Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease
Abstract
:1. Introduction
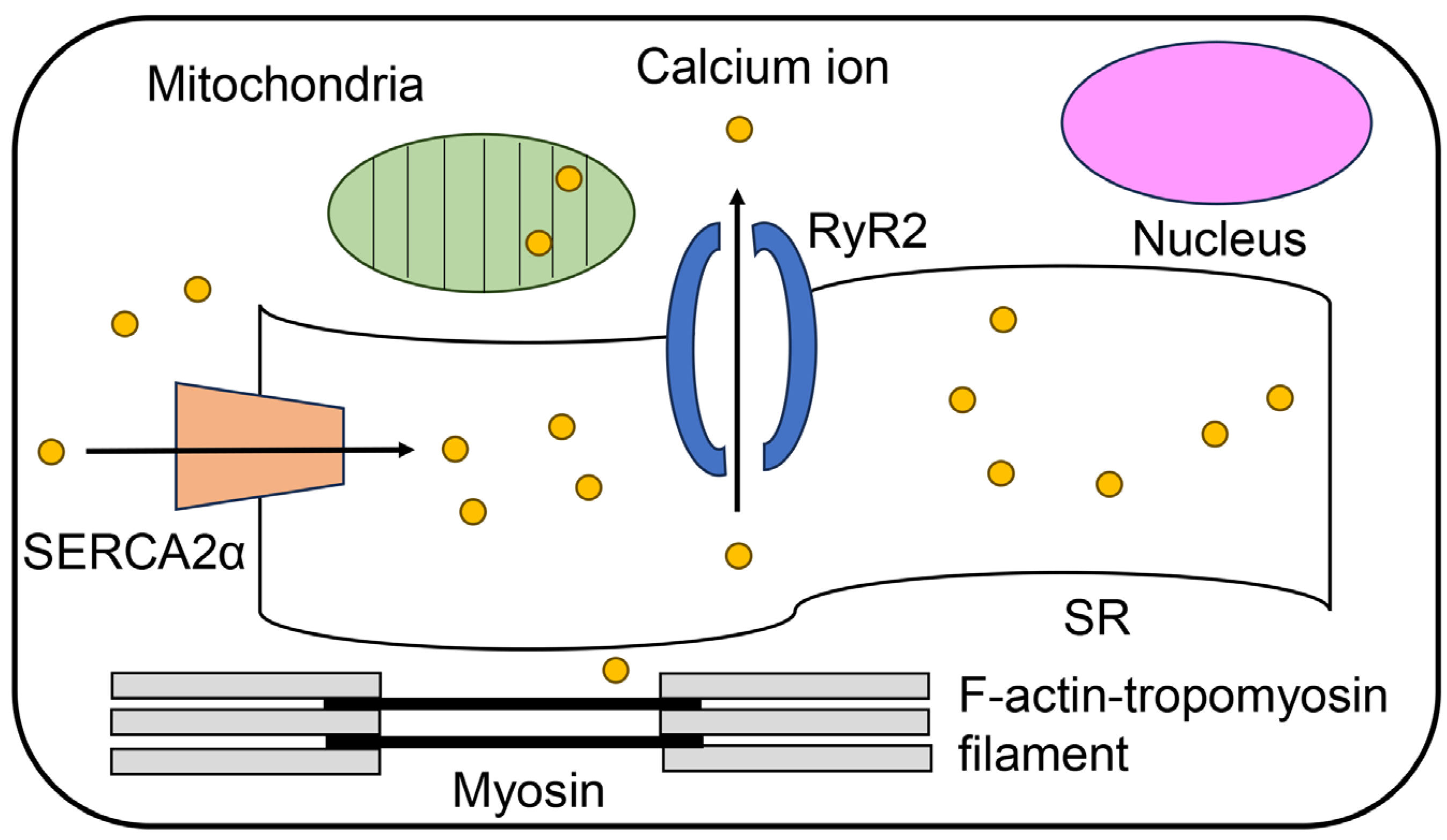
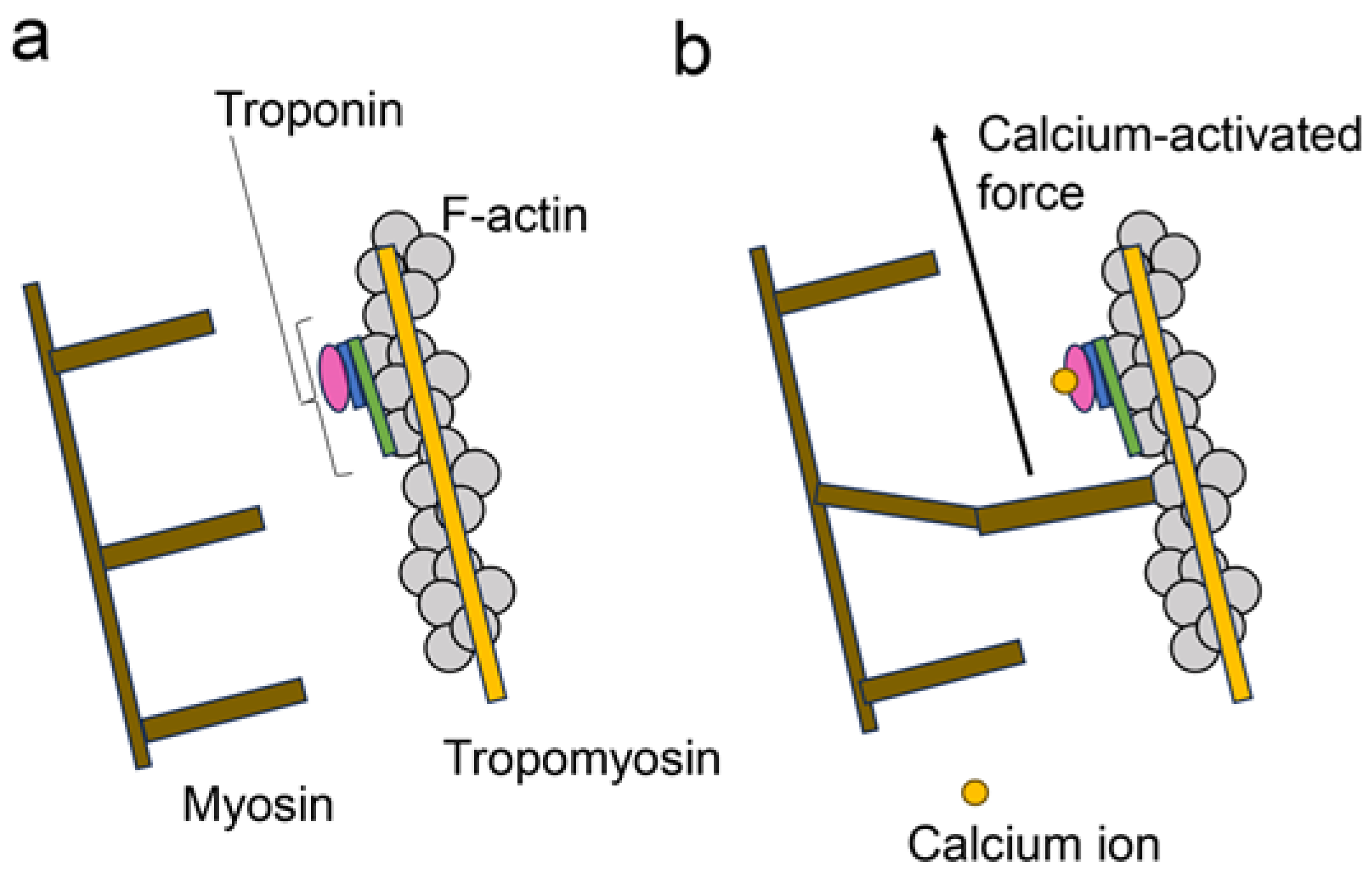
2. Regulatory Mechanisms of Contracting Cardiomyocytes
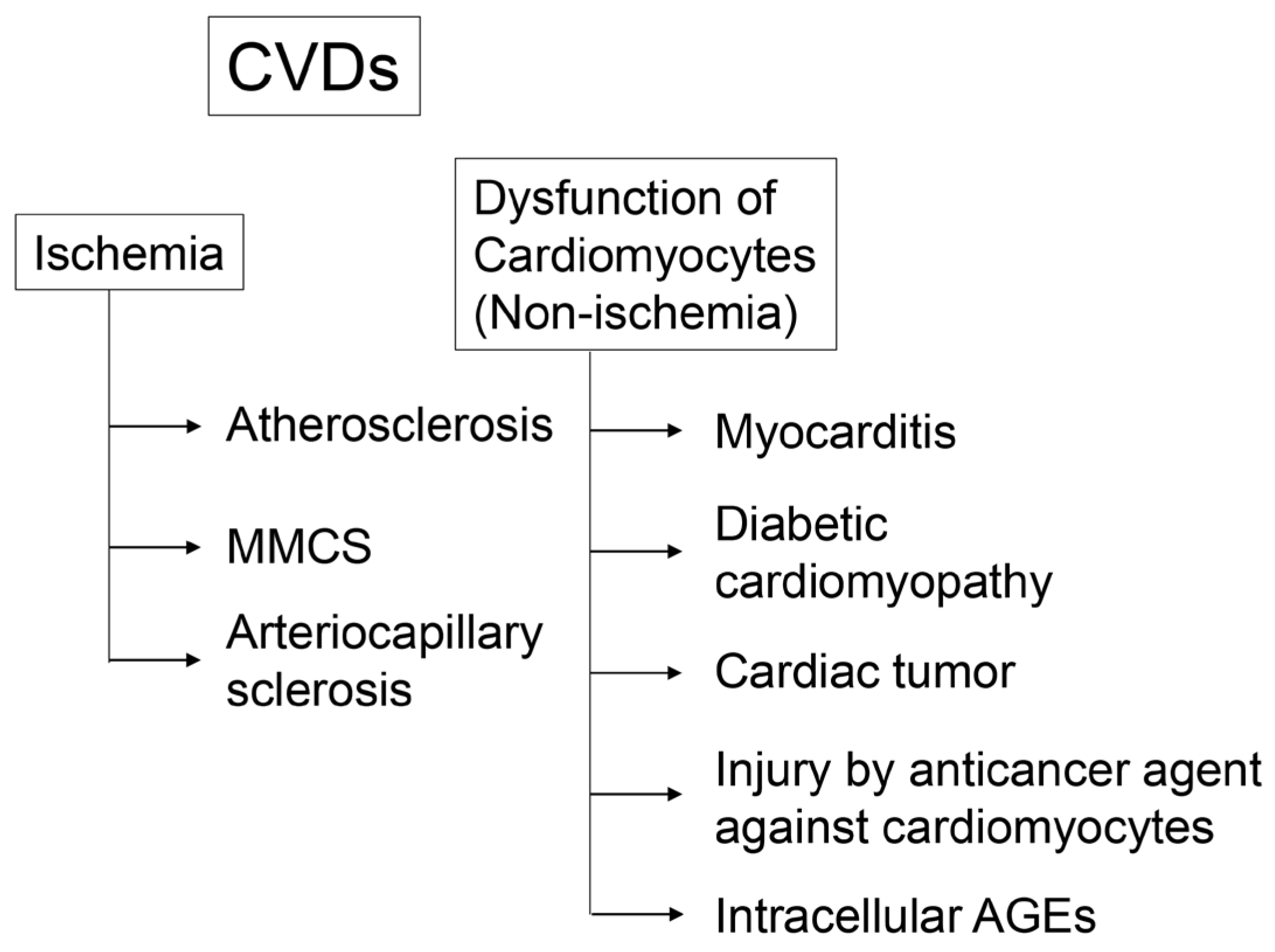
3. CVD
3.1. Cardiomyocyte Dysfunction through Ischemia
3.2. Cardiomyocyte Dysfunction through Non-Ischemia
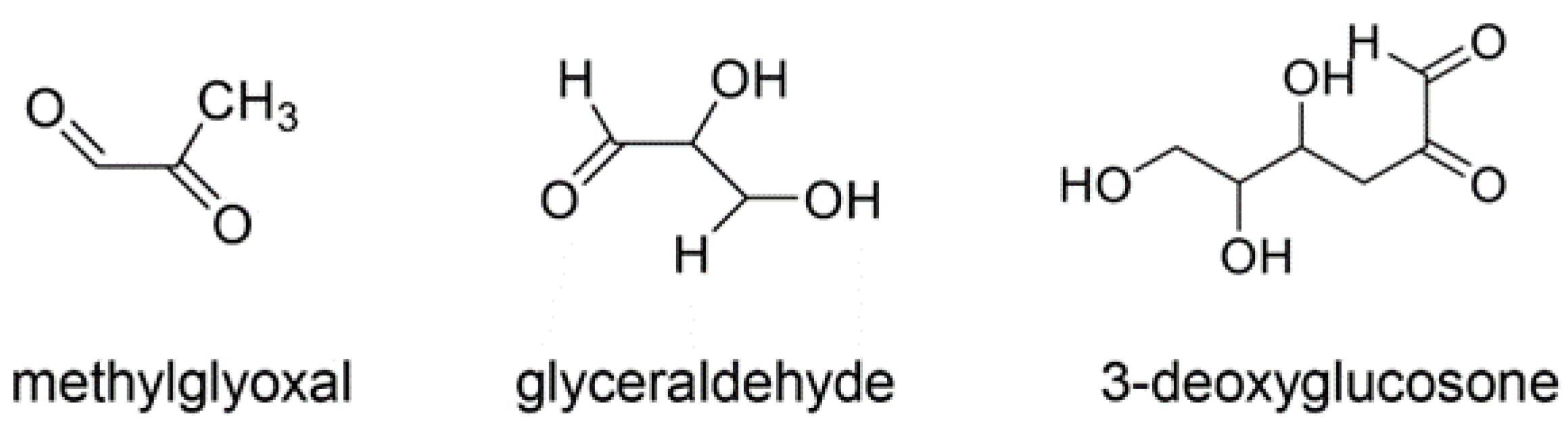
4. AGEs
4.1. Various AGE Types
4.2. Crude, Diverse, and Multiple AGE Patterns
4.3. Intracellular AGEs and LSRDs
4.4. Extracellular AGEs and LSRDs
4.4.1. AGEs in the Body Fluids
4.4.2. AGEs in the Extracellular Matrix
4.4.3. Dietary AGEs
5. Types of Identification and Quantification Technologies for AGEs
5.1. Fluorimetry
5.2. Immunostaining
5.3. Western Blotting
5.4. Slot Blotting
5.5. ELISA
5.6. GC–MS
5.7. ESI–MS and MALDI–MS
5.8. NMR
6. Intracellular AGEs and Cardiomyocytic Dysfunction
6.1. Role of AGEs in the Pathophysiology of DM and Hypertension
6.2. Various Glycations in RyR2 in the Cardiomyocytes in DM
6.3. Intracellular AGEs Contain Glycated RyR2 in the Cardiomyocytes of the Senescence Model
6.4. Intracellular AGEs Contain Glycated Myosin and F-actin in Cardiomyocytes in Diabetes and Heart Failure
6.5. Generation of TAGE in Cardiomyocytes
6.6. Highlights of the Novel Aspects of Our Research Compared to the Previous Investigations
6.7. The Possibility That Extracellular AGEs Directly Induce Dysfunction of Cardiomyocytes
7. Inhibition of the Generation of AGEs by Natural Compounds in Traditional Medicines
7.1. Traditional Medicines
7.2. Various Natural Compounds Have Been Identified in Traditional Medicines
7.3. Inhibition of the Generation of Intracellular AGEs
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end-products |
| Arg-P | Argpyrimidine |
| CHAPS | 3-[3-(cholamidopropyl)-dimethylammonio]-1-propanesulfonate |
| CEL | Nε-carboxyethyl-lysine |
| CMA | Nω-carboxymethyl-arginine |
| CML | Nε-carboxymethyl-lysine |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| ELISA | Enzyme-linked immunosorbent assay |
| ESI-MS | Electrospray ionization-mass spectrometry |
| GA-AGEs | Glyceraldehyde-derived advanced glycation end-products |
| GC-MS | Gas chromatography-mass spectrometry |
| GH-1 | Glyoxal-derived hydroimidazolone |
| GLAP | Glyceraldehyde-derived pyridinium |
| GLO | Glyoxalase |
| GO-AGEs | Glyoxal-derived advanced glycation end products |
| HPLC | High performance liquid chromatography |
| HPLC-ESI-MS | High performance liquid chromatography electrospray ionization-mass spectrometry |
| LC | Liquid chromatography |
| LC-ESI-MS | Liquid chromatography electrospray ionization-mass spectrometry |
| LSRD | Lifestyle-related disease |
| MAGE | Melibiose-derived advanced glycation end-products |
| MALDI-MS | Matrix assisted laser desorption-mass spectrometry |
| MG-H1 | Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine |
| MGO-AGEs | Methylglyoxal-derived advanced glycation end-products |
| MOLD | Methylglyoxal-lysine dimer |
| NASH | Non-alcoholic steatohepatitis |
| NMR | Nuclear magnetic resonance |
| PPG | Pyrrolopyridinium lysine dimer derived from glyceraldehyde |
| RAGE | Receptor for advanced glycation end-products |
| TAGE | Toxic advanced glycation end-products |
| TLR4 | Tool like receptor 4 |
| Tris | 3-[3-(cholamidepropyl)-tris-(hydroxymethyl)-aminomethane |
References
- Zhang, X.; Lu, J.; Yang, Y.; Cui, J.; Zhang, X.; Xu, W.; Song, L.; Wu, C.; Wang, Q.; Wang, Y.; et al. Cardiovascular disease prevent and mortality across 1 million populations in China: Data from a nationwide population-based study. Lancet Public Health 2022, 7, e1041–e1050. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, R.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Wegener, J.W.; Mitoronova, G.Y.; ElShareif, L.; Quentin, C.; Belov, V.; Pochechueva, T.; Hasenfuss, G.; Ackermann, L.; Lehnart, S.E. A dual-targeted drug inhibits cardiac ryanodine receptor Ca2+ leak but activates SERCA2a Ca2+ uptake. Life Sci. Alliance 2023, 7, e202302278. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Y.; Bahriz, S.M.F.M.; Zhao, M.; Zhu, C.; Xiang, Y.K. Probing spatiotemporal PKA activity at the ryanodine receptor and SERCA2a nanodomains in cardomyocytes. Cell Commun. Signal. 2022, 20, 143. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wang, H.; Zhou, Z.; Yang, C.; Li, Z.; Zhang, Y.; Shi, X.; Zhang, L.; Wang, Y.; et al. Seipin deficiency accelerates heart failure due to calcium handling abnormalities and endoplasmic reticulum stress in mice. Front Cardiovasc. Med. 2021, 8, 644128. [Google Scholar] [CrossRef]
- Mitronova, G.T.; Quentin, C.; Belov, V.N.; Wegener, J.W.; Kiszka, K.A.; Lehnart, S.E. 1,4–Benzothiazpines with cyclopropanol groups and their structural analogues exhibit both RyR2–stabilizing and SERCA2a–stimulating activities. Med. Chem. 2023, 66, 15761–15775. [Google Scholar] [CrossRef]
- Millet, J.; Aguilar–Sanchez, Y.; Kornyeyev, D.; Bazmi, M.; Fainstein, D.; Copello, J.A.; Escobar, A.L. Thermal modulation of epicardial Ca2+ Dynamics uncovers molecular mechanisms of Ca2+ alternans. J. Gen. Physiol. 2021, 153, e202012568. [Google Scholar] [CrossRef]
- Barry, M.E.; Rynkiewicz, M.J.; Payadai, E.; Viana, A.; Lehman, W.; Moore, J.R. Glutamate 139 of tropomyosin is critical for cardiac thin filament blocked–state stabilization. J. Mol. Cell Cardiol. 2024, 188, 30–37. [Google Scholar] [CrossRef]
- Kelly, C.M.; Martin, J.L.; Coseno, M.; Previs, M.J. Visualization of cardiac thick filament dynamics in ex vivo heart preparations. J. Mol. Cell Cardiol. 2023, 185, 88–98. [Google Scholar] [CrossRef]
- Wong, F.L.; Bunch, T.A.; Lepak, V.C.; Steedman, A.L.; Colson, B.A. Cardiac myosin–binding protein C N–terminal interactions with myosin and actin filaments: Opposite effects of phosphorylation and M–domain mutations. J. Mol. Cell Cardiol. 2024, 186, 125–137. [Google Scholar] [CrossRef]
- Takanobu, T.; Sakasai-Sakai, A.; Ueda, T.; Takeuchi, M. Intracellular toxic advanced glycation end–products in cardiomyocytes may cause cardiovascular disease. Sci. Rep. 2019, 9, 2121. [Google Scholar]
- Shin, S.B.; Park, W.K.; Choi, J.W.; Jung, J.P.; Park, C.R.; Lee, Y.J.; Kim, G.S. Surgical management of multiple coronary artery to coronary sinus fistulas with giant left circumflex artery and multivalvular infective endocarditis. J. Cardiovasc. Surg. 2024, 19, 186. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Cui, J.; Zhu, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Oral–gut microbial transmission promotes diabetic coronary heart disease. Cardiovasc. Diabetol. 2024, 23, 123. [Google Scholar] [CrossRef]
