First Immunohistochemical Demonstration of the Expression of a Type-2 Vomeronasal Receptor, V2R2, in Wild Canids
Abstract
:1. Introduction
2. Results
3. Discussion
4. Specific Conclusions and Future Perspectives
Future Research Directions
5. Strengths and Limitations of the Study
6. Materials and Methods
6.1. Immunohistochemistry Methodology
6.2. Primary Antibodies
6.3. Image Capture
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaas, J.H. The Evolution of Brains from Early Mammals to Humans. WIRES Cogn. Sci. 2013, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.; Baldwin, M.W. Evolution of Sensory Systems. Curr. Opin. Neurobiol. 2021, 71, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Woszczyło, M.; Jezierski, T.; Szumny, A.; Niżański, W.; Dzięcioł, M. The Role of Urine in Semiochemical Communication between Females and Males of Domestic Dog (Canis familiaris) during Estrus. Animals 2020, 10, 2112. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Kendrick, K.M. Mammalian Social Odours: Attraction and Individual Recognition. Phil. Trans. R. Soc. B 2006, 361, 2061–2078. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Moreno, C.; Barneo-Muñoz, M.; Ibáñez-Gual, M.V.; Lanuza, E.; Agustín-Pavón, C.; Sánchez-Catalán, M.J.; Martínez-García, F. Becoming a Mother Shifts the Activity of the Social and Motivation Brain Networks in Mice. iScience 2022, 25, 104525. [Google Scholar] [CrossRef]
- Meredith, M. Vomeronasal Organ Removal before Sexual Experience Impairs Male Hamster Mating Behavior. Physiol. Behav. 1986, 36, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Maico, L.M.; Burrows, A.M.; Mooney, M.P.; Siegel, M.I.; Bhatnagar, K.P.; Smith, T.D. Size of the Vomeronasal Organ in Wild Microtus with Different Mating Strategies. Acta Biol. Hung. 2003, 54, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Richardson, B.J.; Wyllie, S.G. Semiochemicals and Social Signaling in the Wild European Rabbit in Australia: I. Scent Profiles of Chin Gland Secretion from the Field. J. Chem. Ecol. 2002, 28, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.V. Mammalian Semiochemicals. In The Chemistry of Pheromones and Other Semiochemicals II; Schulz, S., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 240, pp. 231–278. ISBN 978-3-540-21308-6. [Google Scholar]
- Kimoto, H.; Haga, S.; Sato, K.; Touhara, K. Sex-Specific Peptides from Exocrine Glands Stimulate Mouse Vomeronasal Sensory Neurons. Nature 2005, 437, 898–901. [Google Scholar] [CrossRef]
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From Pheromones to Behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef]
