Activation and Autoinhibition Mechanisms of NLR Immune Receptor Pi36 in Rice
Abstract
:1. Introduction
2. Results
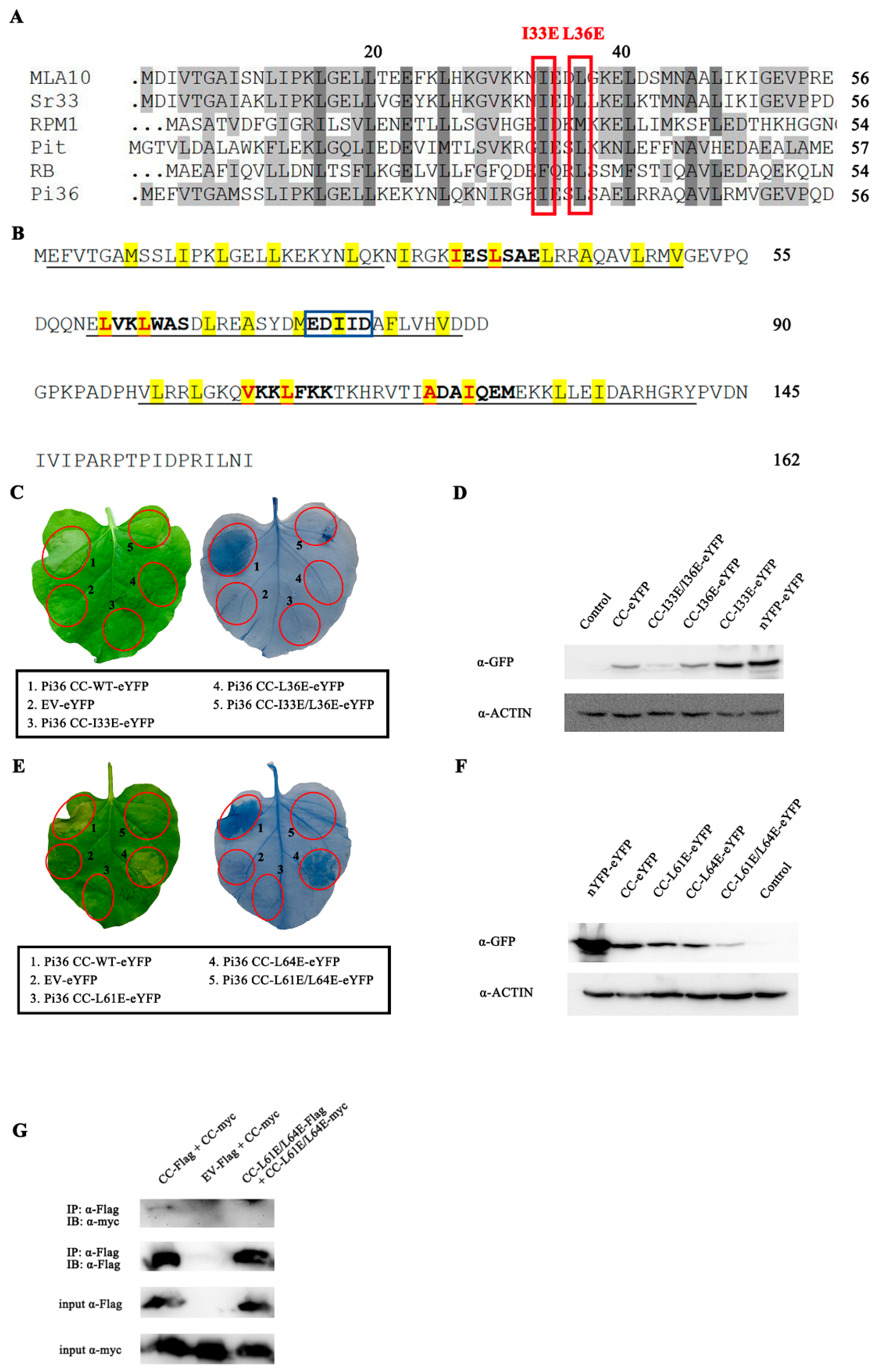
2.1. The Pi36 CC Domain Induces Cell Death
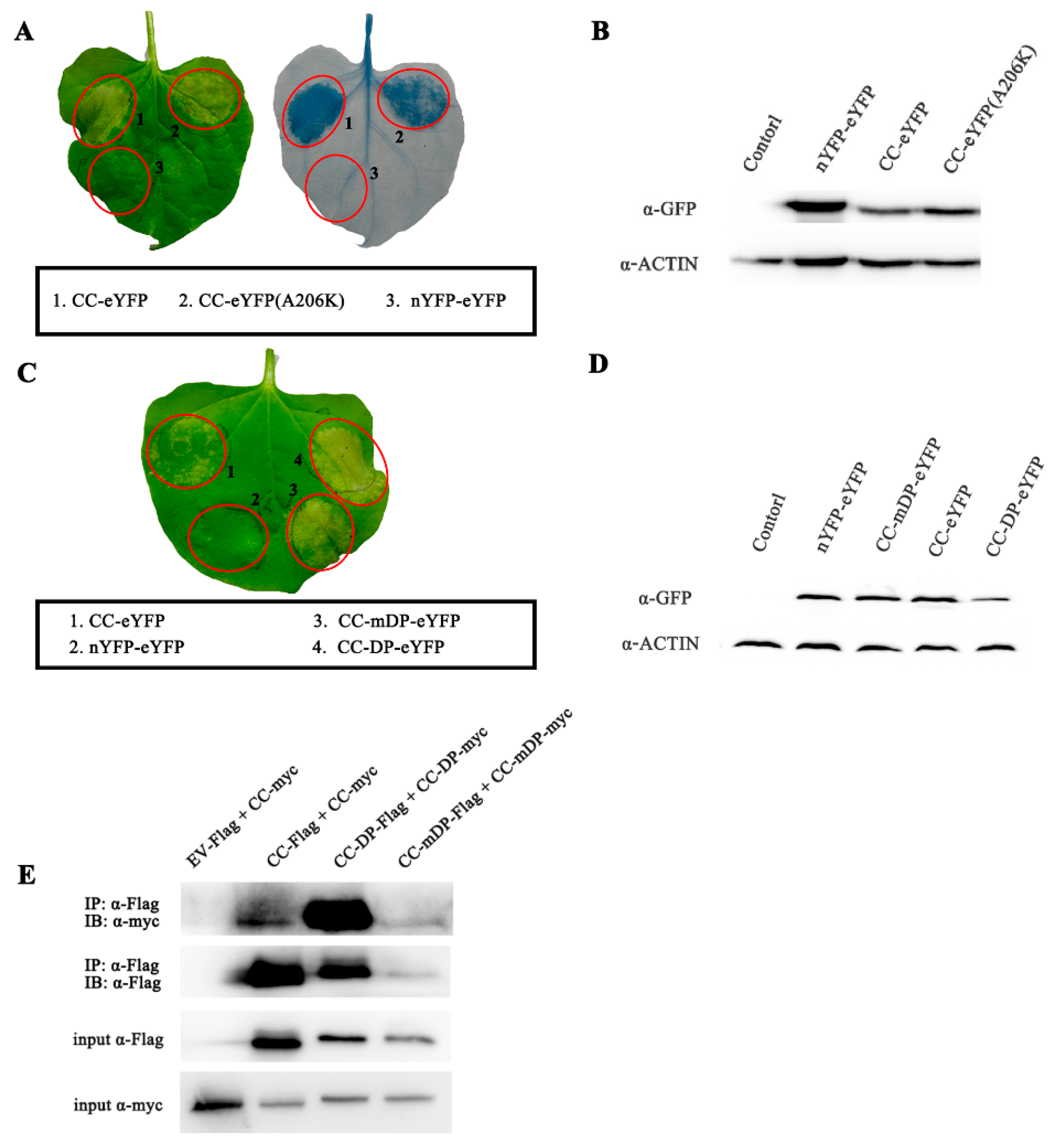
2.2. Cell Death Induced by Pi36 CC Domain Is Dependent on Its Self-Association
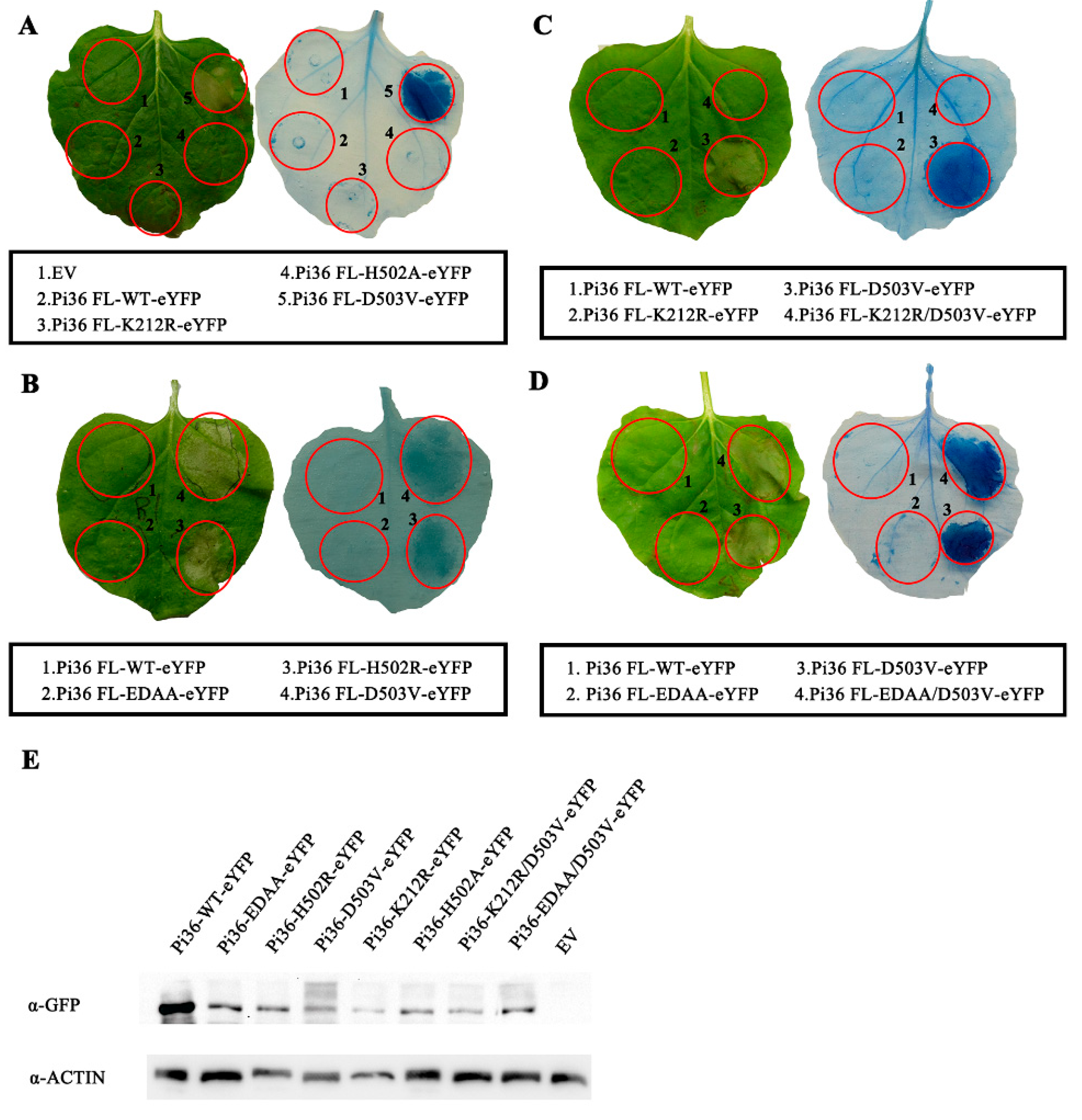
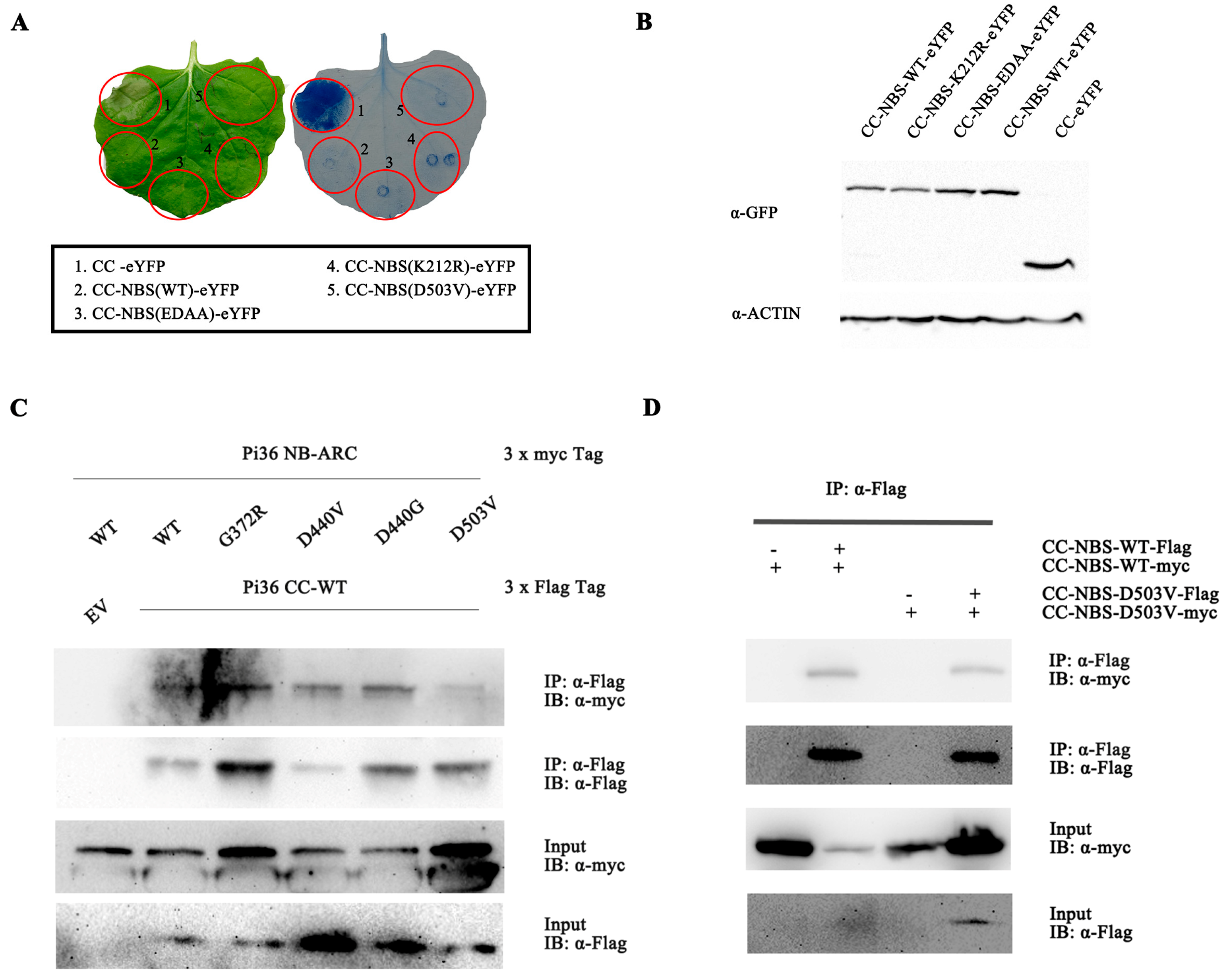
2.3. Pi36 Cell Death-Inducing Activity Is Tightly Regulated by the NB-ARC Domain
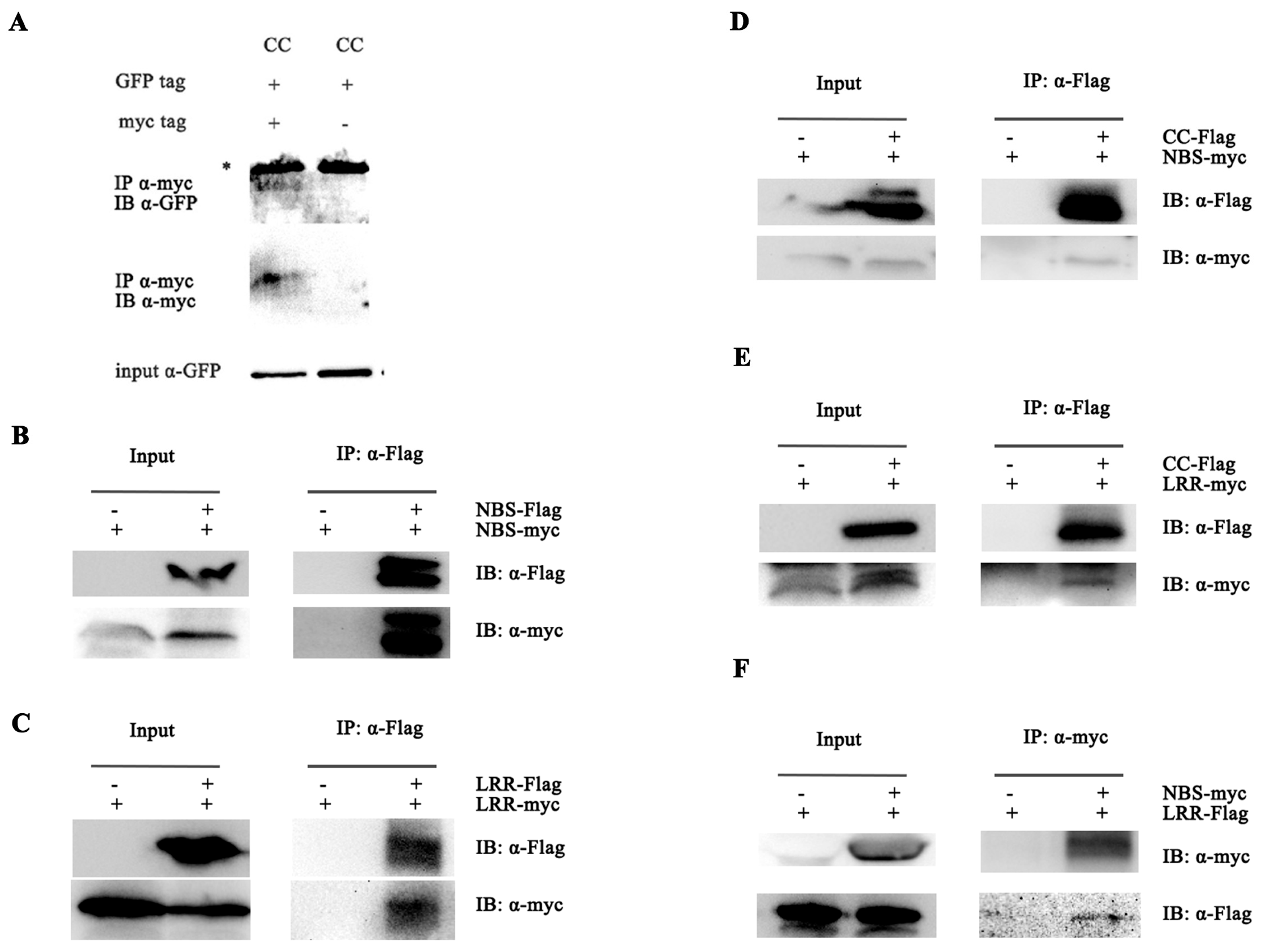
2.4. Pi36 Self-Association Is Constitutive and P-Loop-Dependent
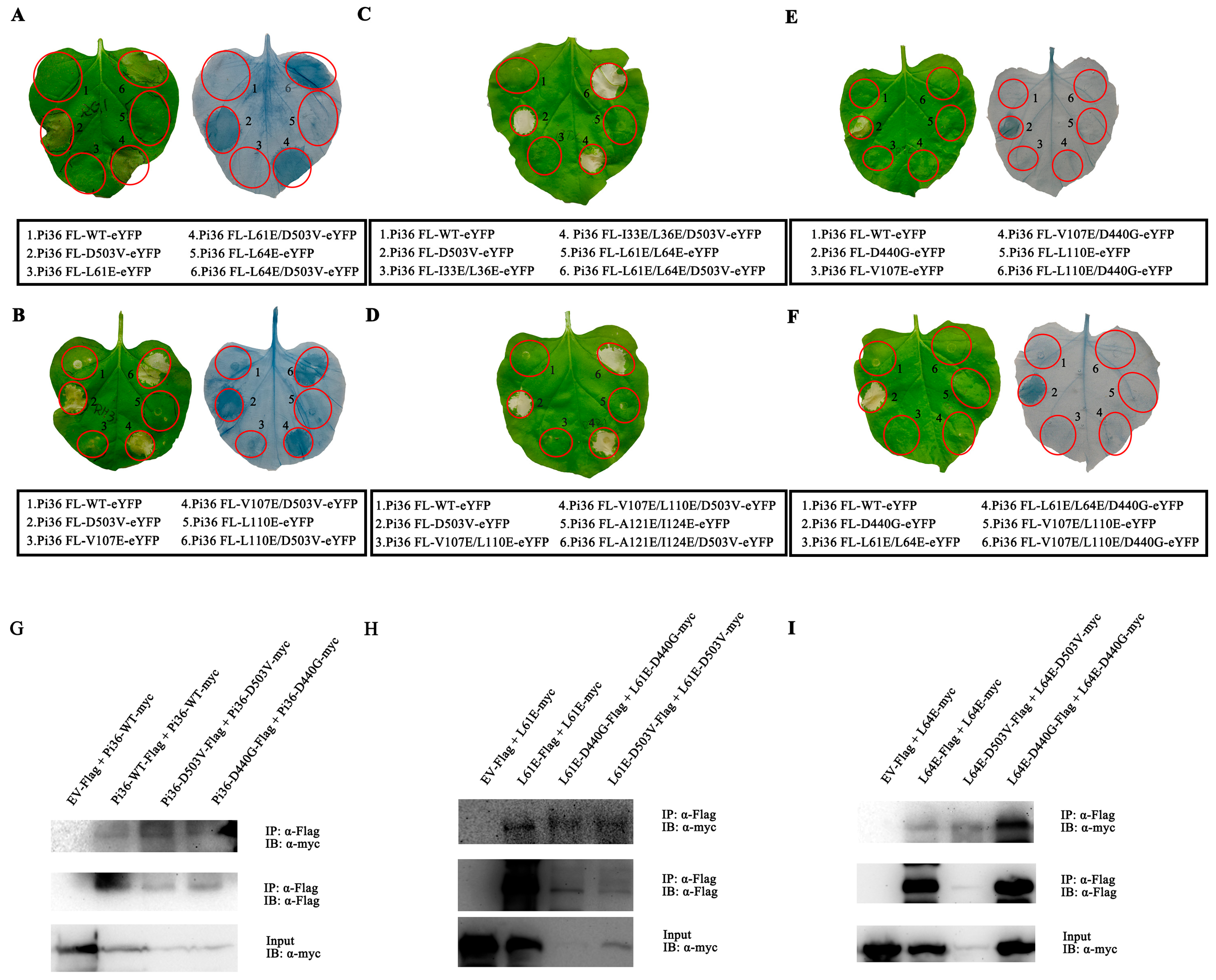
2.5. Intermolecular and Intramolecular Interactions in Pi36
2.6. Autoactivity of Pi36-D503V Is Dependent on the LRR Domain
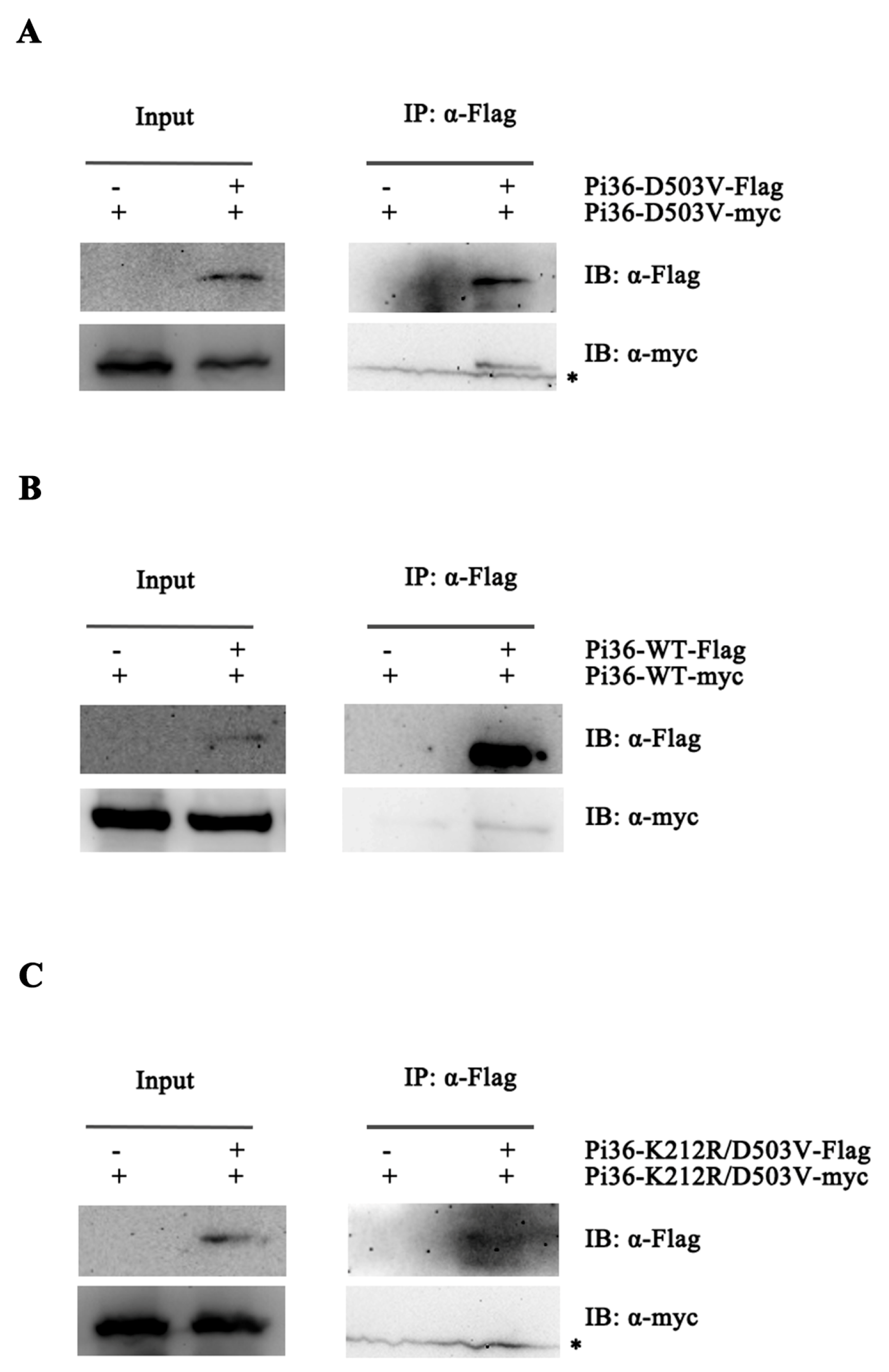
2.7. Self-Association of Pi36 Is Enhanced upon Activation
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction
4.2. Site-Directed Mutagenesis
4.3. Agrobacteria-Mediated Transient Expression
4.4. Protein Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takken, F.L.; Albrecht, M.; Tameling, W.I. Resistance proteins: Molecular switches of plant defence. Curr. Opin. Plant Biol. 2006, 9, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Q.; Xue, J.Y.; Wu, P.; Zhang, Y.M.; Wu, Y.; Hang, Y.Y.; Wang, B.; Chen, J.Q. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016, 170, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wan, L. Molecular insights into the biochemical functions and signalling mechanisms of plant NLRs. Mol. Plant Pathol. 2022, 23, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Cesari, S.; Moore, J.; Chen, C.; Webb, D.; Periyannan, S.; Mago, R.; Bernoux, M.; Lagudah, E.S.; Dodds, P.N. Cytosolic activation of cell death and stem rust resistance by cereal MLA-family CC-NLR proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 10204–10209. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, J.; Chang, C.; Zhang, L.; Maekawa, T.; Wang, Q.; Xiao, W.; Liu, Y.; Chai, J.; Takken, F.L.; et al. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 2012, 8, e1002752. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Ji, J.; El-Kasmi, F.; Dangl, J.L.; Johal, G.; Balint-Kurti, P.J. Molecular and functional analyses of a maize autoactive NB-LRR protein identify precise structural requirements for activity. PLoS Pathog. 2015, 11, e1004674. [Google Scholar] [CrossRef]
- Baudin, M.; Hassan, J.A.; Schreiber, K.J.; Lewis, J.D. Analysis of the ZAR1 Immune Complex Reveals Determinants for Immunity and Molecular Interactions. Plant Physiol. 2017, 174, 2038–2053. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, H.Y.; Choi, E.H.; Park, E.; Kim, J.H.; Moon, K.B.; Kim, H.S.; Choi, D. The Coiled-Coil and Leucine-Rich Repeat Domain of the Potyvirus Resistance Protein Pvr4 Has a Distinct Role in Signaling and Pathogen Recognition. Mol. Plant Microbe Interact. 2018, 31, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Bolus, S.; Akhunov, E.; Coaker, G.; Dubcovsky, J. Dissection of Cell Death Induction by Wheat Stem Rust Resistance Protein Sr35 and Its Matching Effector AvrSr35. Mol. Plant Microbe Interact. 2020, 33, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, E.; Takken, F.L. STANDing strong, resistance proteins instigators of plant defence. Curr. Opin. Plant Biol. 2009, 12, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tameling, W.I.; Vossen, J.H.; Albrecht, M.; Lengauer, T.; Berden, J.A.; Haring, M.A.; Cornelissen, B.J.; Takken, F.L. Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol. 2006, 140, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Hu, M.; Wu, S.; Qi, J.; Wang, G.; Han, Z.; Qi, Y.; Gao, N.; Wang, H.-W.; et al. Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 2019, 364, eaav5868. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; DeYoung, B.J.; Innes, R.W. Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein. Plant Physiol. 