- Suludere, M.A.; Danesh, S.K.; Killeen, A.L.; Crisologo, P.A.; Malone, M.; Siah, M.C.; Lavery, L.A. Mönckeberg’s Medial Calcific sclerosis makes traditional arterial doppler’s unreliable in high–risk patients with diabetes. Int. J. Low Extrem Wounds 2023. [Google Scholar] [CrossRef]
- Odah, A.M.; Khalid, M.O.; Alsaati, A.A.; Alqassab, H.A.; Alkouder, G.R.; Alhejji, M.H.; Alkathem, J.A.; Aleid, A. Mönckeberg’s disease with calcified lower limb ischemia in Saudi Arabia: A rare case report and literature review. Cureus 2023, 15, e38345. [Google Scholar] [CrossRef]
- Tsai, M.; Tseng, W.; Lee, K.; Lin, C.; Ou, S.; Li, A. Associations of urinary fetuin-A with histopathology and kidney events in biopsy-proven kidney disease. Clin. Kidney J. 2024, 17, sfae065. [Google Scholar] [CrossRef]
- Kakarla, S.; Abhilash, S.P.; Valakkade, J.; Namboodiri, N. Feasibility of cardiac magnetic resonance imaging in temporary permanent pacemaker implants in pediatric myocarditis and complete atrioventricular block. J. Arrhythm. 2024, 40, 385–389. [Google Scholar] [CrossRef]
- Aggarwal, N.; Bianchini, D.; Parkar, R.; Turner, J. Immunotherapy-Induced overlap syndrome: Myositis, myasthenia gravis, and myocarditis—A case series. Case. Rep. Med. 2024, 2024, 5399073. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Lei, F.; Jiang, K.; Liao, Y.; Huang, F.; Chen, M. Cholesterol 25-hydroxylase prevents type 2 diabetes mellitus induced cardiomyopathy by alleviating cardiac lipotoxicity. Biochim. Biophys. Acta Mol. Basis. Dis. 2024, 1870, 167158. [Google Scholar] [CrossRef]
- Ma, X.; Mei, S.; Wuyun, Q.; Zhou, L.; Sun, D.; Yan, J. Epigenetics in diabetic cardiomyopathy. Clin. Epigenetics 2024, 16, 52. [Google Scholar] [CrossRef]
- Chachar, T.; Yousif, N.; Noor, H.A.; Makwana, D.; Klkhayat, M.K.; Tareif, H.; Arekat, Z.R.; Amin, H. Epidemiology of Cardiac Myxoma in the Kingdom of Bahrain. Cureus 2024, 16, e55704. [Google Scholar] [CrossRef]
- Zimpfer, A.; Abel, L.M.; Alozie, A.; Etz, C.D.; Schneider, B. Frequent protein kinase A regulatory subunit A1 mutations but no GNAS mutations as potential driver in sporadic cardiac myxomas. Cardiovasc. Pathol. 2024, 71, 107632. [Google Scholar] [CrossRef]
- Bjørnstad, R.; Reiten, I.N.; Knudsen, K.S.; Schiøtt, J.; Herfindal, L. A liposomal formulation of simvastatin and doxorubicin for improved cardioprotective and anti-cancer effect. Int. J. Pharm. 2022, 629, 122379. [Google Scholar] [CrossRef]
- Qiu, M.; Ke, L.; Zhang, A.; Zeng, X.; Fang, Z.; Liu, J. JS-K, a GST-activated nitric oxide donor prodrug, enhances chemo-sensitivity in renal carcinoma cells and prevents cardiac myocytes toxicity induced by doxorubicin. Cancer Chemother. Pharmacol. 2017, 80, 275–286. [Google Scholar] [CrossRef]
- LeWinter, M.M.; Taatjes, D.; Ashikaga, T.; Palmer, B.; Bishop, N.; VanBuren, P.; Bell, S.; Donaldson, C.; Meyer, M.; Margulies, K.B.; et al. Abundance, localization, and functional correlates of the advanced glycation end-product carboxymethyl lysine in human myocardium. Physiol. Rep. 2017, 5, e13462. [Google Scholar] [CrossRef]
- Ruiz–Meana, M.; Minguet, M.; Bou–Teen, D.; Miro–Casas, E.; Castans, C.; Castellano, J.; Bonzon–Kulichenko, E.; Igual, A.; Rodriguez–Lecoq, R.; Vázquez, J.; et al. Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation 2019, 139, 949–964. [Google Scholar] [CrossRef]
- Papadaki, M.; Holewinski, R.J.; Previs, S.B.; Martin, T.G.; Stachowski, M.J.; Li, A.; Blair, C.A.; Moravec, C.S.; Eyk, J.E.V.; Campbell, K.S.; et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI Insight 2018, 3, e121264. [Google Scholar] [CrossRef]
- Wang, X.; Lau, W.B.; Yuan, Y.; Wang, Y.; Yi, W.; Christopher, T.A.; Lopez, B.L.; Liu, H.; Ma, X. Methylglyoxal increases cardiomyocyte ischemia-reperfusion injury via glycative inhibition of thioredoxin activity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E207–E214. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef]
- Mucci, L.; Santilli, F.; Cuccurullo, C.; Davì, G. Cardiovascular risk and dietary sugar intake: Is the link so sweet? Intern. Emerg. Med. 2012, 7, 313–322. [Google Scholar] [CrossRef]
- Grasser, E.K.; Dullo, A.; Montani, J. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: Does glucose attenuate the blood pressure-elevating effect of fructose? Br. J. Nutr. 2014, 112, 183–192. [Google Scholar] [CrossRef]
- Brahma, M.K.; Pepin, M.E.; Wende, A.R. My sweetheart is broken: Role of glucose in diabetic cardiomyopathy. Diabetes Metab. J. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Mapanga, R.F.; Essop, M.F. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am. J. Physiol. Heart. Circ. Physiol. 2016, 310, 153–173. [Google Scholar] [CrossRef]
- Xing, D.; Wu, Y. Effect of phosphorus deficiency on photosynthetic inorganic carbon assimilation of three climber plant species. Bot. Stud. 2014, 55, 60. [Google Scholar] [CrossRef]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the medicinal value, bioactive compounds, and pharmacological activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef]
- Du, H.; Liu, Z.; Lu, S.; Jiang, L.; Zhou, L.; Liu, J. Genomic evidence for human-mediated introgressive hybridization and selection in the developed breed. BMC Genom. 2024, 25, 331. [Google Scholar] [CrossRef]
- Nishimura, T.; Yamaguchi, C.; Miyazaki, K.; Chikaraishi, R. The role of lipids in enhancing the koku perception of pork sausage. J. Oleo. Sci. 2024, 73, 503–508. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known chinese tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Luo, Y.; Meng, X.; Pan, G.; Zhang, H.; Li, Y.; Zhang, B. The molecular basis of the anti-inflammatory property of Astragaloside IV for the treatment of diabetes and its complications. Drug Des Devel. Ther. 2023, 17, 771–790. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Yang, M. Evidence and potential mechanisms of Jin-Gui Shen-Qi Wan as a treatment for Type 2 Diabetes Mellitus: A systematic review and meta-analysis. Front. Pharmacol. 2021, 12, 699932. [Google Scholar] [CrossRef]
- Sun, G.; Li, C.; Cui, W.; Guo, Q.; Dong, C.; Zou, H.; Liu, S.; Dong, W.; Miao, L. Review of herbal Traditional Chinese Medicine for the treatment of diabetic nephropathy. J. Diabetes Res. 2016, 2016, 5749857. [Google Scholar] [CrossRef]
- Ezaki, H.; Ayaori, M.; Sato, H.; Maeno, Y.; Taniwaki, M.; Miyake, T.; Sakurada, M. Effects of Mokuboito, a Japanese Kampo medicine, on symptoms in patients hospitalized for acute decompensated heart failure—A prospective randomized pilot study. J. Cardiol. 2019, 74, 412–417. [Google Scholar] [CrossRef]