- Fortes-Marco, L.; Lanuza, E.; Martinez-Garcia, F. Of Pheromones and Kairomones: What Receptors Mediate Innate Emotional Responses?: Pheromones and Kairomones. Anat. Rec. 2013, 296, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, R.M.; Ghirardi, F.; Tirindelli, R. Lacrimal Gland Removal Impairs Sexual Behavior in Mice. Front. Neuroanat. 2014, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Reep, R.L.; Finlay, B.L.; Darlington, R.B. The Limbic System in Mammalian Brain Evolution. Brain Behav. Evol. 2007, 70, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-de Miguel, S.; Barreiro-Vázquez, J.D.; Sánchez-Quinteiro, P.; Ortiz-Leal, I.; González-Martínez, Á. Behavioural Disorder in a Dog with Congenital Agenesis of the Vomeronasal Organ and the Septum Pellucidum. Vet. Rec. Case Rep. 2023, 11, e571. [Google Scholar] [CrossRef]
- Zilkha, N.; Chuartzman, S.G.; Sofer, Y.; Pen, Y.; Cum, M.; Mayo, A.; Alon, U.; Kimchi, T. Sex-Dependent Control of Pheromones on Social Organization within Groups of Wild House Mice. Curr. Biol. 2023, 33, 1407–1420.e4. [Google Scholar] [CrossRef] [PubMed]
- Del Cerro, M. Role of the Vomeronasal Input in Maternal Behaviour. Psychoneuroendocrinology 1998, 23, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R.W.; Stolzenberg, D.S. Flipping the Parental Switch: From Killing to Caring in Male Mammals. Anim. Behav. 2020, 165, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The Effects of Predator Odors in Mammalian Prey Species: A Review of Field and Laboratory Studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, J.; Moine, F.; Klaey, M.; Nenniger-Tosato, M.; Hurni, N.; Sporkert, F.; Giroud, C.; Broillet, M.-C. Mouse Alarm Pheromone Shares Structural Similarity with Predator Scents. Proc. Natl. Acad. Sci. USA 2013, 110, 4762–4767. [Google Scholar] [CrossRef]
- Stowers, L.; Marton, T.F. What Is a Pheromone? Mammalian Pheromones Reconsidered. Neuron 2005, 46, 699–702. [Google Scholar] [CrossRef]
- Torres, M.V.; Ortiz-Leal, I.; Sanchez-Quinteiro, P. Pheromone Sensing in Mammals: A Review of the Vomeronasal System. Anatomia 2023, 2, 346–413. [Google Scholar] [CrossRef]
- Doyle, W.I.; Meeks, J.P. Excreted Steroids in Vertebrate Social Communication. J. Neurosci. 2018, 38, 3377–3387. [Google Scholar] [CrossRef]
- Xie, W.; Chen, M.; Shen, Y.; Liu, Y.; Zhang, H.; Weng, Q. Vomeronasal Receptors Associated with Circulating Estrogen Processing Chemosensory Cues in Semi-Aquatic Mammals. IJMS 2023, 24, 10724. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Brennan, P.; Widmayer, P.; Chandramani S, P.; Maul-Pavicic, A.; Jäger, M.; Li, X.-H.; Breer, H.; Zufall, F.; Boehm, T. MHC Class I Peptides as Chemosensory Signals in the Vomeronasal Organ. Science 2004, 306, 1033–1037. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Ishii, T.; Chamero, P.; Hendrix, P.; Oboti, L.; Schmid, A.; Kircher, S.; Pyrski, M.; Akiyoshi, S.; Khan, M.; et al. A Family of Nonclassical Class I MHC Genes Contributes to Ultrasensitive Chemodetection by Mouse Vomeronasal Sensory Neurons. J. Neurosci. 2014, 34, 5121–5133. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J. Neurobehavioral Evidence for the Involvement of the Vomeronasal System in Mammalian Reproduction. Neurosci. Biobehav. Rev. 1979, 3, 301–341. [Google Scholar] [CrossRef]