2012, 158, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Michael Weaver, L.; Swiderski, M.R.; Li, Y.; Jones, J.D.G. The Arabidopsis thaliana TIR-NB-LRR R-protein, RPP1A; protein localization and constitutive activation of defence by truncated alleles in tobacco and Arabidopsis. Plant J. 2006, 47, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Howles, P.; Lawrence, G.; Finnegan, J.; McFadden, H.; Ayliffe, M.; Dodds, P.; Ellis, J. Autoactive alleles of the flax L6 rust resistance gene induce non-race-specific rust resistance associated with the hypersensitive response. Mol. Plant Microbe Interact. 2005, 18, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 2020, 370, eabe3069. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Qi, T.; Zhang, H.; Liu, F.; King, M.; Toth, C.; Nogales, E.; Staskawicz, B.J. Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 2020, 370, eabd9993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Liu, M.X.; Chen, T.T.; Ma, X.; Li, Z.K.; Zheng, Z.; Zheng, S.R.; Chen, L.; Li, Y.Z.; Tang, L.R.; et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 2022, 8, eabq5108. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; McAdams, S.A.; Bryan, G.T.; Hershey, H.P.; Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000, 19, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Zhou, J.M. Regulation of Cell Death and Signaling by Pore-Forming Resistosomes. Annu. Rev. Phytopathol. 2021, 59, 239–263. [Google Scholar] [CrossRef] [PubMed]
- Takken, F.L.; Goverse, A. How to build a pathogen detector: Structural basis of NB-LRR function. Curr. Opin. Plant Biol. 2012, 15, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Ade, J.; DeYoung, B.J.; Golstein, C.; Innes, R.W. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc. Natl. Acad. Sci. USA 2007, 104, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Moffett, P. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 2002, 21, 4511–4519. [Google Scholar] [CrossRef] [PubMed]