- Han, K.; Kwon, O.; Oark, H.; Jung, S.; Yang, C.; Sou, C. Effect of Daesiho-tang on obesity with non-alcoholic fatty liver disease: A study protocol for a randomised, double-blind, placebo-controlled pilot trial. Trials 2020, 21, 128. [Google Scholar] [CrossRef]
- Fujimoto, M.; Tsuneyama, K.; Kinoshita, H.; Goto, H.; Takano, Y.; Selmi, C.; Keen, C.L.; Gershwin, M.E.; Shimada, Y. The traditional Japanese formula keishibukuryogan reduces liver injury and inflammation in patients with nonalcoholic fatty liver disease. Ann. N. Y. Acad. Sci. 2010, 1190, 151–158. [Google Scholar] [CrossRef]
- Kubota, K.; Imai, Y.; Okuyama, T.; Ishiyama, Y.; Ueno, S.; Kairo, K. dramatically improved severe pulmonary arterial hypertension caused by Qing-Dai (Chinese Herbal Drug) for ulcerative colitis. Int. Heart. J. 2023, 64, 316–320. [Google Scholar] [CrossRef]
- Añazco, C.; Riedelsberger, J.; Vega–Montoto, L.; Rojas, A. exploring the interplay between polyphenols and lysyl oxidase enzymes for maintaining extracellular matrix homeostasis. Int. J. Mol. Sci. 2023, 24, 10985. [Google Scholar] [CrossRef]
- Yadav, N.; Palkhede, J.D.; Kim, S. Anti-Glucotoxicity Effect of Phytoconstituents via Inhibiting MGO-AGEs Formation and Breaking MGO-AGEs. Int. J. Mol. Sci. 2023, 24, 7672. [Google Scholar] [CrossRef]
- Takata, T. Is the novel slot blot a useful method for quantification of intracellular advanced glycation end-products? Metabolites 2023, 13, 564. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J. Effects of Toxic AGEs (TAGE) on Human Health. Cells 2022, 11, 2178. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; BiałKowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biol. Rep. 2021, 14, 46. [Google Scholar] [CrossRef]
- Shorey-Kendrick, L.E.; Crosland, B.A.; Spindel, E.R.; McEvoy, C.T.; Wilmarth, P.A.; Reddy, A.P.; Zientek, K.D.; Roberts, V.H.; D’Mello, R.J.; Ryan, K.S.; et al. The amniotic fluid proteome changes across gestation in humans and rhesus macaques. Sci. Rep. 2023, 13, 17039. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Lian, W.; Bai, Y.; Wang, L.; Zhao, F.; Li, H.; Wang, D.; Pang, Q. TMT-based quantitative proteomics reveals the targets of andrographolide on LPS-induced liver injury. BMC Vet. Res. 2023, 19, 199. [Google Scholar]
- Niwa, T. Mass spectrometry for the study of protein glycation in disease. Mass. Spectrom.Rev. 2006, 25, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Kűhn, J.; Pennodorf, I.; Knoll, K.; Henle, T. N-Terminal pyrazinones: A new class of peptide-bound advanced glycation end-products. Amino Acids 2004, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Murakami, Y.; Miyake, H.; Hayase, F.; Watanabe, H. Identification of a novel advanced glycation end product derived from lactaldehyde. Biosci. Biotechnol. Biochem. 2019, 83, 1136–1145. [Google Scholar] [CrossRef]
- Ohno, R.; Ichimura, K.; Tanaka, S.; Sugawa, H.; Katsuta, N.; Sakake, S.; Tominaga, Y.; Ban, I.; Shirakawa, J.; Yamaguchi, Y.; et al. Glucoselysine is derived from fructose and accumulates in the eye lens of diabetic rats. J. Biol. Chem. 2019, 294, 17326–17338. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, T.; Sasamoto, K.; Yamamoto, T. Glyceraldehyde-derived advanced glycation end-products having pyrrolopyridinium-based crosslinks. Biochem. Biophys. Rep. 2021, 26, 100963. [Google Scholar] [CrossRef] [PubMed]
- Litwinowicz, K.; Waszczuk, E.; Kuzan, A.; Branowicka-Szydełko, A.; Gostomska-Pampuch, K.; Naqporowski, P.; Gamian, A. Alcoholic liver disease is associated with elevated plasma levels of novel advanced glycation end-products: A preliminary study. Nutrients 2022, 14, 5266. [Google Scholar] [CrossRef]
- Baskal, S.; Tsikas, D. Free L-lysine and its methyl ester react with glyoxal and methylglyoxal in phosphate buffer (100 mM, pH 7.4) to form Nε-carboxymethyl-lysine, Nε-carboxyethyl-lysine and Nε-hydroxymethyl-lysine. Int. J. Mol. 2022, 23, 3446. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann. N. Y. Acad. Sci. 2005, 1043, 260–266. [Google Scholar] [CrossRef]
- Hayase, F.; Usui, T.; Nishiyama, K.; Sasaki, S.; Shirahashi, Y.; Tsuchiya, N.; Numata, N.; Watanabe, H. Chemistry and biological effects of melanoidins and glyceraldehyde-derived pyridinium as advanced glycation end products. Ann. N. Y. Acad. Sci. 2005, 1043, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Mashima, T.; Yamamoto, K.; Tsuruo, T. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J. Biol. Chem. 2002, 277, 45770–45775. [Google Scholar] [CrossRef] [PubMed]
- Manfredelli, D.; Pariano, M.; Costantini, C.; Graziani, A.; Bozza, S.; Romani, L.; Puccetti, P.; Talesa, V.N.; Antognelli, C. Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) spike protein S1 induces methylglyoxal-derived hydroimidazolone/receptor for advanced glycation end products (MG-H1/RAGE) activation to promote inflammation in human bronchial BEAS-2B cells. Int. J. Mol. Sci. 2023, 24, 14868. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Watanabe, H.; Hayase, F. Isolation and identification of 5-methyl-imidazolin-4-one derivative as glyceraldehyde-derived advanced glycation end product. Biosci. Biotechnol. Biochem. 2006, 70, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Ohguchi, M.; Watanabe, H.; Hayase, F. The formation of argpyrimidine in glyceraldehyde-related glycation. Biosci. Bioctechnol. Biochem. 2008, 72, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Hayase, F. Isolation and identification of the 3-hydroxy-5-hydroxymethyl-pyridinium compound as a novel advanced glycation end product on glyceraldehyde-related Maillard reaction. Biosci. Bitrchnol. Biochem. 2003, 67, 930–932. [Google Scholar] [CrossRef]
- Usui, T.; Shimohira, K.; Watanabe, H.; Hayase, F. Detection and determination of glyceraldehyde-derived pyridinium-type advanced glycation end product in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2007, 71, 442–448. [Google Scholar] [CrossRef]
- Tessier, F.J.; Monnier, V.M.; Sayre, L.M.; Kornfield, J.A. Triosidines: Novel Maillard reaction products and cross-links from the reaction of triose sugars with lysine and arginine residues. Biochem. J. 2003, 369, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Suzuki, H.; Takeda, K.; Sakai-Sakasai, A. Toxic advanced glycation end-products (TAGE) are major structures of cytotoxic AGEs derived from glyceraldehyde. Med. Hyp. 2024, 183, 111248. [Google Scholar] [CrossRef]
