Modifications of Nanobubble Therapy for Cancer Treatment
Abstract
:1. Introduction
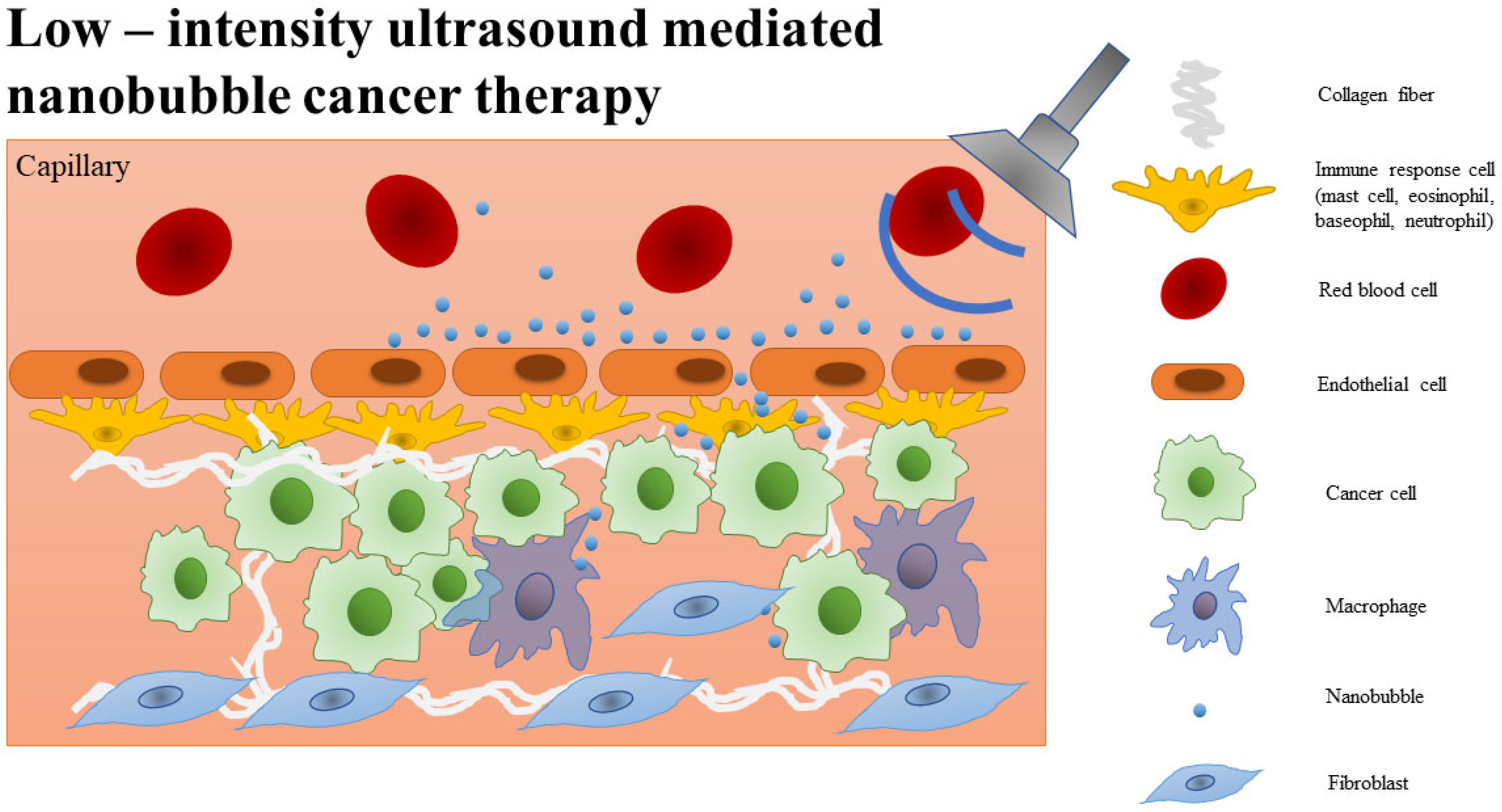
2. Nanobubble Characteristics
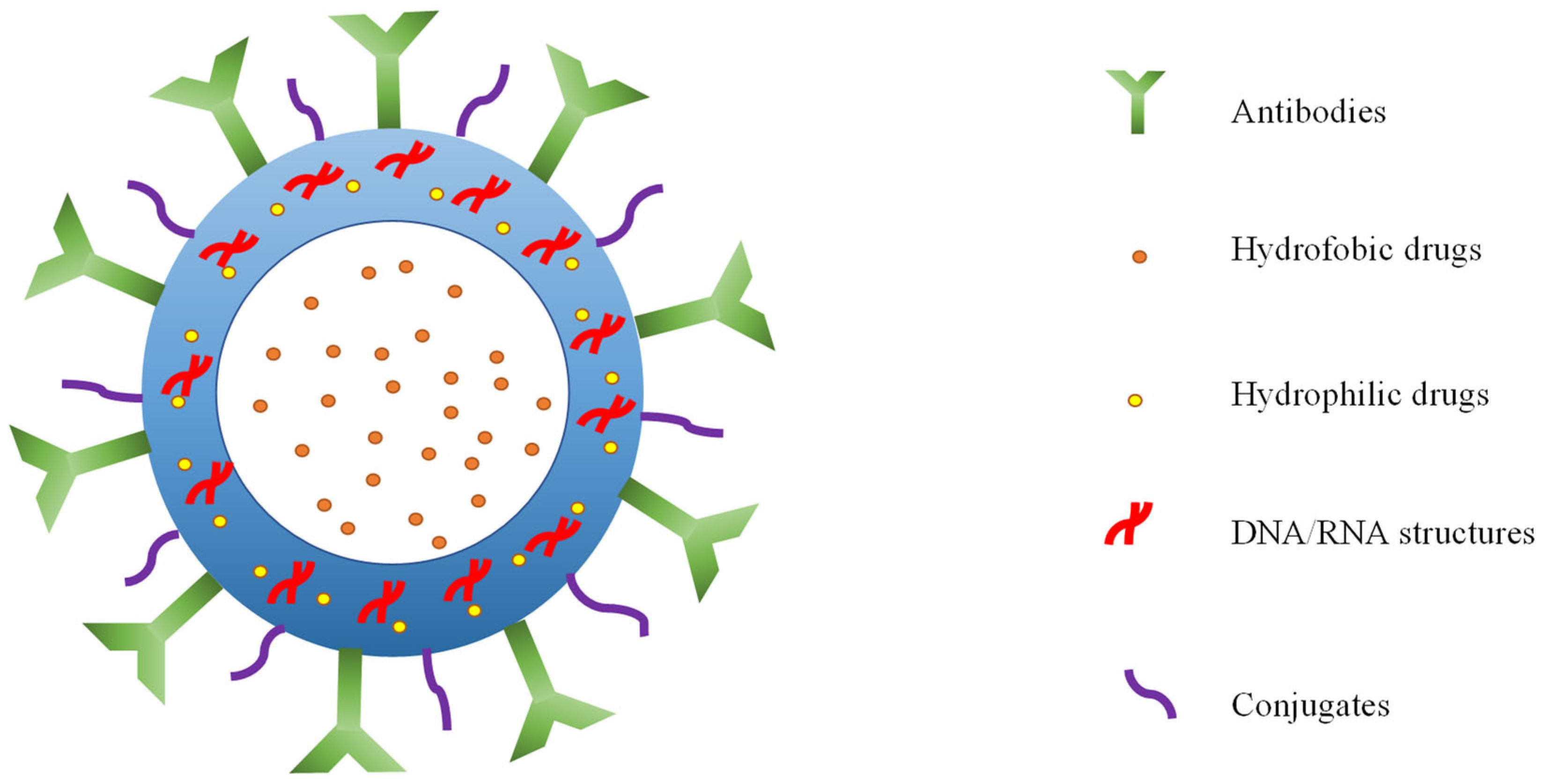
3. Construction of Nanobubbles in Biomedicine
4. Nanobubble Modifications against Cancer
| Drug/Oxygen/Antibody Delivery Technique | Analyzed Material | Measurement Techniques | Outcomes | References |
|---|---|---|---|---|
| Chitosan nanobubbles with a perfluoropropane core and doxorubicin hydrochloride combined with ultrasound (DOX-NBs + US) | MCF-7 cells (Michigan Cancer Foundation-7 cells), breast cancer cells, in vitro | Confocal images with Annexin V staining, flow cytometry |
| [54] |
| Doxorubicin hydrochloride-loaded oxygen core nanobubbles with phospholipid shell | MDA-MB-231 (MD Anderson-Metastatic Breast-231 cancer cells), HeLa cervical cancer cells, in vitro | Reactive oxygen species assays (ROS), confocal images, fluorescence, DAPI staining |
| [57] |
| Paclitaxel-loaded nanobubbles with anti-pro-gastrin-releasing peptide antibody | SCLC (small cell lung cancer), H446 lung cancer cells, in vitro, in vivo | Reverse-transcription polymerase chain reaction (RT-PCR), Western blot, immunohistochemical detection, CCk-8 assay, flow cytometry, cell scratch test, tumor-burden nude mice models |
| [63] |
| Targeting nanobubbles conjugated with NET-1 (Neuroepithelial cell-transforming gene 1) siRNA by shear wave elastography | Hepatocellular carcinoma (HepG2)-bearing mice model, in vivo | Ultrasound, shear wave elastography (SWE), immunohistochemical analysis |
| [67] |
| Hematoporphyrin monomethyl ether (HMME) with Lonidamine(LND) liposome nanobubbles (NBs) with perfluorocarbone core in combination with US (C3F8) HMME-LND@C3F8-NBs | HCC (Hepatocellular carcinoma) Huh7 and HepG2 cancer cell lines, in vitro | CCk-8 assay, intracellular ROS generation detection and mitochondrial membrane potential assay, cell apoptosis assay, measurement of whole transcriptome library, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) |
| [81] |
| Chitosan-shelled nanobubble for the delivery of siRNA against Nrf2 in combination with US | M14 melanoma cancer cells, in vitro | Fluorescence microscopy, viability analysis, Western blot, cytofluorometric evaluation |
| [92] |
5. Conclusions and Future Perspectives in Cancer Treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy. |
| ALS | amyotrophic lateral sclerosis. |
| ARE | antioxidant response element. |
| ASRs | age-specific rates. |
| ATF4 | activating transcription factor 4. |
| BAPTA | 1:2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. |
| BBB | blood-brain barrier. |
| BNBs | bulk nanobubbles. |
| BRAF | activating mutations in cytoplasmic serine/threonine kinase B-Raf gene. |
| CCk-8 | cell counting kit-8. |