- El Kasmi, F.; Chung, E.H.; Anderson, R.G.; Li, J.; Wan, L.; Eitas, T.K.; Gao, Z.; Dangl, J.L. Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc. Natl. Acad. Sci. USA 2017, 114, E7385–E7394. [Google Scholar] [CrossRef]
- Rairdan, G.J.; Moffett, P. Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell 2006, 18, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Slootweg, E.J.; Spiridon, L.N.; Roosien, J.; Butterbach, P.; Pomp, R.; Westerhof, L.; Wilbers, R.; Bakker, E.; Bakker, J.; Petrescu, A.J.; et al. Structural determinants at the interface of the ARC2 and leucine-rich repeat domains control the activation of the plant immune receptors Rx1 and Gpa2. Plant Physiol. 2013, 162, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, T.; Han, M.; Qian, L.; Li, J.; Wu, M.; Han, T.; Cao, J.; Nagalakshmi, U.; Rathjen, J.P.; et al. Plant NLR immune receptor Tm-22 activation requires NB-ARC domain-mediated self-association of CC domain. PLoS Pathog. 2020, 16, e1008475. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Han, Z.; Chai, J. Resistosome and inflammasome: Platforms mediating innate immunity. Curr. Opin. Plant Biol. 2020, 56, 47–55. [Google Scholar] [CrossRef]
- Hu, Z.; Chai, J. Assembly and Architecture of NLR Resistosomes and Inflammasomes. Annu. Rev. Biophys. 2023, 52, 207–228. [Google Scholar] [CrossRef]
- Williams, S.J.; Sornaraj, P.; deCourcy-Ireland, E.; Menz, R.I.; Kobe, B.; Ellis, J.G.; Dodds, P.N.; Anderson, P.A. An autoactive mutant of the M flax rust resistance protein has a preference for binding ATP, whereas wild-type M protein binds ADP. Mol. Plant Microbe Interact. 2011, 24, 897–906. [Google Scholar] [CrossRef]
- van Ooijen, G.; Mayr, G.; Kasiem, M.M.; Albrecht, M.; Cornelissen, B.J.; Takken, F.L. Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J. Exp. Bot. 2008, 59, 1383–1397. [Google Scholar] [CrossRef]
- Roberts, M.; Tang, S.; Stallmann, A.; Dangl, J.L.; Bonardi, V. Genetic requirements for signaling from an autoactive plant NB-LRR intracellular innate immune receptor. PLoS Genet. 2013, 9, e1003465. [Google Scholar] [CrossRef]