- Halpern, M.; Martínez-Marcos, A. Structure and Function of the Vomeronasal System: An Update. Prog. Neurobiol. 2003, 70, 245–318. [Google Scholar] [CrossRef] [PubMed]
- Swaney, W.T.; Keverne, E.B. The Evolution of Pheromonal Communication. Behav. Brain Res. 2009, 200, 239–247. [Google Scholar] [CrossRef]
- Salazar, I.; Barrios, A.W.; Sánchez-Quinteiro, P. Revisiting the Vomeronasal System From an Integrated Perspective. Anat. Rec. 2016, 299, 1488–1491. [Google Scholar] [CrossRef]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A Putative Pheromone Receptor Gene Expressed in Human Olfactory Mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef]
- Trinh, K.; Storm, D.R. Vomeronasal Organ Detects Odorants in Absence of Signaling through Main Olfactory Epithelium. Nat. Neurosci. 2003, 6, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hagino-Yamagishi, K. Diverse Systems for Pheromone Perception: Multiple Receptor Families in Two Olfactory Systems. Zool. Sci. 2008, 25, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.S. Morphological Studies on the Rodent Main and Accessory Olfactory Systems: The Regio Olfactoria and Vomeronasal Organ. Ann. Anat.-Anat. Anz. 1993, 175, 425–446. [Google Scholar] [CrossRef]
- Chamero, P.; Katsoulidou, V.; Hendrix, P.; Bufe, B.; Roberts, R.; Matsunami, H.; Abramowitz, J.; Birnbaumer, L.; Zufall, F.; Leinders-Zufall, T. G Protein Gαo Is Essential for Vomeronasal Function and Aggressive Behavior in Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 12898–12903. [Google Scholar] [CrossRef]
- Ibarra-Soria, X.; Levitin, M.O.; Saraiva, L.R.; Logan, D.W. The Olfactory Transcriptomes of Mice. PLoS Genet. 2014, 10, e1004593. [Google Scholar] [CrossRef]
- Mechin, V.; Pageat, P.; Teruel, E.; Asproni, P. Histological and Immunohistochemical Characterization of Vomeronasal Organ Aging in Mice. Animals 2021, 11, 1211. [Google Scholar] [CrossRef]
- Portalés, A.; Chamero, P.; Jurado, S. Natural and Pathological Aging Distinctively Impacts the Pheromone Detection System and Social Behavior. Mol. Neurobiol. 2023, 60, 4641–4658. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Asano, T.; Kato, K. Differential Localization of G-Proteins Gi and Go in the Accessory Olfactory Bulb of the Rat. J. Neurosci. 1992, 12, 1275–1279. [Google Scholar] [CrossRef]
- Halpern, M.; Shapiro, L.S.; Jia, C. Differential Localization of G Proteins in the Opossum Vomeronasal System. Brain Res. 1995, 677, 157–161. [Google Scholar] [CrossRef]
- Jia, C.; Halpern, M. Subclasses of Vomeronasal Receptor Neurons: Differential Expression of G Proteins (Giα2 and Goα) and Segregated Projections to the Accessory Olfactory Bulb. Brain Res. 1996, 719, 117–128. [Google Scholar] [CrossRef]