| DAPI | 4′:6-diamidino-2-phenylindole dihydrochloride. |
| DOX | doxorubicin. |
| EMT | epithelialmesenchymal transition. |
| GCL | glutamate cysteine ligase |
| GRP | gastrin–releasing peptide. |
| GSH | glutathione. |
| GST | glutathione-s-transferase. |
| H446 | lung cancer cell line. |
| HCC | hepatocellular carcinoma. |
| HeLa | cervical cancer cell line. |
| HepG2 | hepatocellular carcinoma cell line. |
| HIF1α | hypoxia-inducible factor. |
| HMME | hematoporphyrin monomethyl ether. |
| HMME-LND@C3F8-NBs | hematoporphyrin monomethyl ether–lonidamine perflurorocarbon nannobubbles. |
| HMME-PDT | hematoporphyrin monomethyl ether photodynamic therapy. |
| HO-1 | heme oxygenase-1. |
| Huh7 | hepatocellular carcinoma cell line. |
| IL-2 | interleukin-2. |
| ISO | international organization for standardization. |
| Keap1 | kelch-like ECH-associated protein. |
| LND | lonidamine. |
| M14 | melanoma cancer cell line. |
| MCF-7 | breast cancer cell line. |
| MDA-MB-231 | breast cancer cell line. |
| MDR | multidrug resistance. |
| mTOR | mammalian target of rapamycin. |
| NB–nanobubble. | |
| NET-1 | neuroepithelial-transforming protein 1 gene. |
| NET-2 | neuroepithelial-transforming protein 2 gene. |
| NET-7 | neuroepithelial-transforming protein 7 gene. |
| NET-x | neuroepithelial-transforming protein gene family. |
| NK | natural killer cells. |
| NRF2, Nrf2 | nuclear factor erythroid 2 protein. |
| NFE2L2 | NFE2 Like BZIP transcription factor 2 gene. |
| PDT | photodynamic therapy. |
| PLA | poly(lactic acid). |
| PLGA | poly(lactic-co-glycolic acid). |
| ProGRP | pro-gastrin-releasing peptide. |
| qRT-PCR | quantitative reverse transcription-polymerase chain reaction. |
| ROS | reactive oxygen species. |
| RT-PCR | reverse-transcripton polymerase chain reaction. |
| SCLC | small cell lung cancer. |
| SDT | sonodynamic therapy. |
| siRNA | small interfering RNA. |
| SNBs | surface nanobubbles. |
| SREBP2 | sterol regulatory element-binding protein 2. |
| SWE | shear wave elastography. |
| TM4SF | tetraspan superfamily gene. |
| TME | tumor microenvironment. |
| US | ultrasound. |
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Malvezzi, M.; Santucci, C.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2023 with a focus on lung cancer. Ann. Oncol. 2023, 34, 410–419. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Nakahara, R.; Maeda, K.; Aki, S.; Osawa, T. Metabolic adaptations of cancer in extreme tumor microenvironments. Cancer Sci. 2023, 114, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, C.; Hou, Y.; Tian, Y.; Li, Y.; Zhang, H.; Zhang, L.; Li, W. Molecular mechanisms of Thrombospondin-2 modulates tumor vasculogenic mimicry by PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2023, 167, 115455. [Google Scholar] [CrossRef]