- Bendahmane, A.; Farnham, G.; Moffett, P.; Baulcombe, D.C. Constitutive gain-of-function mutants in a nucleotide binding site–leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 2002, 32, 195–204. [Google Scholar] [CrossRef]
- Tornero, P.; Chao, R.A.; Luthin, W.N.; Goff, S.A.; Dangl, J.L. Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 2002, 14, 435–450. [Google Scholar] [CrossRef]
- Dinesh-Kumar, S.P.; Tham, W.H.; Baker, B.J. Structure-function analysis of the tobacco mosaic virus resistance gene N. Proc. Natl. Acad. Sci. USA 2000, 97, 14789–14794. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef]
- Wang, G.; Roux, B.; Feng, F.; Guy, E.; Li, L.; Li, N.; Zhang, X.; Lautier, M.; Jardinaud, M.-F.; Chabannes, M.; et al. The Decoy Substrate of a Pathogen Effector and a Pseudokinase Specify Pathogen-Induced Modified-Self Recognition and Immunity in Plants. Cell Host Microbe 2015, 18, 285–295. [Google Scholar] [CrossRef]
- Qi, T.; Seong, K.; Thomazella, D.P.T.; Kim, J.R.; Pham, J.; Seo, E.; Cho, M.-J.; Schultink, A.; Staskawicz, B.J. NRG1 functions downstream of EDS1 to regulate TIR-NLR-mediated plant immunity in Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA 2018, 115, E10979–E10987. [Google Scholar] [CrossRef]
- Borrill, P.; Mago, R.; Xu, T.; Ford, B.; Williams, S.J.; Derkx, A.; Bovill, W.D.; Hyles, J.; Bhatt, D.; Xia, X.; et al. An autoactive NB-LRR gene causes Rht13 dwarfism in wheat. Proc. Natl. Acad. Sci. USA 2022, 119, e2209875119. [Google Scholar] [CrossRef]
- Yan, J.; Liu, Y.; Huang, X.; Li, L.; Hu, Z.; Zhang, J.; Qin, Q.; Yan, L.; He, K.; Wang, Y.; et al. An unreported NB-LRR protein SUT1 is required for the autoimmune response mediated by type one protein phosphatase 4 mutation (topp4-1) in Arabidopsis. Plant J. 2019, 100, 357–373. [Google Scholar] [CrossRef]