- Berghard, A.; Buck, L. Sensory Transduction in Vomeronasal Neurons: Evidence for G Alpha o, G Alpha I2, and Adenylyl Cyclase II as Major Components of a Pheromone Signaling Cascade. J. Neurosci. 1996, 16, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C.; Axel, R. A Novel Family of Genes Encoding Putative Pheromone Receptors in Mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Herrada, G.; Dulac, C. A Novel Family of Putative Pheromone Receptors in Mammals with a Topographically Organized and Sexually Dimorphic Distribution. Cell 1997, 90, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Buck, L.B. A Multigene Family Encoding a Diverse Array of Putative Pheromone Receptors in Mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Ryba, N.J.P.; Tirindelli, R. A New Multigene Family of Putative Pheromone Receptors. Neuron 1997, 19, 371–379. [Google Scholar] [CrossRef]
- Takigami, S.; Mori, Y.; Ichikawa, M. Projection Pattern of Vomeronasal Neurons to the Accessory Olfactory Bulb in Goats. Chem. Senses 2000, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Takigami, S. Morphological Evidence for Two Types of Mammalian Vomeronasal System. Chem. Senses 2004, 29, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Cifuentes, J.M.; Sánchez-Quinteiro, P. Morphological and Immunohistochemical Features of the Vomeronasal System in Dogs. Anat. Rec. 2013, 296, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Quinteiro, P.S.; Alemañ, N.; Cifuentes, J.M.; Troconiz, P.F. Diversity of the Vomeronasal System in Mammals: The Singularities of the Sheep Model. Microsc. Res. Tech. 2007, 70, 752–762. [Google Scholar] [CrossRef]
- Suárez, R.; Fernández-Aburto, P.; Manger, P.R.; Mpodozis, J. Deterioration of the Gαo Vomeronasal Pathway in Sexually Dimorphic Mammals. PLoS ONE 2011, 6, e26436. [Google Scholar] [CrossRef]
- Salazar, I.; Sánchez-Quinteiro, P. A Detailed Morphological Study of the Vomeronasal Organ and the Accessory Olfactory Bulb of Cats. Microsc. Res. Tech. 2011, 74, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Watanabe, K.; Nishihara, K.; Ono, Y.S.; Nakamura, K.G.; Yuhara, K.; Tomikawa, S.; Sugimoto, M.; Kobayashi, S.; Horiuchi, N.; et al. Histological Properties of Main and Accessory Olfactory Bulbs in the Common Hippopotamus. Brain Behav. Evol. 2017, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. Neuroanatomical and Immunohistological Study of the Main and Accessory Olfactory Bulbs of the Meerkat (Suricata suricatta). Animals 2021, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, D.; Kawai, Y.K.; Watanabe, K.; Muranishi, Y. Artiodactyl Livestock Species Have a Uniform Vomeronasal System with a Vomeronasal Type 1 Receptor (V1R) Pathway. Tissue Cell 2022, 77, 101863. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. The Vomeronasal Organ of the Tammar Wallaby. J. Anat. 2008, 213, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. Does a Third Intermediate Model for the Vomeronasal Processing of Information Exist? Insights from the Macropodid Neuroanatomy. Brain. Struct. Funct. 