- Aki, S.; Nakahara, R.; Maeda, K.; Osawa, T. Cancer metabolism within tumor microenvironments. Biochim. Biophys. Acta 2023, 1867, 130330. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug resistance in cancer: Understanding molecular mechanisms, immunoprevention and therapeutic approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef]
- Batchelor, D.V.B.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; Mclaughlan, J.R.; Coletta, P.L.; Evans, S.D. Nanobubbles for therapeutic delivery: Production, stability and current prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101456. [Google Scholar] [CrossRef]
- ISO 20480-1:2017; Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles—Part 1: Terminology. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 18 March 2024).
- Thi Phan, K.K.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Zhu, J.; An, H.; Alheshibri, M.; Liu, L.; Terpstra, P.M.J.; Liu, G.; Craig, V.S.J. Cleaning with bulk nanobubbles. Langmuir 2016, 32, 11203–11211. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Dong, Y.; Mao, H.; Sun, J.; Chen, S.; Craig, V.S.J.; Hu, J. Cleaning using nanobubbles: Defouling by electrochemical generation of bubbles. J. Colloid Interface Sci. 2008, 328, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro-nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci. Total Environ. 2021, 800, 149627. [Google Scholar] [CrossRef]
- Sharif, P.M.; Hairuddin, A.A.; As’arry, A.; Rezali, K.A.M.; Noor, M.M.; Norhafana, M.; Shareef, S.M. Nano gas bubbles dissolve in gasoline fuel and its influence on engine combustion performance. IOP Conf. Ser. Mater. Sci. Eng. 2019, 469, 012062. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chen, F.; Gu, N. Drug delivery system based on nanobubbles. Interdiscip. Mater. 2022, 1, 471–494. [Google Scholar] [CrossRef]
- Parker, J.L.; Claesson, P.M.; Attard, P. Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces. J. Phys. Chem. 1994, 98, 8468–8480. [Google Scholar] [CrossRef]
- Mita, M.; Matsushima, H.; Ueda, M.; Ito, H. In-situ high-speed atomic force microscopy observation of dynamic nanobubbles during water electrolysis. J. Colloid Interface Sci. 2022, 614, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T. Preparation method and application of nanobubbles: A review. Coatings 2023, 13, 1510. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Li, Y.; Gu, R.; Yan, X. Characterization of lipid-based nanomedicines at the single-particle level. Fundam. Res. 2023, 3, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficient tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.