- Maekawa, T.; Cheng, W.; Spiridon, L.N.; Toller, A.; Lukasik, E.; Saijo, Y.; Liu, P.; Shen, Q.H.; Micluta, M.A.; Somssich, I.E.; et al. Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe 2011, 9, 187–199. [Google Scholar] [CrossRef]
- Baudin, M.; Schreiber, K.J.; Martin, E.C.; Petrescu, A.J.; Lewis, J.D. Structure–function analysis of ZAR1 immune receptor reveals key molecular interactions for activity. Plant J. 2020, 101, 352–370. [Google Scholar] [CrossRef]
- Gao, A.; Hu, M.; Gong, Y.; Dong, R.; Jiang, Y.; Zhu, S.; Ji, J.; Zhang, D.; Li, S.; He, H. Pm21 CC domain activity modulated by intramolecular interactions is implicated in cell death and disease resistance. Mol. Plant Pathol. 2020, 21, 975–984. [Google Scholar] [CrossRef]
- Mestre, P.; Baulcombe, D.C. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell 2006, 18, 491–501. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Bentham, A.; Williams, S.J.; Kobe, B.; Staskawicz, B.J. Multiple Domain Associations within the Arabidopsis Immune Receptor RPP1 Regulate the Activation of Programmed Cell Death. PLoS Pathog. 2016, 12, e1005769. [Google Scholar] [CrossRef]
- Forderer, A.; Li, E.; Lawson, A.W.; Deng, Y.N.; Sun, Y.; Logemann, E.; Zhang, X.; Wen, J.; Han, Z.; Chang, J.; et al. A wheat resistosome defines common principles of immune receptor channels. Nature 2022, 610, 532–539. [Google Scholar] [CrossRef]
- Liu, X.; Lin, F.; Wang, L.; Pan, Q. The in silico map-based cloning of Pi36, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 2007, 176, 2541–2549. [Google Scholar] [CrossRef]
- Zacharias, D.A.; Violin, J.D.; Newton, A.C.; Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002, 296, 913–916. [Google Scholar] [CrossRef]
- Adachi, H.; Contreras, M.P.; Harant, A.; Wu, C.H.; Derevnina, L.; Sakai, T.; Duggan, C.; Moratto, E.; Bozkurt, T.O.; Maqbool, A.; et al. An N-terminal motif in NLR immune receptors is functionally conserved across distantly related plant species. Elife 2019, 8, e49956. [Google Scholar] [CrossRef]
