.; Yıldız, M.R. Microbiota and parasite relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.W.; Jeon, C.O. Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef]
- Dubik, M.; Pilecki, B.; Moeller, J.B. Commensal Intestinal Protozoa—Underestimated Members of the Gut Microbial Community. Biology 2022, 11, 1742. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Huwe, T.; Prusty, B.K.; Ray, A.; Lee, S.; Ravindran, B.; Michael, E. Interactions between parasitic infections and the human gut microbiome in Odisha, India. Am. J. Trop. Med. Hyg. 2019, 100, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Delgadinho, M.; Veiga, L.; Ginete, C.; Santos, B.; Miranda, A.; Vasconcelos, J.N.; Brito, M. Differential expression of adhesion molecules in sickle cell anemia and gut microbiome effect. Ann. Hematol. 2023, 103, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Blangé, R.A.; Templeton, K.; Schinkel, J.; Brienen, E.A.T.; Van Rooyen, M.A.A.; Van Lieshout, L.; Polderman, A.M. Simultaneous Detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in Fecal Samples by Using Multiplex Real-Time PCR. J. Clin. Microbiol. 2004, 42, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Köller, T.; Hahn, A.; Altangerel, E.; Verweij, J.J.; Landt, O.; Kann, S.; Dekker, D.; May, J.; Loderstädt, U.; Podbielski, A.; et al. Comparison of commercial and in-house real-time PCR platforms for 15 parasites and microsporidia in human stool samples without a gold standard. Acta Trop. 2020, 207, 105516. [Google Scholar] [CrossRef] [PubMed]
- Basuni, M.; Muhi, J.; Othman, N.; Verweij, J.J.; Ahmad, M.; Miswan, N.; Rahumatullah, A.; Aziz, F.A.; Zainudin, N.S.; Noordin, R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2011, 84, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.M.V.; Senra, C.; de Oliveira, A.A.; Carneiro NF de, F.; Gomes, L.I.; Rabello, A.; Coelho, P.M.Z.; Oliveira, E. A Real-Time PCR Assay for the Diagnosis of Intestinal Schistosomiasis and Cure Assessment After the Treatment of Individuals With Low Parasite Burden. Front. Immunol. 2021, 11, 620417. [Google Scholar] [CrossRef]
- Kaisar, M.M.M.; Brienen, E.A.T.; Djuardi, Y.; Sartono, E.; Yazdanbakhsh, M.; Verweij, J.J.; Supali, T.; Van Lieshout, L. Improved diagnosis of Trichuris trichiura by using a bead-beating procedure on ethanol preserved stool samples prior to DNA isolation and the performance of multiplex real-Time PCR for intestinal parasites. Parasitology 2017, 144, 965–974. [Google Scholar] [CrossRef]
| Giardia (+) | Entamoeba (+) | Ascaris (+) | Enterobius (+) | |
|---|---|---|---|---|
| ↓ Lactobacillus_uc | p = 0.010 | p = 0.031 | n.s. | p = 0.042 |
| ↓ Bifidobacterium_uc | n.s. | p < 0.001 | p = 0.001 | n.s. |
| ↓ Cuneatibacter | p = 0.002 | n.s. | p < 0.001 | p = 0.029 |
| ↓ Bacteroides uniformis | p = 0.025 | p = 0.017 | p = 0.021 | n.s. |
| ↓ Roseburia_uc | n.s. | p = 0.026 | p = 0.001 | p = 0.040 |
| ↓ Shuttleworthia_uc | p = 0.011 | p < 0.001 | p = 0.003 | n.s. |
| Target Parasite | Molecular Target | Oligonucleotides | Sequence (5′-3′) | Melting Temperature | References |
|---|---|---|---|---|---|
| Entamoeba histolytica | SSU rRNA | FW | ATTGTCGTGGCATCCTAACTCA | 76.5 °C | [46] |
| REV | GCGGACGGCTCATTATAACA | ||||
| Hymenolepis nana | ITS1 | FW | CATTGTGTACCAAATTGATGATGAGTA | 79.5 °C | [47] |
| REV | CAACTGACAGCATGTTTCGATATG | ||||
| Strongyloides stercoralis | 18S | FW | GAATTCCAAGTAAACGTAAGTCATTAGC | 81.5 °C | [48] |
| REV | TGCCTCTGGATATTGCTCAGTTC | ||||
| Ascaris lumbricoides | ITS1 | FW | GTAATAGCAGTCGGCGGTTTCTT | [48] | |
| REV | GCCCAACATGCCACCTATTC | ||||
| Probe | FAM-TTGGCGGACAATTGCATGCGAT-BHQ1 | ||||
| Giardia intestinalis | SSU rRNA | FW | GACGGCTCAGGACAACGGTT | [46] | |
| REV | TTGCCAGCGGTGTCCG | ||||
| Probe | FAM-CCCGCGGCGGTCCCTGCTAG-BHQ1 | ||||
| Schistosoma mansoni | 90 bp fragment of a highly repetitive 121 bp sequence of S. mansoni | FW | CCGACCAACCGTTCTATGA | [49] | |
| REV | CACGCTCTCGCAAATAATCTAAA | ||||
| Probe | FAM-TCGTTGTATCTCCGAAACCACTGGACG-BHQ1 | ||||
| Enterobius vermicularis | ITS1 | FW | CGGTGTAATTTTGTTGGTGTCTATG | [47] | |
| REV | TGGCAGCATTGCAAACTAATG | ||||
| Probe | FAM-TGTGCCAGTCAACGCCTAAACCGTC-BHQ1 | ||||
| Trichuris trichiura | 18S | FW | TTGAAACGACTTGCTCATCAACTT | [50] | |
| REV | CTGATTCTCCGTTAACCGTTGTC | ||||
| Probe | FAM-CGATGGTACGCTACGTGCTTACCATGG-BHQ1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgadinho, M.; Ginete, C.; Santos, B.; de Vasconcelos, J.N.; Arez, A.P.; Brito, M. Sickle Cell Disease and Gut Health: The Influence of Intestinal Parasites and the Microbiome on Angolan Children. Int. J. Mol. Sci. 2024, 25, 7258. https://doi.org/10.3390/ijms25137258
Delgadinho M, Ginete C, Santos B, de Vasconcelos JN, Arez AP, Brito M. Sickle Cell Disease and Gut Health: The Influence of Intestinal Parasites and the Microbiome on Angolan Children. International Journal of Molecular Sciences. 2024; 25(13):7258. https://doi.org/10.3390/ijms25137258
Chicago/Turabian StyleDelgadinho, Mariana, Catarina Ginete, Brígida Santos, Jocelyne Neto de Vasconcelos, Ana Paula Arez, and Miguel Brito. 2024. "Sickle Cell Disease and Gut Health: The Influence of Intestinal Parasites and the Microbiome on Angolan Children" International Journal of Molecular Sciences 25, no. 13: 7258. https://doi.org/10.3390/ijms25137258
APA StyleDelgadinho, M., Ginete, C., Santos, B., de Vasconcelos, J. N., Arez, A. P., & Brito, M. (2024). Sickle Cell Disease and Gut Health: The Influence of Intestinal Parasites and the Microbiome on Angolan Children. International Journal of Molecular Sciences, 25(13), 7258. https://doi.org/10.3390/ijms25137258