-J.; Hong, J.W.; Choi, J. Oxygen-carrying micro/nanobubbles: Composition, synthesis techniques and potential prospects in photo-triggered theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, P.; Deng, Y.; Liu, Y. Mechanistic insights and therapeutic delivery through micro/nanobubble-assisted ultrasound. Pharmaceutics 2022, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, M.; Aronson, L.; Pattilachan, T.; Sautto, F.; Daines, B.; Thommes, D.; Shar, A.; Razavi, M. Bubble-based drug delivery systems: Next-generation diagnosis to therapy. J. Funct. Biomater. 2023, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, M.; Xu, X.; Sun, C. On the role of surface charge and surface tension tuned by surfactant in stabilizing bulk nanobubbles. App. Surf. Sci. 2023, 608, 155232. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Koshiyama, K.; Wada, S. Collapse of a lipid-coated nanobubble and subsequent liposome formation. Sci. Rep. 2016, 6, 28164. [Google Scholar] [CrossRef]
- Liwang, S.; Jinqiu, Z.; Man, Z.; Shukun, T.; Xu, C.; Wenyuan, Z.; Wenhua, L.; Xiaoying, L.; Haisheng, P.; Qun, W. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar]
- Hamano, N.; Kamoshida, S.; Kikkawa, Y.; Yano, Y.; Kobayashi, T.; Endo-Takahashi, Y.; Suzuki, R.; Maruyama, K.; Ito, Y.; Nomizu, M.; et al. Development of antibody-modified nanobubbles using Fc-region-binding polypeptides for ultrasound imaging. Pharmaceutics 2019, 11, 283. [Google Scholar] [CrossRef]
- Park, B.; Yoon, S.; Choi, Y.; Jang, J.; Park, S.; Choi, J. Stability of engineered micro or nanobubbles for biomedical applications. Pharmaceutics 2020, 12, 1089. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Shkirin, A.V.; Penkov, N.V.; Goltayev, M.V.; Ignatiev, P.S.; Gudkov, S.V.; Izmailov, A.Y. Effect of gas type and its pressure on nanobubble generation. Front. Chem. 2021, 9, 630074. [Google Scholar] [CrossRef] [PubMed]
- Lohse, D.; Zhang, X. Pinning and gas oversaturation imply stable single surface nanobubbles. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2015, 91, 031003. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, J.E.; Yoon, S.; Lee, D.J.; Mai, H.N.; Ida-Yonemochi, H.; Choi, J.; Jung, H.S. Hypoxia-responsive oxygen nanobubbles for tissues-targeted delivery in developing tooth germs. Front. Cell Dev. Biol. 2021, 9, 626224. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, Y.; Kohei, F.; Hao, H.; Peng, G.; Cheng, C.; Ye, J. Nanobubble hydrogen water: An effective therapeutic agent against inflammation related disease caused by viral infection in zebrafish model. Virol. Sin. 2022, 37, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Feng, Z.; Yang, F.; Gu, N. Bulk nanobubbles fabricated by repeated compression of microbubbles. Langmuir 2019, 35, 4238–4245. [Google Scholar] [CrossRef]
- Miller, M.A.; Sletten, E.M. Perfluorocarbons in chemical biology. ChemBioChem 2020, 21, 3451–3462. [Google Scholar] [CrossRef]
- Hwang, T.L.; Lin, Y.K.; Chi, C.H.; Huang, T.H.; Fang, J.Y. Development and evaluation of perfluorocarbon nanobubbles for apomorphine delivery. J. Pharm. Sci. 2009, 98, 3735–3747. [Google Scholar] [CrossRef] [PubMed]