- Takata, T.; Masauji, T.; Motoo, Y. Analysis of crude, diverse, and multiple advanced glycation end-product patterns may be important and beneficial. Metabolites 2024, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Oya-Ito, T.; Naito, Y.; Takagi, T.; Handa, O.; Matsui, H.; Yamada, M.; Shima, K.; Yoshikawa, T. Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim. Biophys. Acta 2011, 1812, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Senavirathna, L.; Ma, C.; Chen, C.; Pan, S. Proteomic investigation of glyceraldehyde-derived intracellular AGEs and their potential influence on pancreatic ductal cells. Cells 2021, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Ueda, T.; Sakasai-Sakai, A.; Takeuchi, M. Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion. World. J. Gastroenterol. 2017, 23, 4910–4919. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collino, M.; Nigro, D.; Chiazza, F.; D’Antona, G.; Aragno, M.; Minetto, M.A. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PLoS ONE 2015, 10, e0119587. [Google Scholar] [CrossRef]
- Mastrocola, R.; Nigro, D.; Chiazza, F.; Medana, C.; Bello, F.D.; Boccuzzi, G.; Collino, M.; Aragno, M. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free Radic. Biol. Med. 2016, 91, 224–235. [Google Scholar] [CrossRef]
- Matsui, T.; Joo, H.D.; Lee, J.M.; Ju, S.M.; Tao, W.H.; Higashimoto, Y.; Fukami, K.; Yamaguchi, S. Development of a monoclonal antibody-based ELISA system for glyceraldehyde-derived advanced glycation end products. Immunol. Lett. 2015, 167, 141–146. [Google Scholar] [CrossRef]
- Kato, S.; Sugawa, H.; Tabe, K.; Ito, K.; Nakashima, H.; Nagai, R. Rapid pretreatment for multi-sample analysis of advanced glycation end products and their role in nephropathy. J. Clin. Biochem. Nutr. 2022, 70, 256–261. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, S.; Kim, H.R.; Ryu, H.Y.; Kim, Y.H.; Kim, J. Advanced glycation end products increase salivary gland hypofunction in d-galactose-induced aging rats and its prevention by physical exercise. Curr. Issues Mol. Biol. 2021, 43, 2059–2067. [Google Scholar] [CrossRef]
- Kashiwabara, S.; Hosoe, H.; Ohno, R.; Nagai, R.; Shiraki, M. Development and evaluation of novel ELISA for determination of urinary pentosidine. J. Nutr. Sci. Vitaminol. 2019, 65, 526–533. [Google Scholar] [CrossRef]
- Hayashi, K.; Sato, K.; Ochi, S.; Kawano, S.; Munesue, S.; Harashima, A.; Oshima, Y.; Kimura, K.; Kyoi, T.; Yamamoto, Y. Inhibitory effects of Saururus chinensis extract on receptor for advanced glycation end-products-dependent inflammation and diabetes-induced dysregulation of vasodilation. Int. J. Mol. Sci. 2022, 23, 5757. [Google Scholar] [CrossRef]
- González-Guerrero, D.E.; Lazo-De-la-Vega-Monroy, M.; Gómez-Ojeda, A.; Luévano-Contreras, C.; Rojas-Rubio, A.; Garay-Sevilla, M.E. Polymorphisms -374 T/A and -429 T/C of the receptor for advanced glycation end-products (RAGE) and serum levels of RAGE (sRAGE) are not associated with metabolic syndrome. Metabolites 2023, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, H.; Ma, Y.; Wang, T.; Zhu, H.; Zhu, L.; Pan, S.; Min, N.; Wamg, X.; Liu, Z. Matrine inhibits advanced glycation end products-induced macrophage M1 polarization by reducing DNMT3a/b-mediated DNA methylation of GPX1 promoter. Eur. J. Tharmacol. 2022, 926, 175039. [Google Scholar] [CrossRef]
- Liu, K.; Deng, T.; Qu, H.; Qu, H.; Huang, Q.; Gao, B.; Li, X.; Wei, N. Gastric protective effect of Alpinia officinarum flavonoids: Mediating TLR4/NF-κB and TRPV1 signalling pathways and gastric mucosal healing. Pharm. Biol. 2023, 61, 50–60. [Google Scholar]
- Quansah, E.; Shaik, T.A.; Çevik, E.; Wang, X.; Höppener, C.; Meyer-Zedler, T.; Deckert, V.; Schmitt, M.; Popp, J.; Krafft, C. Investigating biochemical and structural changes of glycated collagen using multimodal multiphoton imaging, Raman spectroscopy, and atomic force microscopy. Anal. Bioanal. Chem. 2023, 415, 6257–6267. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, S.; Liao, K.; Ho, C.; Hung, W. Quantitation of α-dicarbonyls, lysine- and arginine-derived advanced glycation end products, in commercial canned meat and seafood products. J. Agric. Food Chem. 2023, 71, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Tokimitsu, N.; Nagata, C. Dietary advanced glycation end products and cancer risk in Japan: From the Takayama study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef]
- Yan, Y.; Hemmler, D.; Schmitt-Kopplin, P. HILIC-MS for untargeted profiling of the free glycation product diversity. Metabolites 2022, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Phuong-Nguyen, K.; McNeill, B.A.; Aston-Mourney, K.; Rivera, L.R. Advanced glycation end-products and their effects on gut health. Nutrients 2023, 15, 405. [Google Scholar] [CrossRef]
- Chen, J.; Radiabzadeh, D.; Medina-Gomez, C.; Voortman, T.; van Meurs, J.B.J.; Ikram, M.A.; Uitterlinden, A.G.; Kraaij, R.; Zillikens, M.C. Advanced glycation end products (AGEs) in diet and skin in relation to stool microbiota: The Rotterdam Study. Nutrients 2023, 15, 2567. [Google Scholar] [CrossRef]
- Pint, R.S.; Ferreira, G.S.; Silxestre, G.C.R.; de Fátima M Santana, M.; Nunes, V.S.; Ledesma, L.; Pint, P.R.; de Assis, S.L.S.; Machade, U.F.; de Silva, E.S.; et al. Plasma advanced glycation end products and soluble receptor for advanced glycation end products as indicators of sterol content in human carotid atherosclerotic plaques. Diab. Vasc. Dis. Res. 2022, 19, 14791641221085269. [Google Scholar] [CrossRef]
- Kehm, R.; Rückriemen, J.; Weber, D.; Deubel, S.; Geune, T.; Höhn, A. Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr. Diabetes. 2019, 9, 9. [Google Scholar] [CrossRef]
- Chang, G.; Yeh, Y.; Chen, W.; Ko, Y.; Pang, J.S.; Lee, H. Inhibition of advanced glycation end products formation attenuates cardiac electrical and mechanical remodeling and vulnerability to tachyarrhythmias in diabetic rats. J. Pharmacol. Exp. Ther. 2019, 368, 66–78. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Impact of intracellular toxic advanced glycation end-products (TAGE) on murine myoblast cell death. Diabetol. Metab. Syndr. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular Toxic Advanced Glycation-End Prodcuts May Induce Cell Death and Suppress Cardiac Fibroblasts. Metabolites 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Murayama, H.; Masauji, T. Slot Blot Analysis of Intracellular Glyceraldehyde-Derived Glycation End-Products Using a Novel Lysis Buffer and Polyvinylidene Difluoride Membrane. Bio. Protocl. in press.