2022, 227, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Grus, W.E.; Shi, P.; Zhang, Y.-p.; Zhang, J. Dramatic Variation of the Vomeronasal Pheromone Receptor Gene Repertoire among Five Orders of Placental and Marsupial Mammals. Proc. Natl. Acad. Sci. USA 2005, 102, 5767–5772. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Trask, B.J. V2R Gene Families Degenerated in Primates, Dog and Cow, but Expanded in Opossum. Trends Genet. 2007, 23, 212–215. [Google Scholar] [CrossRef]
- Grus, W.E.; Zhang, J. Distinct Evolutionary Patterns between Chemoreceptors of 2 Vertebrate Olfactory Systems and the Differential Tuning Hypothesis. Mol. Biol. Evol. 2008, 25, 1593–1601. [Google Scholar] [CrossRef]
- Dong, D.; Jin, K.; Wu, X.; Zhong, Y. CRDB: Database of Chemosensory Receptor Gene Families in Vertebrate. PLoS ONE 2012, 7, e31540. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; López-Beceiro, A.; Sanchez-Quinteiro, P. The Vomeronasal Organ of Wild Canids: The Fox (Vulpes vulpes) as a Model. J. Anat. 2020, 237, 890–906. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Barreiro-Vázquez, J.; López-Beceiro, A.; Fidalgo, L.; Shin, T.; Sanchez-Quinteiro, P. The Vomeronasal System of the Wolf (Canis lupus signatus): The Singularities of a Wild Canid. J. Anat. 2024, 245, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Hohenbrink, P.; Mundy, N.I.; Zimmermann, E.; Radespiel, U. First Evidence for Functional Vomeronasal 2 Receptor Genes in Primates. Biol. Lett. 2013, 9, 20121006. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.C.; Allgier, J.G.; Desouza, L.S.; Eward, W.C.; Morrison, E.E. Immunohistochemistry of the Canine Vomeronasal Organ. J. Anat. 2003, 202, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Silvotti, L.; Shirazi, A.; Ryba, N.J.P.; Tirindelli, R. Co-Expression of Putative Pheromone Receptors in the Sensory Neurons of the Vomeronasal Organ. J. Neurosci. 2001, 21, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Silvotti, L. The Vomeronasal Receptor V2R2 Does Not Require Escort Molecules for Expression in Heterologous Systems. Chem. Senses 2005, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Silvotti, L.; Moiani, A.; Gatti, R.; Tirindelli, R. Combinatorial Co-expression of Pheromone Receptors, V2Rs. J. Neurochem. 2007, 103, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Antunes, A. Vomeronasal Receptors in Vertebrates and the Evolution of Pheromone Detection. Annu. Rev. Anim. Biosci. 2017, 5, 353–370. [Google Scholar] [CrossRef]
- Mier Quesada, Z.; Portillo, W.; Paredes, R.G. Behavioral Evidence of the Functional Interaction between the Main and Accessory Olfactory System Suggests a Large Olfactory System with a High Plastic Capability. Front. Neuroanat. 2023, 17, 1211644. [Google Scholar] [CrossRef]