- Prato, M.; Magnetto, C.; Jose, J.; Khadjavi, A.; Cavallo, F.; Quaglino, E.; Panariti, A.; Rivolta, I.; Benintende, E.; Varetto, G.; et al. 2H,3H-decafluoropentane-based nanodroplets: New perspectives for oxygen delivery to hypoxic cutaneous tissues. PLoS ONE 2015, 10, e0119769. [Google Scholar] [CrossRef]
- Abu-Nab, A.K.; Abu-Bakr, A.F. On the theory of multiple encapsulated microbubbles interaction: Effect of lipid shell thickness. Case Stud. Therm. Eng. 2023, 45, 102901. [Google Scholar] [CrossRef]
- Li, M.; Ma, X.; Eisener, J.; Pfeiffer, P.; Ohl, C.-D.; Sun, C. How bulk nanobubbles are stable over a wide range of temperatures. J. Colloid Interface Sci. 2021, 596, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef]
- Kida, H.; Nishimura, K.; Ogawa, K.; Watanabe, A.; Feril, L.B.; Irie, Y.; Endo, H.; Kawakami, S.; Tachibana, K. Nanobubble mediated gene delivery in conjunction with a hand-held ultrasound scanner. Front. Pharmacol. 2020, 11, 363. [Google Scholar] [CrossRef]
- Park, Y.; Shin, S.; Shukla, N.; Kim, K.; Park, M.H. Effects of nanobubbles in dermal delivery of drugs and cosmetics. Nanomaterials 2022, 12, 3286. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, R.; Bai, W.; Ye, T.; Wang, S. Oxygen-based nanocarriers to modulate tumor hypoxia for ameliorated anti-tumor therapy: Fabrications, properties, and future directions. Front. Mol. Biosci. 2021, 8, 683519. [Google Scholar] [CrossRef] [PubMed]
- Ficiarà, E.; Ansari, S.A.; Argenziano, M.; Cangemi, L.; Monge, C.; Cavalli, R.; D’Agata, F. Beyond oncological hyperthermia: Physically drivable magnetic nanobubbles as novel multipurpose theranostic carriers in the central nervous system. Molecules 2020, 25, 2104. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, V.R.; da Cunha Santos, M.A.; de Sousa, V.B.; de Araújo Neves, G.; de Lima Santana, N.L.; Menezes, R.R. A review on chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, L.; Shi, D.; Duan, S.; Li, J. Biocompatible chitosan nanobubbles for ultrasound-mediated targeted delivery of doxorubicin. Nanoscale Res. Lett. 2019, 14, 24. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, K.; He, Q.; Gu, X.; Jiang, C.; Wu, J. Hypoxia signaling in cancer: Implications for therapeutic interventions. MedComm 2023, 4, e203. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Comito, G.; Giannoni, E.; Chiarugi, P. Time-dependent stabilization of hypoxia inducible factor-1α by different intracellular sources of reactive oxygen species. PLoS ONE 2012, 7, e38388. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Seo, Y.; Jeon, H.; Hong, J.W.; Choi, J. Anti-tumor drug-loaded oxygen nanobubbles for the degradation of HIF-1α and the upregulation of reactive oxygen species in tumor cells. Cancers 2019, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, R.; Wang, S.; Dong, Z. Paclitaxel: New uses for an old drug. Drug Des. Dev. Ther. 2014, 8, 279–284. [Google Scholar]