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular toxic advanced glycation end-products in 1.4E7 cell line induce death with reduction of microtubule-associated protein 1 light chain 3 and p62. Nutrients 2022, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Masauji, T.; Motoo, Y. Potential of the Novel slot blot method with a PVDF membrane for protein identification and quantification in Kampo Medicines. Membranes 2023, 13, 896. [Google Scholar] [CrossRef]
- Baskal, S.; Büttner, P.; Werner, S.; Besler, C.; Lurz, P.; Thiele, H.; Tsikas, D. Profile of urinary amino acids and their post-translational modifications (PTM) including advanced glycation end-products (AGEs) of lysine, arginine and cysteine in lean and obese ZSF1 rats. Amino Acids 2022, 54, 643–652. [Google Scholar] [CrossRef]
- Kuang, L.; Jing, Z.; Wang, J.; Ma, L.; Liu, Z.; Yang, J. Quantitative determination of ɛ-N-carboxymethyl-L-lysine in human plasma by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 1–6. [Google Scholar] [CrossRef]
- Ban, I.; Sugawa, H.; Nagai, R. Protein modification with ribose generates Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine. Int. J. Mol. Sci. 2022, 23, 1224. [Google Scholar] [CrossRef] [PubMed]
- Nokin, M.J.; Durieux, F.; Peixoto, P.; Chavaria, B.; Peulen, O.; Blomme, A.; Turtoi, A.; Costanza, B.; Smargiasso, N.; Baiwm, D.; et al. Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP-mediated tumor growth and metastasis. eLife 2016, 5, e19375. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Tian, C.; Ouyang, S.; Moore, C.J.; Alomar, F.; Nemet, I.; D’Souza, A.; Nagai, R.; Kutty, S.; Rozanski, G.J.; et al. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol. Pharmacol. 2012, 82, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Espín, J.; Nilsson, O.; Bernfur, K.; Giudice, R.D.; Lagerstedt, J.O. Site-specific glycations of apolipoprotein A-I lead to differentiated functional effects on lipid-binding and on glucose metabolism. Biochemi. Biophys. Acta. Mol. Basis. Dis. 2018, 1864, 2822–2834. [Google Scholar] [CrossRef]
- Argirov, O.K.; Lin, B.; Ortwerthm, B.J. 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J. Bio. Chem. 2004, 279, 6487–6495. [Google Scholar] [CrossRef]
- Humsae, C.; Gifford, K.; Lian, W.; Liu, H.; Radziejewski, C.H.; Zhou, Z.S. Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal. Chem. 2013, 85, 11401–11409. [Google Scholar] [CrossRef]
- Scheckhuber, C.Q. Arg354 in the catalytic centre of bovine liver catalase is protected from methylglyoxal-mediated glycation. BMC Res. Notes 2015, 8, 830. [Google Scholar] [CrossRef]
- Nuti, F.; Gallo, A.; Real-Frenandes, F.; Rentier, C.; Rossi, G.; Piarulli, F.; Traldi, P.; Carganico, S.; Rovero, P.; Lapolla, A.; et al. Study of aberrant modifications in peptides as a test bench to investigate the immunological response to non-enzymatic glycation. Folia Biol. 2019, 65, 195–202. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gastrointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Q.; Zhao, P.; Lv, X.; Gong, C.; Gao, J.; Liu, J. Effect of transferrin glycation induced by high glucose on HK-2 cells in vitro. Front. Endocrinol. 2022, 13, 1009507. [Google Scholar] [CrossRef]
- Linder, M.D.; Morkunaite-Haimi, S.; Kinnunen, P.K.J.; Bernardi, P.; Eriksson, O. Ligand-selective modulation of the permeability transition pore by arginine modification. Opposing effects of p-hydroxyphenylglyoxal and phenylglyoxal. Biol. Chem. 2002, 277, 937–942. [Google Scholar] [CrossRef]
- Takazaki, S.; Abe, Y.; Yamaguchi, T.; Yagi, M.; Ueda, T.; Kang, D.; Hamasaki, N. Arg 901 in the AE1 C-terminal tail is involved in conformational change but not in substrate binding. Biochim. Biophys. Acta 2011, 18, 658–665. [Google Scholar] [CrossRef]
- Pianeda-Alemán, R.; Cabarcas-Herrera, C.; Alviz-Amador, A.; Galindo-Murillo, R.; Pérez-Gonzalez, H.; Rodríguez-Cavallo, E.; Méndez-Cuadro, D. Molecular dynamics of structural effects of reactive carbonyl species derivate of lipid peroxidation on bovine serum albumin. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130613. [Google Scholar] [CrossRef]
- Papadaki, M.; Kampaengsri, T.; Barrick, S.K.; Campbell, S.G.; von Lewinski, D.; Rainer, P.P.; Harris, S.P.; Greenberg, M.J.; Kirk, J.A. Myofilament glycation in diabetes reduces contractility by inhibiting tropomyosin movement, is rescued by cMyBPC domains. J. Mol. Cell. Cardiol. 2022, 162, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Huang, C.; Huang, B.; Liang, Q. Rapamycin protects cardiomyocytes against anoxia/reoxygenation injury by inducing autophagy through the PI3k/Akt pathway. J. Huazhong Univ. Sci. Tehnol. Med. Sci. 2015, 35, 10–15. [Google Scholar] [CrossRef]
