Skeletal Muscle UCHL1 Negatively Regulates Muscle Development and Recovery after Muscle Injury
Abstract
1. Introduction
2. Results
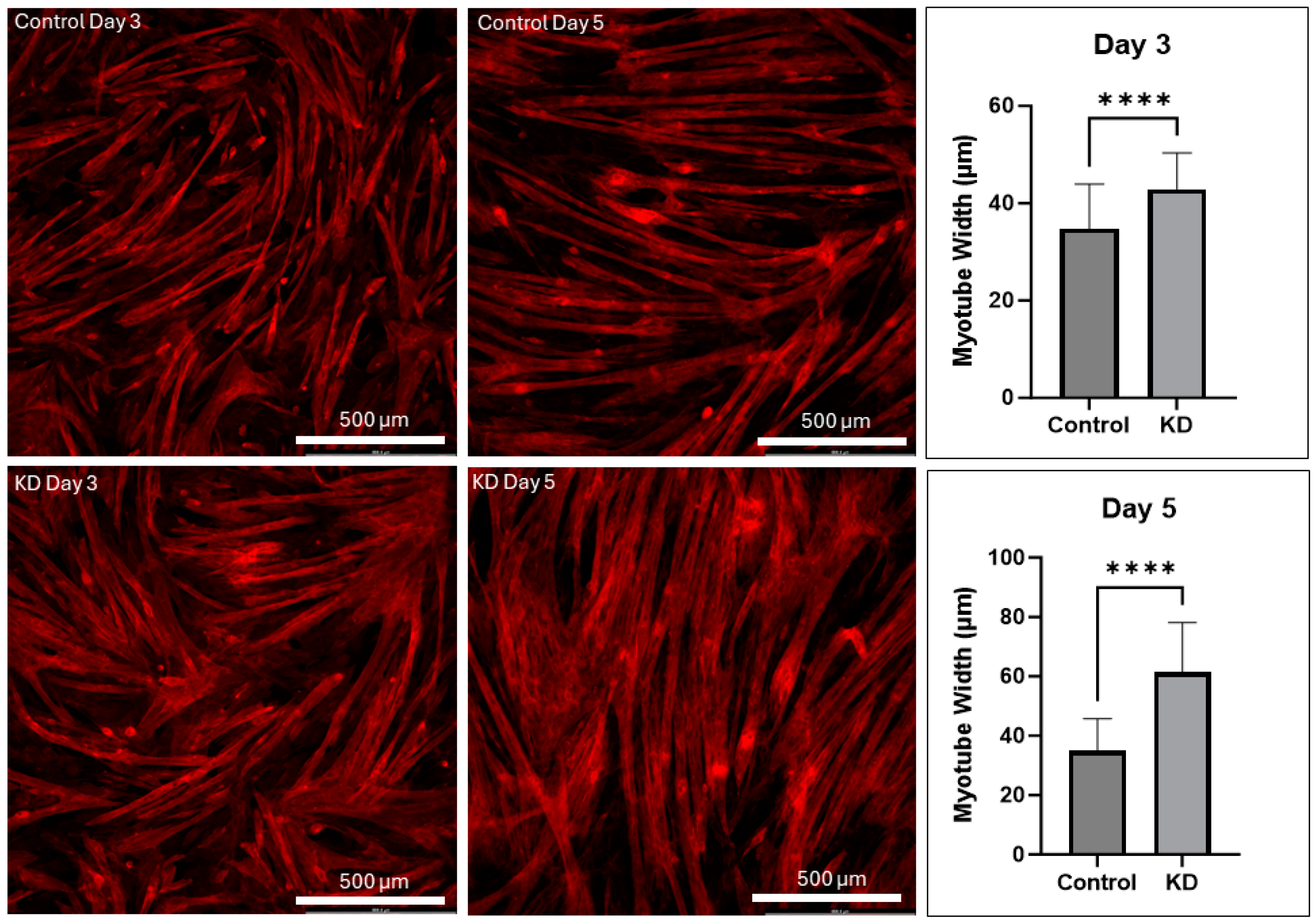