- Gowda, J.I.; Nandibewoor, S.T. Electrochemical behavior of paclitaxel and its determination at glassy carbon electrode. Asian J. Pharm. Sci. 2014, 9, 42–49. [Google Scholar] [CrossRef]
- Gorbsky, G.J. The spindle checkpoint and chromosome segregation in meiosis. FEBS J. 2015, 282, 2471–2487. [Google Scholar] [CrossRef]
- Merali, Z.; McIntosh, J.; Anisman, H. Role of bombesin-related peptides in the control of food intake. Neuropeptides 1999, 33, 376–386. [Google Scholar] [CrossRef]
- Sausville, E.A.; Lebacq-Verheyden, A.M.; Spindel, E.R.; Cuttitta, F.; Gazdar, A.F.; Battey, J.F. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J. Biol. Chem. 1986, 261, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yan, J.P.; Xu, J.; Yin, T.H.; Zheng, R.Q.; Wang, W. Paclitaxel-loaded nanobubble targeted to pro-gastrin-releasing peptide inhibits the growth of small cell lung cancer. Cancer Manag. Res. 2019, 11, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Serru, V.; Dessen, P.; Boucheix, C.; Rubinstein, E. Sequence and expression of seven new tetraspans. Biochim. Biophys. Acta 2000, 1478, 159–163. [Google Scholar] [CrossRef]
- Chen, L.; Shen, A.G.; Wang, G.L.; Lu, P.; Li, X.Y. Expression of NET-1 gene and protein in hepatocellular carcinoma and related tissues. Ai Zheng 2006, 25, 320–325. [Google Scholar] [PubMed]
- Chen, L.; Li, X.Y.; Wang, Y.; Zhu, Y.Y.; Zhu, J.W. Expression and significance of NET-1 protein in hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2007, 29, 917–921. [Google Scholar]
- Shang, H.; Wu, B.; Liang, X.; Sun, Y.; Han, X.; Zhang, L.; Wang, Q.; Cheng, W. Evaluation of therapeutic effect of targeting nanobubbles conjugated with NET-1 siRNA by shear wave elastography: An in vivo study of hepatocellular carcinoma bearing mice model. Drug Deliv. 2019, 26, 944–951. [Google Scholar] [CrossRef]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Morton, C.A.; Sidoroff, A.; Braathen, L.R. Photodynamic therapy for non-melanoma skin cancer. Acta Derm. Venereol. 2005, 85, 483–490. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of anti-tumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Li, X.; Diao, P.; Liu, L.; Zhou, H.; Yang, Y.; Han, C.; Jiang, X. Hematoporphyrin monomethyl ether photodynamic therapy for the treatment of Sturge-Weber syndrome and large segmental facial port-wine stain. Dermatol. Ther. 2022, 35, e15404. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.C.; Glazner, G.F.; Duffy, M.; Scherrer, L.; Pendyala, S.; Li, B.; Wang, X.; Wang, H.; Huang, Z. Optical properties of hematoporphyrin monomethyl ether (HMME), a PDT photosensitizer. Photodiagn. Photodyn. Ther. 2012, 9, 232–242. [Google Scholar] [CrossRef]