- Torres, M.V.; Ortiz-Leal, I.; Villamayor, P.R.; Ferreiro, A.; Rois, J.L.; Sanchez-Quinteiro, P. The Vomeronasal System of the Newborn Capybara: A Morphological and Immunohistochemical Study. Sci. Rep. 2020, 10, 13304. [Google Scholar] [CrossRef]
- Suárez, R.; Santibáñez, R.; Parra, D.; Coppi, A.A.; Abrahão, L.M.B.; Sasahara, T.H.C.; Mpodozis, J. Shared and Differential Traits in the Accessory Olfactory Bulb of Caviomorph Rodents with Particular Reference to the Semiaquatic Capybara: The AOB of Capybaras and Other Caviomorphs. J. Anat. 2011, 218, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, P.R.; Cifuentes, J.M.; Quintela, L.; Barcia, R.; Sanchez-Quinteiro, P. Structural, Morphometric and Immunohistochemical Study of the Rabbit Accessory Olfactory Bulb. Brain Struct. Funct. 2020, 225, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Hohenbrink, P.; Dempewolf, S.; Zimmermann, E.; Mundy, N.I.; Radespiel, U. Functional Promiscuity in a Mammalian Chemosensory System: Extensive Expression of Vomeronasal Receptors in the Main Olfactory Epithelium of Mouse Lemurs. Front. Neuroanat. 2014, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, J.; Kondoh, D.; Sakamoto, H.; Matsumoto, N.; Sasaki, M.; Kitamura, N.; Haneda, S.; Matsui, M. Morphological and Histological Features of the Vomeronasal Organ in the Brown Bear. J. Anat. 2017, 231, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, J.; Korzekwa, A.; Kawai, Y.K.; Robstad, C.A.; Rosell, F.; Kondoh, D. The Vomeronasal System in Semiaquatic Beavers. J. Anat. 2022, 241, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nikaido, M. Inactivation of ancV1R as a Predictive Signature for the Loss of Vomeronasal System in Mammals. Genome Biol. Evol. 2020, 12, 766–778. [Google Scholar] [CrossRef]
- Yohe, L.R.; Krell, N.T. An Updated Synthesis of and Outstanding Questions in the Olfactory and Vomeronasal Systems in Bats: Genetics Asks Questions Only Anatomy Can Answer. Anat. Rec. 2023, 306, 2765–2780. [Google Scholar] [CrossRef] [PubMed]
- Rawson, N.E.; Eberwine, J.; Dotson, R.; Jackson, J.; Ulrich, P.; Restrepo, D. Expression of mRNAs Encoding for Two Different Olfactory Receptors in a Subset of Olfactory Receptor Neurons. J. Neurochem. 2000, 75, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P. Odorant Receptor Gene Choice in Olfactory Sensory Neurons: The One Receptor–One Neuron Hypothesis Revisited. Curr. Opin. Neurobiol. 2004, 14, 31–36. [Google Scholar] [CrossRef]
- Horgue, L.F.; Assens, A.; Fodoulian, L.; Marconi, L.; Tuberosa, J.; Haider, A.; Boillat, M.; Carleton, A.; Rodriguez, I. Transcriptional Adaptation of Olfactory Sensory Neurons to GPCR Identity and Activity. Nat. Commun. 2022, 13, 2929. [Google Scholar] [CrossRef]