- Teng, A.C.T.; Miyake, T.; Yokoe, S.; Zhang, L.; Rezende Jr, L.M.; Sharma, P.; Maclennan, D.H.; Liu, P.P.; Gramolini, A.O. Metformin increases degradation of phospholamban via autophagy in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 7165–7170. [Google Scholar] [CrossRef]
- Hu, P.; Zhou, H.; Lu, M.; Dou, L.; Bo, G.; Wu, J.; Huang, S. Autophagy plays a protective role in advanced glycation end product-induced apoptosis in cardiomyocytes. Cell Physiol. Biochem. 2015, 37, 697–706. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Q.; Shi, W.; Peng, L.; Jiang, Y.; Zhu, M.; Guo, J.; Peng, D.; Wang, M.; Men, L.; et al. ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury. Int. J. Mol. Sci. 2023, 24, 1667. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Z.; Zhang, Y.; Qui, C.; Zhu, L.; Zhao, N.; Liu, Z. Matrine alleviates AGEs- induced cardiac dysfunctions by attenuating calcium overload via reducing ryanodine receptor 2 activity. Eur. J. Pharmacol. 2019, 842, 118–124. [Google Scholar] [CrossRef]
- Motoo, Y.; Yasui, H. Analysis of Kampo case reports from the viewpoint of “Yasui Classification”. Tradit. Kampo Med. 2024, 11, 60–64. [Google Scholar] [CrossRef]
- Motoo, Y.; Seki, T.; Tsutani, K. Traditional Japanese medicine, Kampo: Its history and current status. Chin. J. Integr. Med. 2011, 17, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Yuasa, M.; Chen, F.; Yukawa, K.; Motoo, Y.; Arai, I. The status of education for integrative medicine in Japanese medical universities with special reference to Kampo medicines. Tradit. Kampo Med. 2023, 10, 123–131. [Google Scholar] [CrossRef]
- Motoo, Y.; Hakamatsuka, T.; Kawahara, N.; Arai, I.; Tsutani, K. Standards of Reporting Kampo Products (STORK) in research articles. J. Integr. Med. 2017, 15, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Z.; Wang, T.; Liu, Z.; Zuo, Z. Using ultra-performance liquid chromatography with linear ion trap-electrostatic field orbitrap mass spectrometry, network pharmacology, and molecular docking to explore the constituent targets and action mechanisms of decoction of Angelica sinensis, Zingiberis Rhizoma Recens, and Mutton in the treatment of diarrhea-predominant irritable bowel syndrome. J. Pharm. Pharmacol. 2024, 76, 462–478. [Google Scholar] [PubMed]
- Zhi, J.; Yin, L.; Zhang, Z.; Lv, Y.; Wu, F.; Yang, Y.; Zhang, E.; Li, H.; Lu, N.; Zhou, M.; et al. Network pharmacology-based analysis of Jin-Si-Wei on the treatment of Alzheimer’s disease. J. Ethnopharmacol. 2024, 319, 117291. [Google Scholar] [CrossRef]
- Yamakawa, J.; Moriya, J.; Takeuchi, K.; Nakatou, M.; Motoo, Y.; Kobayashi, J. Significance of Kampo, Japanese traditional medicine, in the treatment of obesity: Basic and clinical evidence. Evi. Based Complement. Aternat. Med. 2013, 2013, 943075. [Google Scholar] [CrossRef] [PubMed]
- Uneda, K.; Kawai, Y.; Yamada, T.; Kaneko, A.; Saito, R.; Chen, L.; Ishigami, T.; Namiki, T.; Mitsuma, T. Japanese traditional Kampo medicine bofutsushosan improves body mass index in participants with obesity: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0266917. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y. Role of Kampo medicine in modern cancer therapy: Towards completion of standard treatment. J. Nippon Med. Sch. 2022, 89, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Arai, I.; Kawahara, N. Kampo pharmaceutical products in the Kapanese health-care system: Legal status and quality assurance. Tradi. Kampo Med. 2019, 6, 3–11. [Google Scholar] [CrossRef]
- Arai, I. Clinical studies of traditional Japanese herbal medicines (Kampo): Need for evidence by the modern scientific methodology. Integr. Med. Res. 2021, 10, 100722. [Google Scholar] [CrossRef]
- Motoo, Y.; Cameron, S. Kampo medicines for supportive care of patients with cancer: A brief review. Integr. Med. Res. 2022, 11, 100839. [Google Scholar] [CrossRef]
- Tagashira, H.; Abe, F.; Sato-Numata, K.; Aizawa, K.; Hirasawa, K.; Kure, Y.; Iwata, D.; Numata, T. Cardioprotective effects of Moku-boi-to and its impact on AngII-induced cardiomyocyte hypertrophy. Front. Cell Dev. Biol. 2023, 7, 1264076. [Google Scholar] [CrossRef]