- Yan, S.; Lu, M.; Ding, X.; Chen, F.; He, X.; Xu, C.; Zhou, H.; Wang, Q.; Hao, L.; Zou, J. Hematoporphyrin monomethyl ether polymer contrast agent for ultrasound/photoacoustic dual-modality imaging-guided synergistic high intensity focused ultrasound (HIFU) therapy. Sci. Rep. 2016, 6, 31833. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liun, Q.; Wang, X.; Wang, P.; Zhang, J.; Cao, B. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009, 49, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Q.; Liu, F.; Zhou, P.; Gu, Y.; Zeng, J.; An, J.; Dai, W.; Li, X. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004, 216, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Floridi, A.; Paggi, M.G.; Marcante, M.L.; Silvestrini, B.; Caputo, A.; De Martino, C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J. Natl. Cancer Inst. 1981, 66, 497–499. [Google Scholar] [PubMed]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Brawer, M.K. Lonidamine: Basic science and rationale for treatment of prostatic proliferative disorders. Rev. Urol. 2005, 7 (Suppl. 7), S21–S26. [Google Scholar] [PubMed]
- Cheng, G.; Zhang, Q.; Pan, J.; Lee, Y.; Ouari, O.; Hardy, M.; Zielonka, M.; Myers, C.R.; Zielonka, J.; Weh, K.; et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 2019, 10, 2205. [Google Scholar] [CrossRef]
- Shang, H.; Chen, Y.; Wang, C.; Zhang, S.; Wu, B.; Liang, X.; Liu, Z.; Wang, Q.; Cheng, W. RNA-Seq technology reveals the mechanism of SDT combined with novel nanobubbles against HCC. Front. Mol. Biosci. 2022, 8, 791331. [Google Scholar] [CrossRef]
- Smolková, K.; Mikó, E.; Kovács, T.; Leguina-Ruzzi, A.; Sipos, A.; Bai, P. Nuclear factor erythroid 2-related factor 2 in regulating cancer metabolism. Antioxid. Redox Signal. 2020, 33, 966–997. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Kitamura, H.; Motohashi, H. NRF2 addiction in cancer cells. Cancer Sci. 2018, 109, 900–911. [Google Scholar] [CrossRef]
- Eades, G.; Yang, M.; Yao, Y.; Zhang, Y.; Zhou, Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J. Biol. Chem. 2011, 286, 40725–40733. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.; Nedjadi, T.; Kitteringham, N.R.; Campbell, F.; Costello, E.; Lloyd, B.; Copple, I.M.; Williams, S.; Owen, A.; Neoptolemos, J.P.; et al. Nrf2 is overexpressed in pancreatic cancer: Implications for cell proliferation and therapy. Mol. Cancer 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Teppo, H.R.; Skarp, S.; Haapasaari, K.M.; Porvari, K.; Vuopala, K.; Kietzmann, T.; Karihtala, P. NRF1 and NRF2 mRNA and protein expression decrease early during melanoma carcinogenesis: An insight into survival and microRNAs. Oxid. Med. Cell. Longev. 2019, 2019, 2647068. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.; Kajitani, G.S.; Quinet, A.; Fortunato, R.S.; Menck, C.F. NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells. Oncotarget 2016, 7, 48081–48092. [Google Scholar] [CrossRef] [PubMed]