- Speca, D.J.; Lin, D.M.; Sorensen, P.W.; Isacoff, E.Y.; Ngai, J.; Dittman, A.H. Functional Identification of a Goldfish Odorant Receptor. Neuron 1999, 23, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Borowsky, B.; Tamm, J.A.; Craig, D.A.; Durkin, M.M.; Dai, M.; Yao, W.-J.; Johnson, M.; Gunwaldsen, C.; Huang, L.-Y.; et al. GABAB Receptors Function as a Heteromeric Assembly of the Subunits GABABR1 and GABABR2. Nature 1998, 396, 674–679. [Google Scholar] [CrossRef] [PubMed]
- White, J.H.; Wise, A.; Main, M.J.; Green, A.; Fraser, N.J.; Disney, G.H.; Barnes, A.A.; Emson, P.; Foord, S.M.; Marshall, F.H. Heterodimerization Is Required for the Formation of a Functional GABAB Receptor. Nature 1998, 396, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Can Domestication Shape Canidae Brain Morphology? The Accessory Olfactory Bulb of the Red Fox as a Case in Point. Ann. Anat. 2022, 240, 151881. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Leal, I.; Torres, M.V.; Vargas-Barroso, V.; Fidalgo, L.E.; López-Beceiro, A.M.; Larriva-Sahd, J.A.; Sánchez-Quinteiro, P. The Olfactory Limbus of the Red Fox (Vulpes vulpes). New Insights Regarding a Noncanonical Olfactory Bulb Pathway. Front. Neuroanat. 2023, 16, 1097467. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, T.; Ensminger, J.; Papet, L.E. (Eds.) Canine Olfaction Science and Law: Advances in Forensic Science, Medicine; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-367-77811-8. [Google Scholar]
- Bird, D.J.; Jacquemetton, C.; Buelow, S.A.; Evans, A.W.; Van Valkenburgh, B. Domesticating Olfaction: Dog Breeds, Including Scent Hounds, Have Reduced Cribriform Plate Morphology Relative to Wolves. Anat. Rec. 2021, 304, 139–153. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; López-Callejo, L.N.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Comparative Neuroanatomical Study of the Main Olfactory Bulb in Domestic and Wild Canids: Dog, Wolf and Red Fox. Animals 2022, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mech, L.D.; Boitani, L. (Eds.) Wolves: Behavior, Ecology, and Conservation; Repr.; University of Chicago Press: Chicago, IL, USA, 2003; ISBN 978-0-226-51696-7. [Google Scholar]
- Martín, J.; Barja, I.; López, P. Chemical Scent Constituents in Feces of Wild Iberian Wolves (Canis lupus signatus). Biochem. Syst. Ecol. 2010, 38, 1096–1102. [Google Scholar] [CrossRef]
- Bakker, J.; Leinders-Zufall, T. The Sense of Smell: Role of the Olfactory Systems in Detecting Pheromones. In Neuroscience in the 21st Century: From Basic to Clinical; Pfaff, D.W., Volkow, N.D., Eds.; Springer: New York, NY, USA, 2016; pp. 935–960. ISBN 978-1-4939-3474-4. [Google Scholar]
- Kukekova, A.V.; Johnson, J.L.; Xiang, X.; Feng, S.; Liu, S.; Rando, H.M.; Kharlamova, A.V.; Herbeck, Y.; Serdyukova, N.A.; Xiong, Z.; et al. Red Fox Genome Assembly Identifies Genomic Regions Associated with Tame and Aggressive Behaviours. Nat. Ecol. Evol. 2018, 2, 1479–1491. [Google Scholar] [CrossRef]