- Krasileva, K.V.; Dahlbeck, D.; Staskawicz, B.J. Activation of an Arabidopsis resistance protein is specified by the in planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell 2010, 22, 2444–2458. [Google Scholar] [CrossRef]
- Gururaja, T.L.; Narasimhamurthy, S.; Payan, D.G.; Anderson, D.C. A novel artificial loop scaffold for the noncovalent constraint of peptides. Chem. Biol. 2000, 7, 515–527. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Q.; Deng, Y.; Cheng, C.S.; Kallenbach, N.R.; Lu, M. A seven-helix coiled coil. Proc. Natl. Acad. Sci. USA 2006, 103, 15457–15462. [Google Scholar] [CrossRef]
- Lupas, A. Coiled coils: New structures and new functions. Trends Biochem. Sci. 1996, 21, 375–382. [Google Scholar] [CrossRef]
- Zhao, J.; Song, J. NLR immune receptor RB is differentially targeted by two homologous but functionally distinct effector proteins. Plant Commun. 2021, 2, 100236. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Kosami, K.I.; Liu, C.; Li, J.; Zhang, D.; Miki, D.; Kawano, Y. Three highly conserved hydrophobic residues in the predicted alpha2-helix of rice NLR protein Pit contribute to its localization and immune induction. Plant Cell Environ. 2022, 45, 1876–1890. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, X.; Wang, Y.; Liu, L.; Xu, B.; Li, F.; Fang, J.; Chu, C. Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J. 2011, 66, 996–1007. [Google Scholar] [CrossRef]
- Du, D.; Zhang, C.; Xing, Y.; Lu, X.; Cai, L.; Yun, H.; Zhang, Q.; Zhang, Y.; Chen, X.; Liu, M.; et al. The CC-NB-LRR OsRLR1 mediates rice disease resistance through interaction with OsWRKY19. Plant Biotechnol. J. 2021, 19, 1052–1064. [Google Scholar] [CrossRef]
- Hak, H.; Raanan, H.; Schwarz, S.; Sherman, Y.; Dinesh-Kumar, S.P.; Spiegelman, Z. Activation of Tm-2(2) resistance is mediated by a conserved cysteine essential for tobacco mosaic virus movement. Mol. Plant Pathol. 2023, 24, 838–848. [Google Scholar] [CrossRef]
- Bernoux, M.; Ve, T.; Williams, S.; Warren, C.; Hatters, D.; Valkov, E.; Zhang, X.; Ellis, J.G.; Kobe, B.; Dodds, P.N. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe 2011, 9, 200–211. [Google Scholar] [CrossRef]