- Khamari, R.; Trinh, A.; Gabert, P.E.; Corazao-Rozas, P.; Riveros-Cruz, S.; Balayssac, S.; Malet-Martino, M.; Dekiouk, S.; Joncquel Chevalier Curt, M.; Maboudou, P.; et al. Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 2018, 9, 325. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Z.; Meng, X.; Chen, H.; Fu, G. Migration and invasion in B16-F10 mouse melanoma cells are regulated by Nrf2 inhibition during treatment with ionizing radiation. Oncol. Lett. 2018, 16, 1959–1966. [Google Scholar] [CrossRef]
- Argenziano, M.; Bessone, F.; Dianzani, C.; Cucci, M.A.; Grattarola, M.; Pizzimenti, S.; Cavalli, R. Ultrasound-responsive Nrf2-targeting siRNA-loaded nanobubbles for enhancing the treatment of melanoma. Pharmaceutics 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Mahabady, M.K.; Zabolian, A.; Abbaspour, A.; Fallahzadeh, P.; Noori, M.; Hashemi, F.; Hushmandi, K.; Daneshi, S.; Kumar, A.P.; et al. Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: Current status with an emphasis on delivery systems. Life Sci. 2021, 275, 119368. [Google Scholar] [CrossRef] [PubMed]
- Nikam, R.R.; Gore, K.R. Journey of siRNA: Clinical developments and targeted delivery. Nucleic Acid Ther. 2018, 28, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Jo, S.D.; Lee, Y.K.; Kim, K.; Kim, S.H. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2016, 104, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Mainini, F.; Eccles, M.R. Lipid and polymer-based nanoparticle siRNA delivery systems for cancer therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hou, X.; Zhao, X.; Jing, J.; Sun, L. Tracking adoptive natural killer cells via ultrasound imaging assisted with nanobubbles. Acta Biomater. 2023, 169, 542–555. [Google Scholar] [CrossRef]
- Guo, X.; Lin, J.; Pan, L.; He, K.; Huang, Z.; Chen, J.; Lin, C.; Zeng, B.; Luo, S.; Wang, M. Ultrasound-triggered release of miR-199a-3p from liposome nanobubbles for enhanced hepato-cellular carcinoma treatment. Artif. Cells Nanomed. Biotechnol. 2023, 51, 560–571. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, S.J. Modifications of Nanobubble Therapy for Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 7292. https://doi.org/10.3390/ijms25137292
Terlikowska KM, Dobrzycka B, Terlikowski SJ. Modifications of Nanobubble Therapy for Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(13):7292. https://doi.org/10.3390/ijms25137292
Chicago/Turabian StyleTerlikowska, Katarzyna M., Bozena Dobrzycka, and Slawomir J. Terlikowski. 2024. "Modifications of Nanobubble Therapy for Cancer Treatment" International Journal of Molecular Sciences 25, no. 13: 7292. https://doi.org/10.3390/ijms25137292