- Prieto-Godino, L.L.; Rytz, R.; Bargeton, B.; Abuin, L.; Arguello, J.R.; Peraro, M.D.; Benton, R. Olfactory Receptor Pseudo-Pseudogenes. Nature 2016, 539, 93–97. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Cockerham, R.E.; Michalakis, S.; Biel, M.; Garbers, D.L.; Reed, R.R.; Zufall, F.; Munger, S.D. Contribution of the Receptor Guanylyl Cyclase GC-D to Chemosensory Function in the Olfactory Epithelium. Proc. Natl. Acad. Sci. USA 2007, 104, 14507–14512. [Google Scholar] [CrossRef] [PubMed]
- Larriva-Sahd, J. Cytological Organization of the Alpha Component of the Anterior Olfactory Nucleus and Olfactory Limbus. Front. Neuroanat. 2012, 6, 30295. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Barroso, V.; Peña-Ortega, F.; Larriva-Sahd, J.A. Olfaction and Pheromones: Uncanonical Sensory Influences and Bulbar Interactions. Front. Neuroanat. 2017, 11, 108. [Google Scholar] [CrossRef] [PubMed]
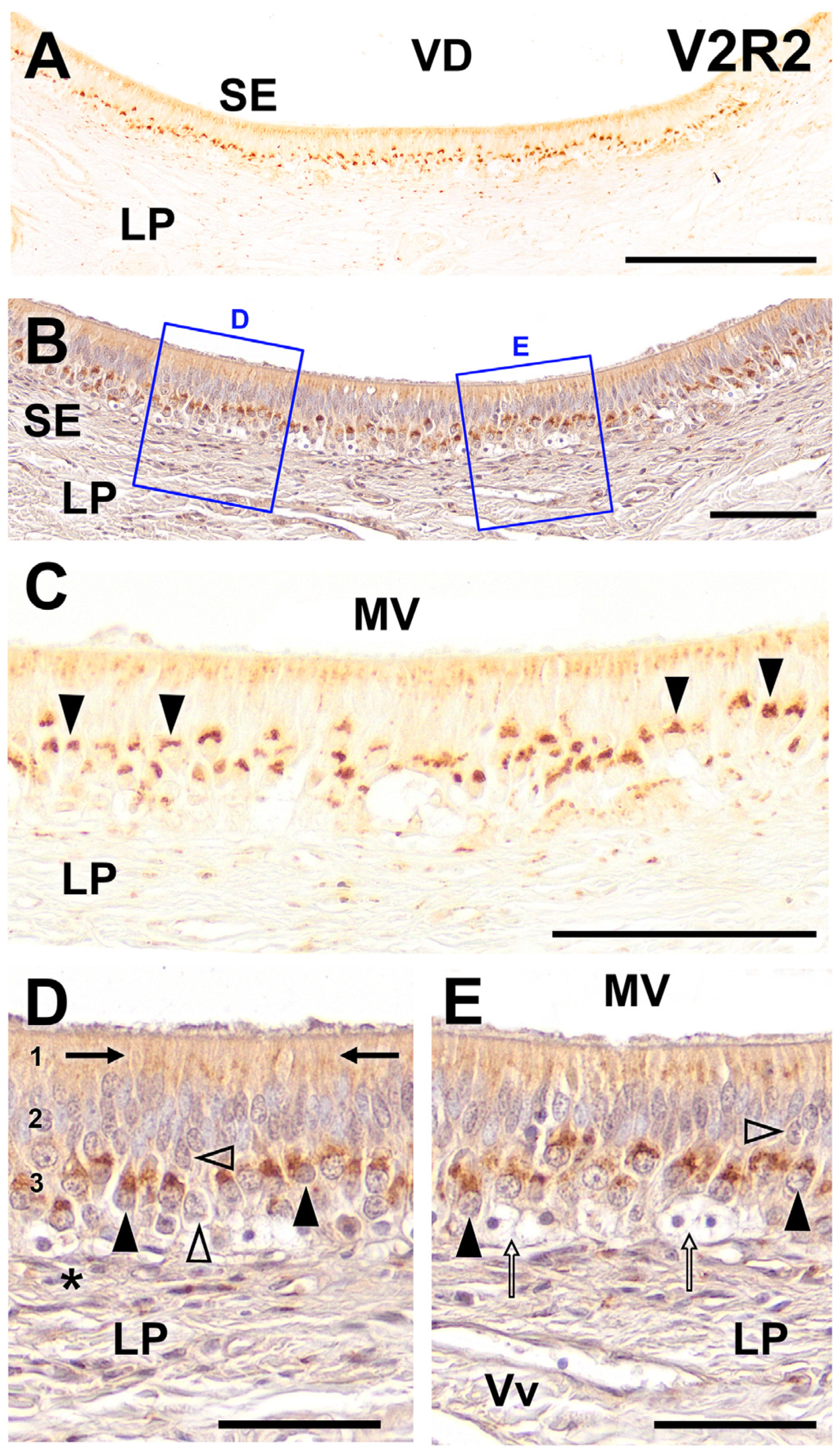
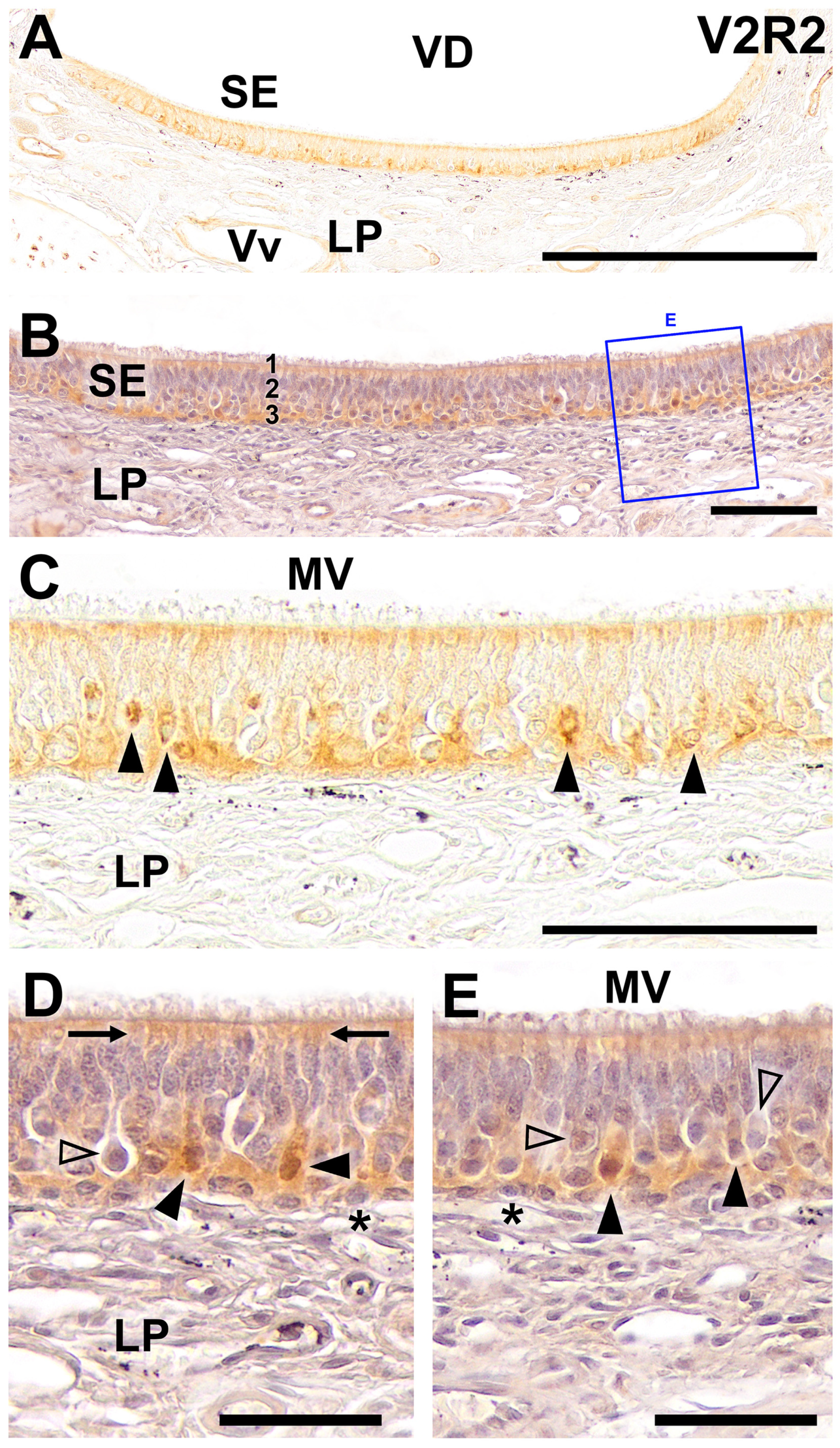
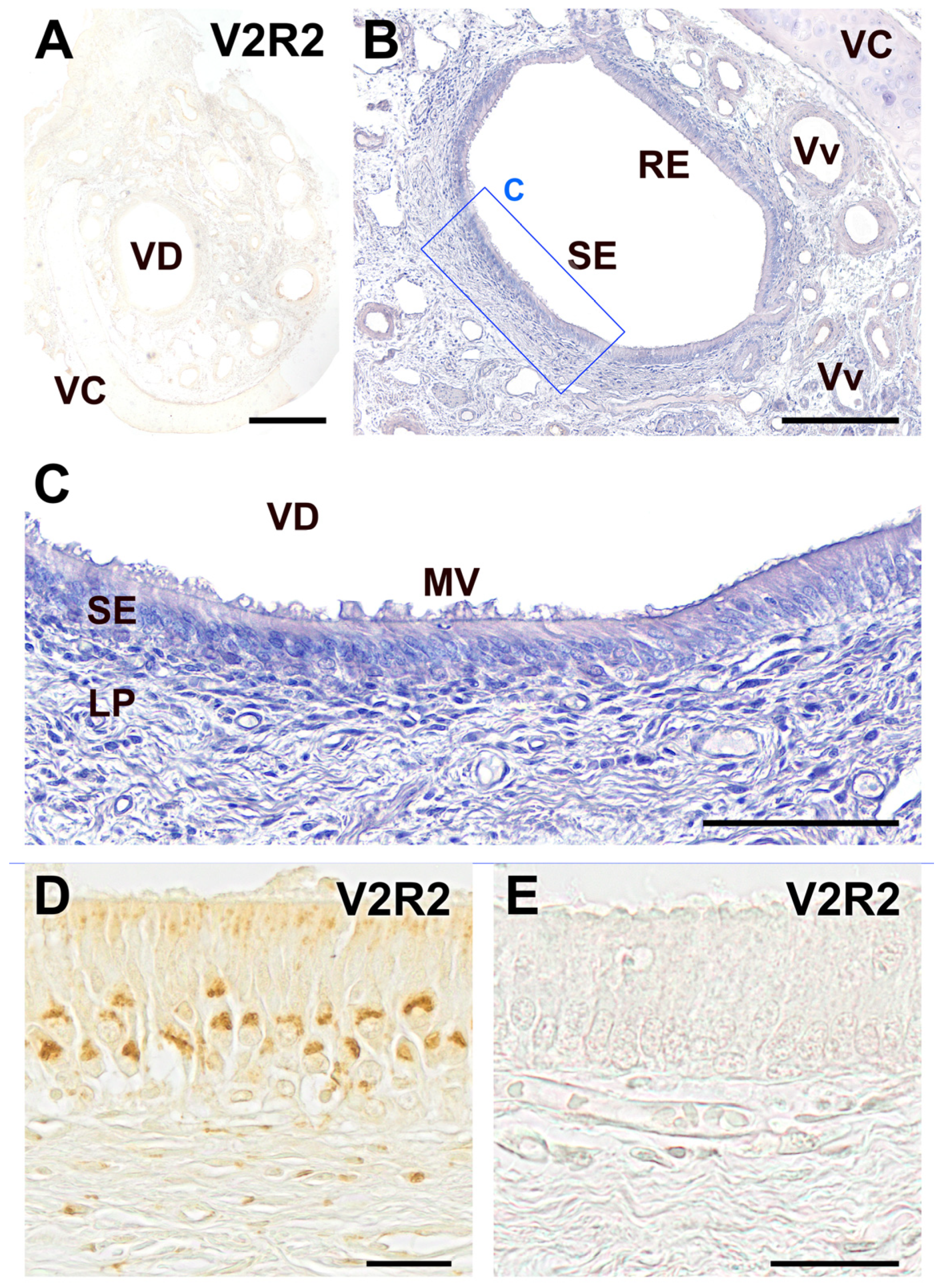

| Species | V2R2-ir in VNO | Gαo-ir in VNO | Observations |
|---|---|---|---|
| Wolf | Positive | Positive | Extensive labeling throughout the VNO neuroepithelium. V2R2 expression is localized mainly to the somas and apical dendritic processes of neuroreceptor cells. |
| Fox | Positive | Positive | Consistent labeling throughout the vomeronasal sensory neuroepithelium. The labeling pattern is similar to that in wolves with strong presence in the somas and apical regions. |
| Dog | Negative | Negative | No immunostaining observed in any part of the VNO, indicating a lack of V2R2 and Gαo expression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Leal, I.; Torres, M.V.; López-Beceiro, A.; Fidalgo, L.; Shin, T.; Sanchez-Quinteiro, P. First Immunohistochemical Demonstration of the Expression of a Type-2 Vomeronasal Receptor, V2R2, in Wild Canids. Int. J. Mol. Sci. 2024, 25, 7291. https://doi.org/10.3390/ijms25137291
Ortiz-Leal I, Torres MV, López-Beceiro A, Fidalgo L, Shin T, Sanchez-Quinteiro P. First Immunohistochemical Demonstration of the Expression of a Type-2 Vomeronasal Receptor, V2R2, in Wild Canids. International Journal of Molecular Sciences. 2024; 25(13):7291. https://doi.org/10.3390/ijms25137291
Chicago/Turabian StyleOrtiz-Leal, Irene, Mateo V. Torres, Ana López-Beceiro, Luis Fidalgo, Taekyun Shin, and Pablo Sanchez-Quinteiro. 2024. "First Immunohistochemical Demonstration of the Expression of a Type-2 Vomeronasal Receptor, V2R2, in Wild Canids" International Journal of Molecular Sciences 25, no. 13: 7291. https://doi.org/10.3390/ijms25137291
APA StyleOrtiz-Leal, I., Torres, M. V., López-Beceiro, A., Fidalgo, L., Shin, T., & Sanchez-Quinteiro, P. (2024). First Immunohistochemical Demonstration of the Expression of a Type-2 Vomeronasal Receptor, V2R2, in Wild Canids. International Journal of Molecular Sciences, 25(13), 7291. https://doi.org/10.3390/ijms25137291