- Motoo, Y.; Arai, I.; Kogure, T.; Tsutani, K. Review of the first 20 years of the Evidence-Based Medicine Committee of Japan Society for Oriental Medicine. Trad. Kampo Med. 2021, 8, 123–139. [Google Scholar] [CrossRef]
- Motoo, Y.; Arai, I.; Tsutani, K. Use of Kampo diagnosis in randomized controlled trials of Kampo products in Japan: A systematic review. PLoS ONE 2014, 9, e104422. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ma, S.; Zhang, S.; Li, X.; Quan, R.; Wan, S.; Duo, X. Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution. Int. J. Biol. Macromol. 2023, 239, 124358. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Kagawa, S.; Arimitsu, J.; Nakanishi, M.; Sakashita, N.; Otsuka, S.; Yoshikawa, H.; Hagihara, K. Go-sha-jinki-Gan (GJG), a traditional Japanese herbal medicine, protects against sarcopenia in senescence-accelerated mice. Phytomedicine 2015, 22, 16–22. [Google Scholar] [CrossRef]
- Nakanishi, M.; Nakae, A.; Kishida, Y.; Baba, K.; Sakashita, N.; Shibata, M.; Yoshikawa, H.; Hagihara, K. Go-sha-jinki-Gan (GJG) ameliorates allodynia in chronic constriction injury-model mice via suppression of TNF-α expression in the spinal cord. Mol. Pain 2016, 12, 1744806916656382. [Google Scholar] [CrossRef]
- Hosogi, S.; Ohsawa, M.; Kato, I.; Kuwahara, A.; Inui, T.; Inui, A.; Marunaka, Y. Improvement of Diabetes Mellitus Symptoms by Intake of Ninjin’yoeito. Front. Nutr. 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Fujii, H.; Hayakawa, Y.; Sakukawa, R.; Yamaura, T.; Sakamoto, T.; Tsukada, K.; Fujimaki, M.; Nunome, S.; Komatsu, Y.; et al. Oral administration of a Kampo (Japanese herbal) medicine Juzen-taiho-to inhibits liver metastasis of colon 26-L5 carcinoma cells. Jpn. J. Cancer Res. 1998, 89, 206–2013. [Google Scholar] [CrossRef]
- Uto, N.S.; Amitani, H.; Atobe, Y.; Sameshima, Y.; Sakaki, M.; Rokot, N.; Ataka, K.; Amitani, M.; Iuoue, A. Herbal Medicine Ninjin’yoeito in the treatment of sarcopenia and frailty. Front. Nutr. 2018, 5, 126. [Google Scholar] [CrossRef]
- Takagi, K.; Sugihara, T.; Kitamura, M.; Kawai, M.; Mitsuguchi, Y.; Tsukamoto, K.; Nakanishi, H.; Makino, T. Inhibitory effect of Bofutsushosan (Fangfengtongshengsan) extract on the absorption of fructose in rats and mice. J. Nat. Med. 2023, 77, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, A.; Ohsawa, M.; Motoo, Y.; Mizukami, H.; Makino, T. Effect of ninjin’yoeito and ginseng extracts on oxaliplatin-induced neuropathies in mice. J. Nat. Med. 2017, 71, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kazachkov, M.; Chen, K.; Babiy, S.; Yu, P.H. Evidence for in vivo scavenging by aminoguanidine of formaldehyde produced via semicarbazide-sensitive amine oxidase-mediated deamination. J. Pharmacol. Exp. Ther. 2007, 322, 1201–1207. [Google Scholar] [CrossRef]
- Sekar, P.; Hsiao, G.; Hsu, S.; Huang, D.; Lin, Q.; Chan, C. Metformin inhibits methylglyoxal-induced retinal pigment epithelial cell death and retinopathy via AMPK-dependent mechanisms: Reversing mitochondrial dysfunction and upregulating glyoxalase 1. Redox. Biol. 2023, 64, 102786. [Google Scholar] [CrossRef]
- Leone, A.; Nigro, C.; Nicolò, A.; Prevenzano, I.; Formisano, P.; Beguinot, F.; Miele, C. The dual-role of methylglyoxal in tumor progression—Novel therapeutic approaches. Front. Oncol. 2021, 11, 6454686. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Sakai-Sakasai, A.; Kajinami, K.; Takeuchi, M. A novel approach: Investigating the intracellular clearance mechanism of glyceraldehyde-derived advanced glycation end-products using the artificial checkpoint kinase 1 d270KD Mutant as a Substrate Model. Cells 2023, 12, 2838. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.Y.; Zuo, G.; Wang, Z.; Lee, J.; Lim, S.S. Anti-glycation, carbonyl trapping and anti-inflammatory activities of chrysin derivatives. Molecules 2018, 23, 1752. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.; Sang, S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.; Yang, C.S.; Ho, C. Tea polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Lee, S.M.; Zheng, L.W.; Jung, Y.; Hwang, G.; Kim, Y. Effects of hydroxycinnamic acids on the reduction of furan and α-dicarbonyl compounds. Food Chem. 2019, 312, 126085. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 30, 679–687. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Lv, Z.; Li, S.; Hu, B.; Guan, Y.; Xie, B.; Sun, Z. Oligomeric procyanidins of lotus seedpod inhibits the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Chem. 2013, 138, 1493–1502. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, X.; Zhu, L.; Wu, G.; Qi, X.; Zhang, H. Trapping of reactive carbonyl species by fiber-bound polyphenols from whole grains under simulated physiological conditions. Food Res. Int. 2022, 156, 111142. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Aspalathin and other Rooibos flavonoids trapped α-Dicarbonyls and inhibited formation of advanced glycation end products in vitro. Int. J. Mol. Sci. 2022, 23, 14738. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I.; Schejien, J.L.J.M.; Ahles, S.; Vangrieken, P.; Schalkwijk, C.G. A citrus and pomegranate complex reduces methylglyoxal in healthy elderly subjects: Secondary analysis of a double-blind randomized cross-over clinical trial. Int. J. Mol. Sci. 2023, 24, 13168. [Google Scholar] [CrossRef]
- Boojar, M.M.A.; Boojar, M.M.A.; Firoozivand, Y. In vitro investigation of the anti-diabetic effects of imperialine on Beta-TC6 pancreatic and C2C12 skeletal muscle cell lines. J. Appl. Biotechnol. Rep. 2021, 8, 337–345. [Google Scholar]
- Schmidt, B.; Ferreira, C.; Passos, C.L.A.; Silva, J.L.; Fialho, E. Resveratrol, curcumin and piperine alter human glyoxalase 1 in MCF-7 breast cancer cells. Int. J. Mol. Sci. 2020, 21, 5244. [Google Scholar] [CrossRef]
- Cha, S.; Hwang, Y.; Heo, S.; Jun, H. Diphlorethohydroxycarmalol attenuates methylglyoxal-induced oxidative stress and advanced glycation end product formation in human kidney cells. Oxid. Med. Cell Longev. 2018, 2018, 3654095. [Google Scholar] [CrossRef]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In vitro antioxidant and anti-glycation activity of resveratrol and its novel triester with trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef]
- Lan, K.; Peng, P.; Chang, T.; Liu, S. Resveratrol alleviates advanced glycation end-products-related renal dysfunction in d-galactose-induced aging mice. Metabolites 2023, 13, 655. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.; Cheng, C.; Hu, M. Trapping of methylglyoxal by curcumin in cell-free systems and in human umbilical vein endothelial cells. J. Agric. Food Chem. 2012, 60, 8190–8196. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhao, G. Flavonoid intake and risk CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, Y.; Yuan, L.; Zhang, D.; Feng, Y.; Hu, H.; Hu, D.; Liu, J. Total dietary flavonoid intake and risk of cardiometabolic disease: A dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2024, 64, 2760–2772. [Google Scholar] [CrossRef]
- Takata, T.; Motoo, Y. Novel In Vitro Assay of the Effects of Kampo Medicines against Intra/Extracellular Advanced Glycation End-Products in Oral, Esophageal, and Gastric Epithelial Cells. Metabolites 2023, 13, 878. [Google Scholar] [CrossRef]


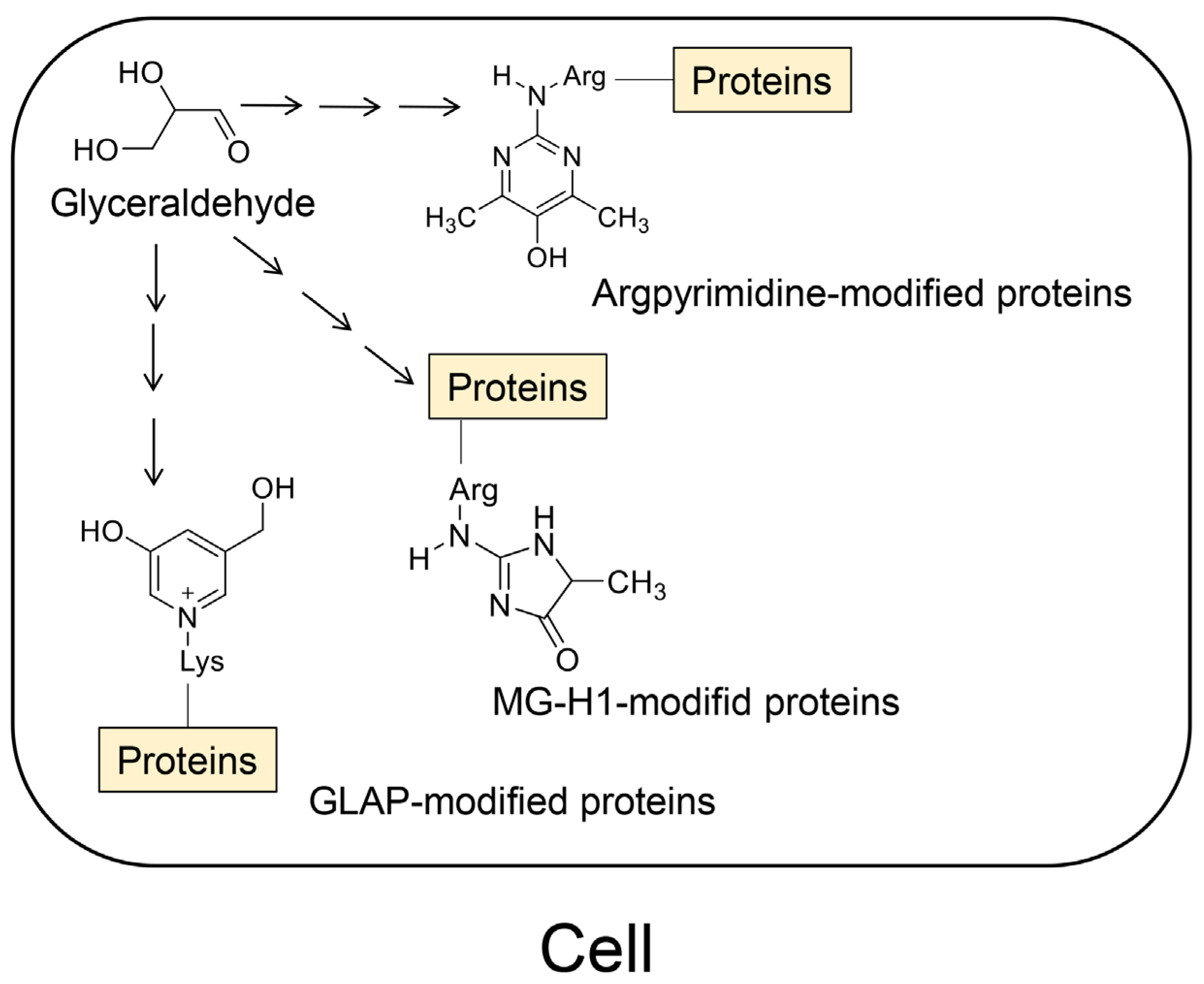
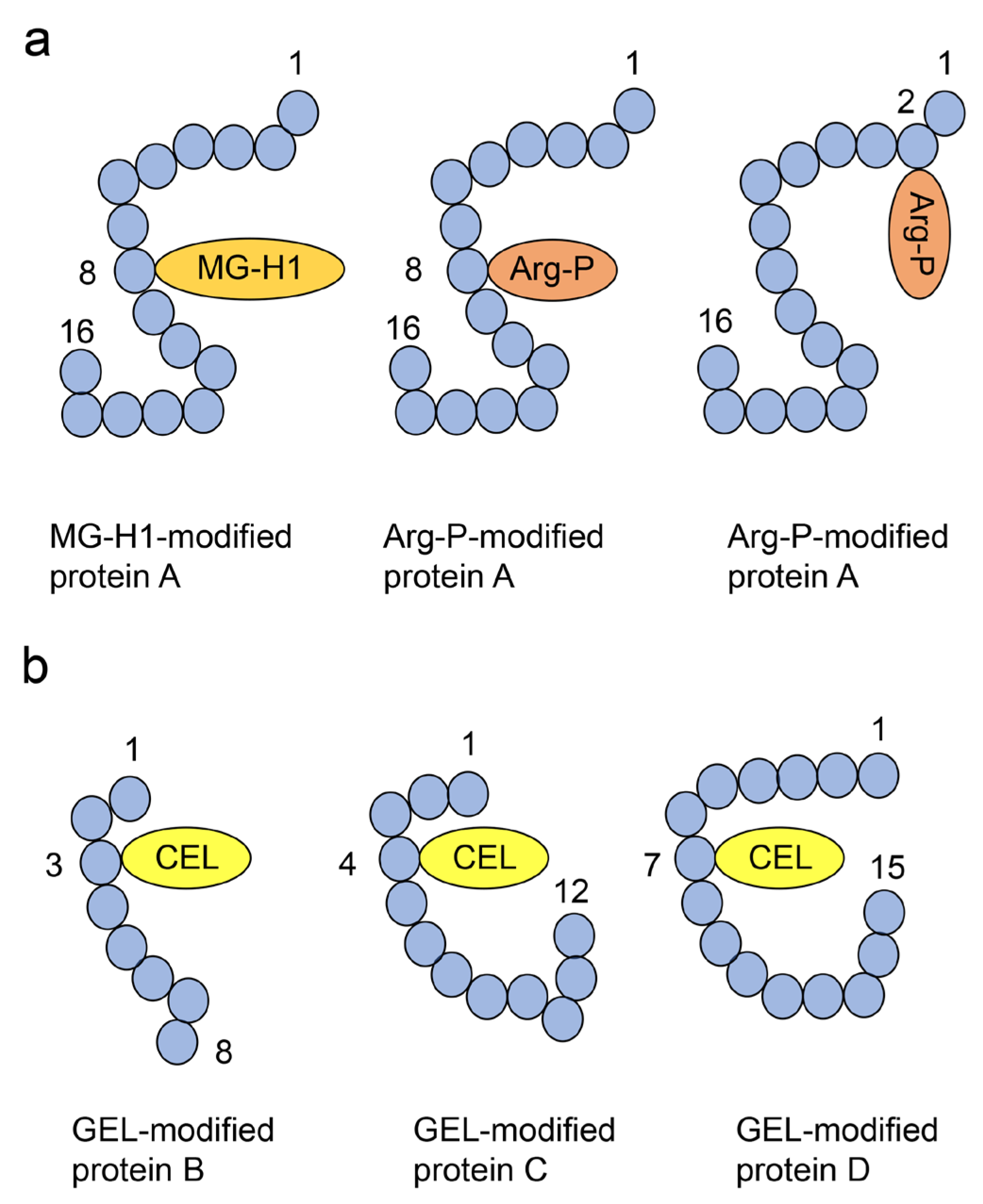
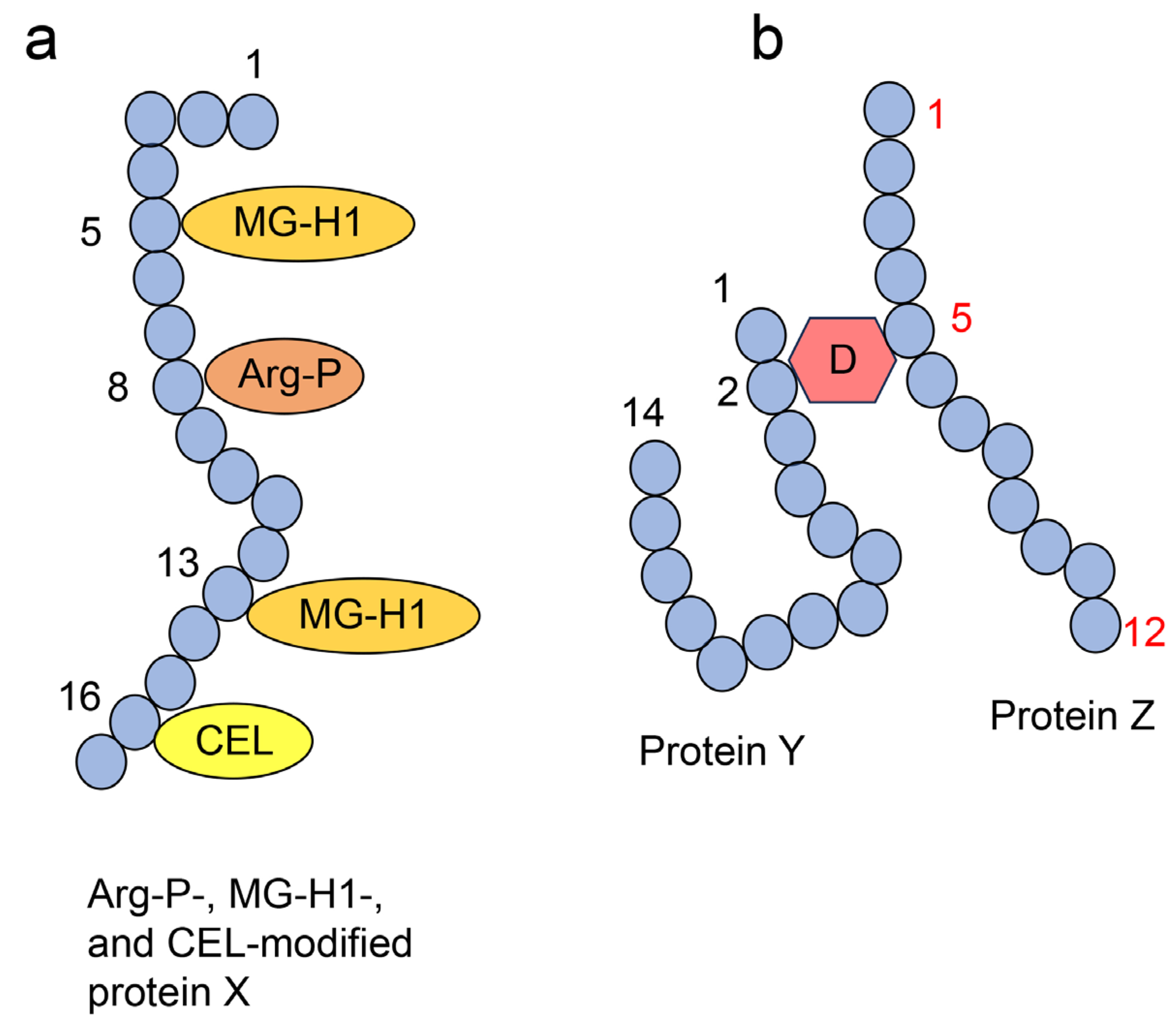

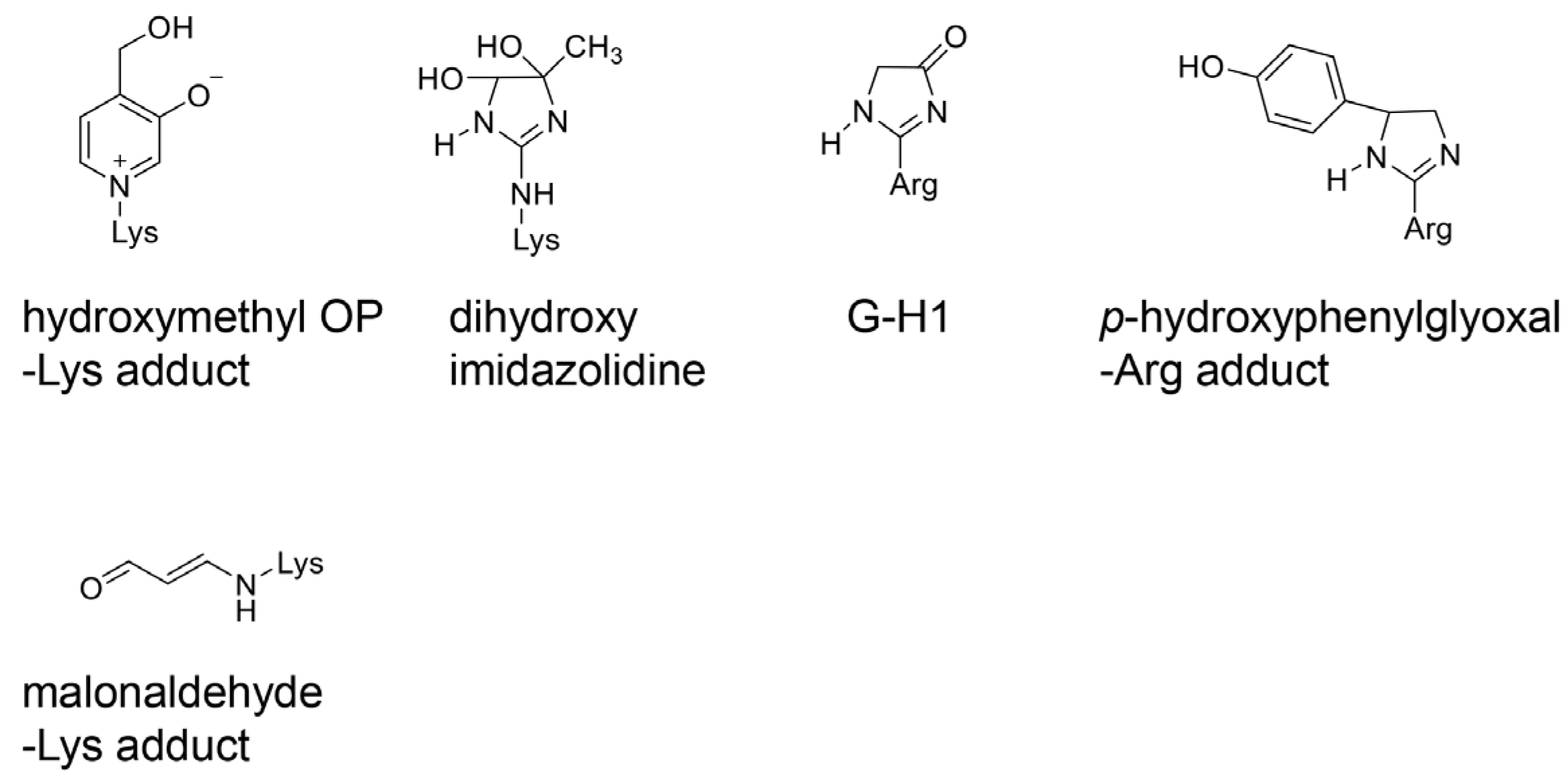
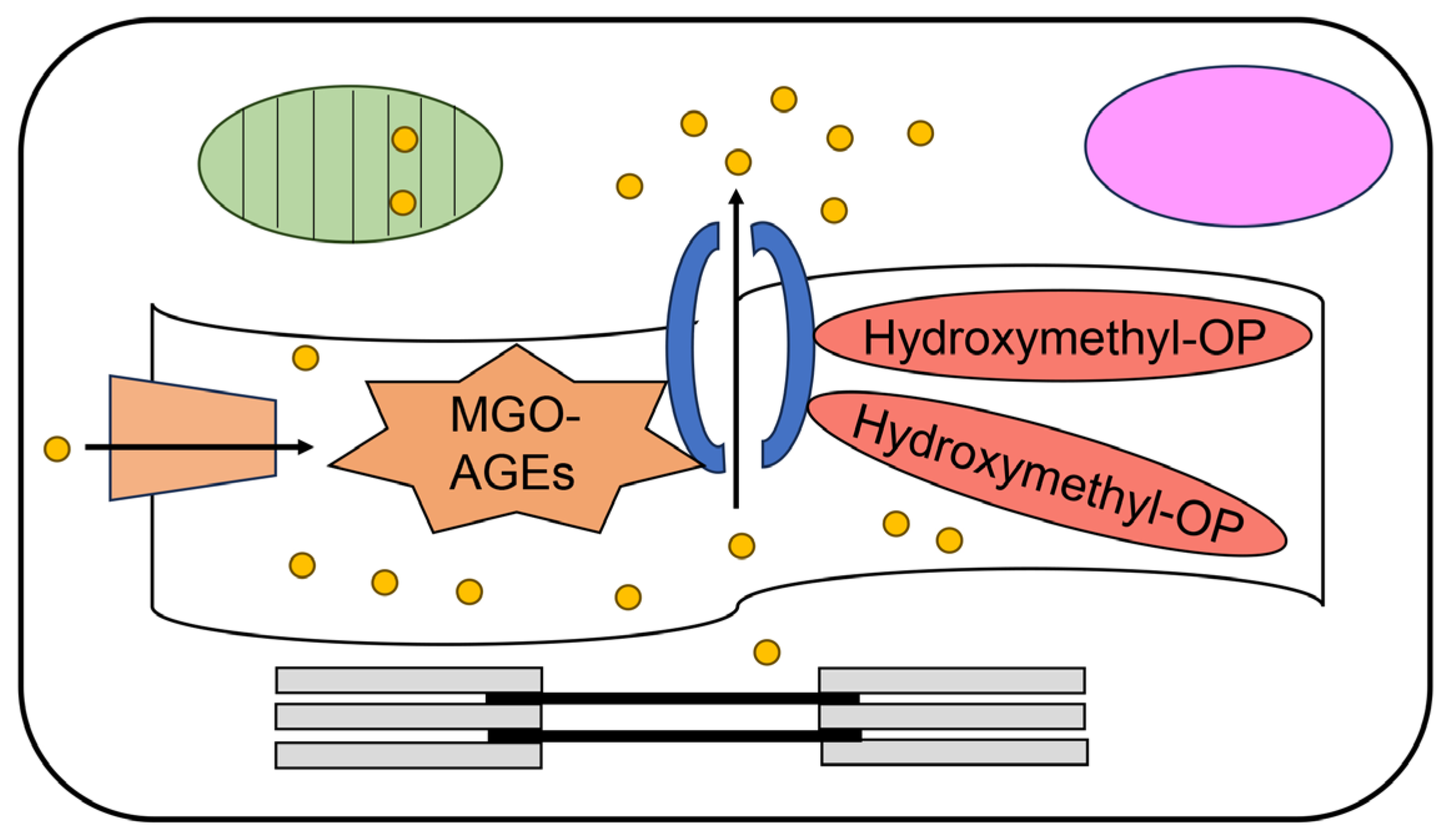
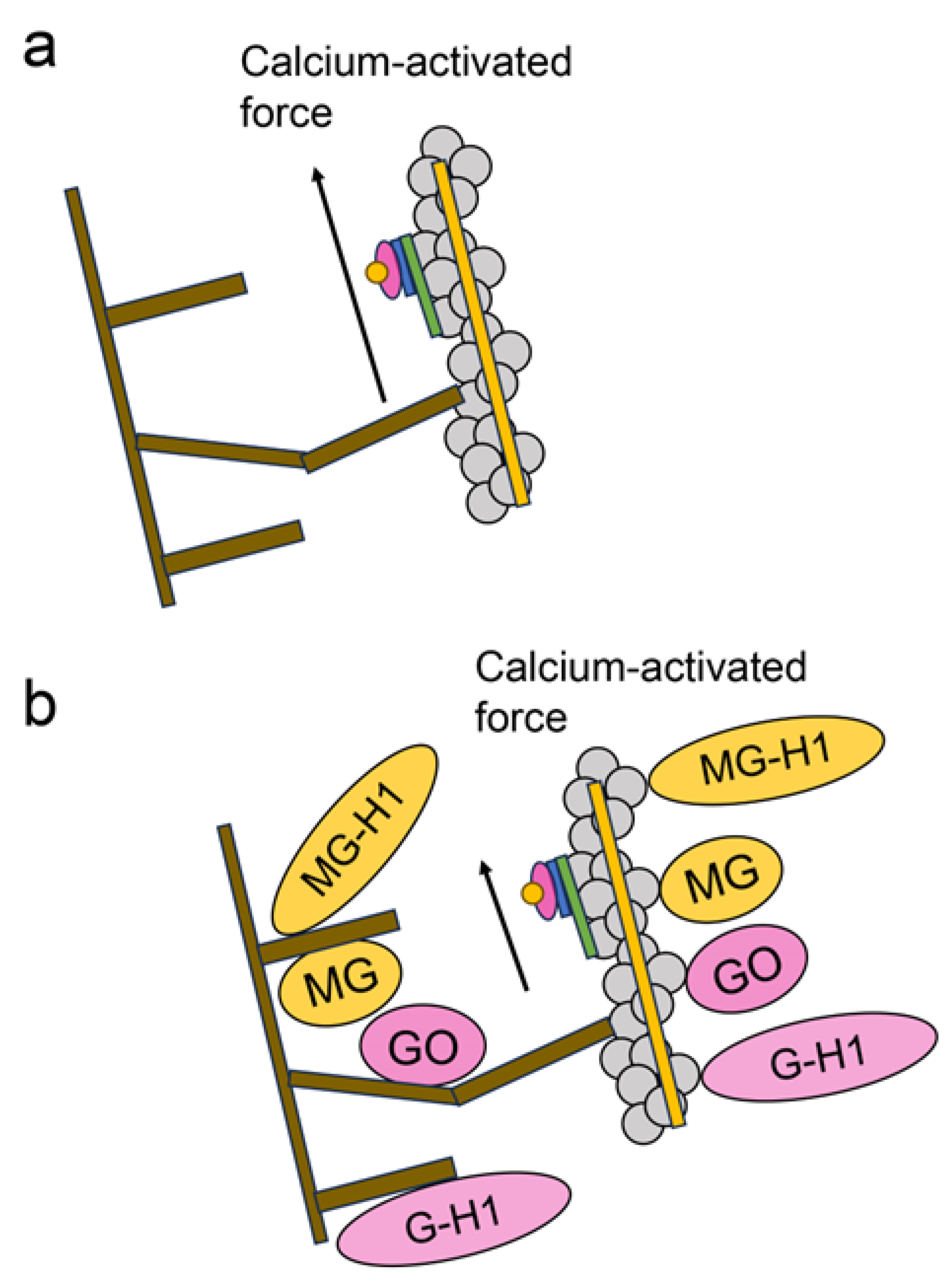

| Category | Origin | AGEs | Reference |
|---|---|---|---|
| AGE-1 | Glucose | Pentosidine | [50] |
| AGE-2 | Glyceraldehyde | Trihydroxy-triosidine | [48,49] |
| GLAP | [48,49] | ||
| AGE-3 | Glycolaldehyde | CML | [50] |
| Hydroxymethyl-OP | [26] | ||
| AGE-4 | Methylglyoxal | MG-H1 | [48,49,50] |
| Argpyrimidine | [48,49,50] | ||
| CEL | [27] | ||
| AGE-5 | Glyoxal | CML | [50] |
| G-H1 | [50] | ||
| AGE-6 | 3-deoxyglucosone | Pyralline | [50] |
| Pentosidine | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, T.; Inoue, S.; Masauji, T.; Miyazawa, K.; Motoo, Y. Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 7319. https://doi.org/10.3390/ijms25137319
Takata T, Inoue S, Masauji T, Miyazawa K, Motoo Y. Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease. International Journal of Molecular Sciences. 2024; 25(13):7319. https://doi.org/10.3390/ijms25137319
Chicago/Turabian StyleTakata, Takanobu, Shinya Inoue, Togen Masauji, Katsuhito Miyazawa, and Yoshiharu Motoo. 2024. "Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease" International Journal of Molecular Sciences 25, no. 13: 7319. https://doi.org/10.3390/ijms25137319
APA StyleTakata, T., Inoue, S., Masauji, T., Miyazawa, K., & Motoo, Y. (2024). Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease. International Journal of Molecular Sciences, 25(13), 7319. https://doi.org/10.3390/ijms25137319