- Duxbury, Z.; Wu, C.H.; Ding, P. A Comparative Overview of the Intracellular Guardians of Plants and Animals: NLRs in Innate Immunity and Beyond. Annu. Rev. Plant Biol. 2021, 72, 155–184. [Google Scholar] [CrossRef] [PubMed]

| Domain | Motif | Mutations Autoactivate Pi36 | Mutations Inactivate Pi36-D503V |
|---|---|---|---|
| NB | P-loop | K212R | |
| RNBS-A | |||
| Walker B | D289A, D290A | ||
| RNBS-B | I313K | ||
| ARC | GLPL | G372R, S382N | |
| RNBS-D | D440G | ||
| MHD | D503V |
| Introduced Mutations | Pi36 CC Domain | Pi36-D503V | Pi36-D440G |
|---|---|---|---|
| HR | HR | — | |
| L61E | Weak HR | HR | — |
| L64E | HR | — | |
| V107E | HR | ||
| L110E | HR | HR | |
| I33E + L36E | HR | — | |
| L61E + L64E | HR | ||
| V107E + L110E | HR | ||
| A121E + I124E | HR | — | |
| L11A | HR | HR | HR |
| L11E | |||
| I12A | HR | HR | HR |
| I12E | HR | HR | |
| G16A | HR | HR | HR |
| G16E | HR | HR | |
| P13A | HR | HR | |
| P13E | HR | HR | |
| K20A | HR | HR | HR |
| K20E | HR | HR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tan, L.; Xu, X.; Tang, Q.; Wang, J.; Xing, S.; Wang, R.; Zou, T.; Wang, S.; Zhu, J.; et al. Activation and Autoinhibition Mechanisms of NLR Immune Receptor Pi36 in Rice. Int. J. Mol. Sci. 2024, 25, 7301. https://doi.org/10.3390/ijms25137301
Yang Y, Tan L, Xu X, Tang Q, Wang J, Xing S, Wang R, Zou T, Wang S, Zhu J, et al. Activation and Autoinhibition Mechanisms of NLR Immune Receptor Pi36 in Rice. International Journal of Molecular Sciences. 2024; 25(13):7301. https://doi.org/10.3390/ijms25137301
Chicago/Turabian StyleYang, Yang, Liu Tan, Xingzhe Xu, Qiaoyi Tang, Ji Wang, Shiyue Xing, Rui Wang, Ting Zou, Shiquan Wang, Jun Zhu, and et al. 2024. "Activation and Autoinhibition Mechanisms of NLR Immune Receptor Pi36 in Rice" International Journal of Molecular Sciences 25, no. 13: 7301. https://doi.org/10.3390/ijms25137301