2.1. UCHL1 Knockdown Promotes Myotube Differentiation In Vitro
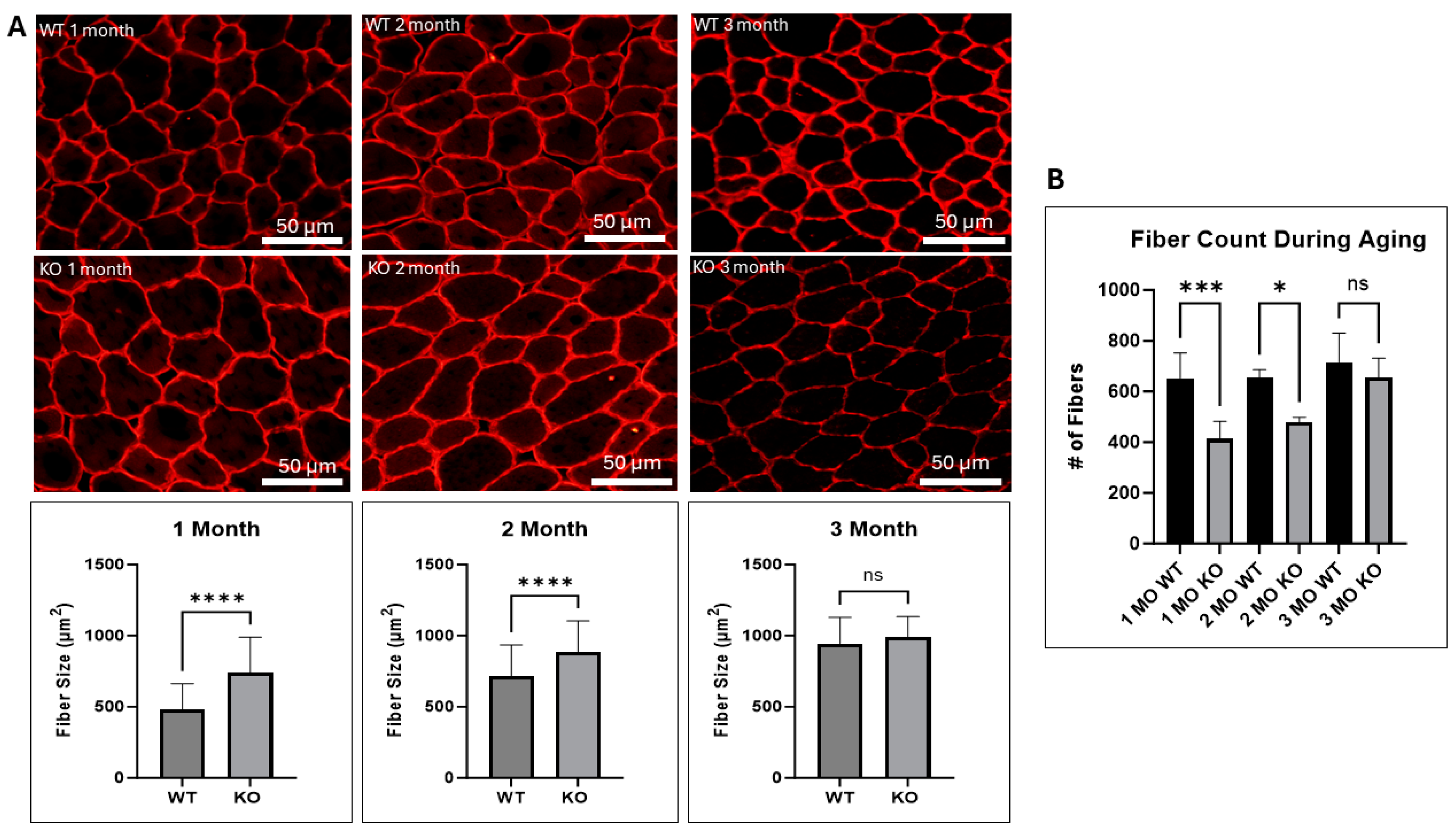
2.2. UCHL1 Negatively Affects Fiber Size In Vivo
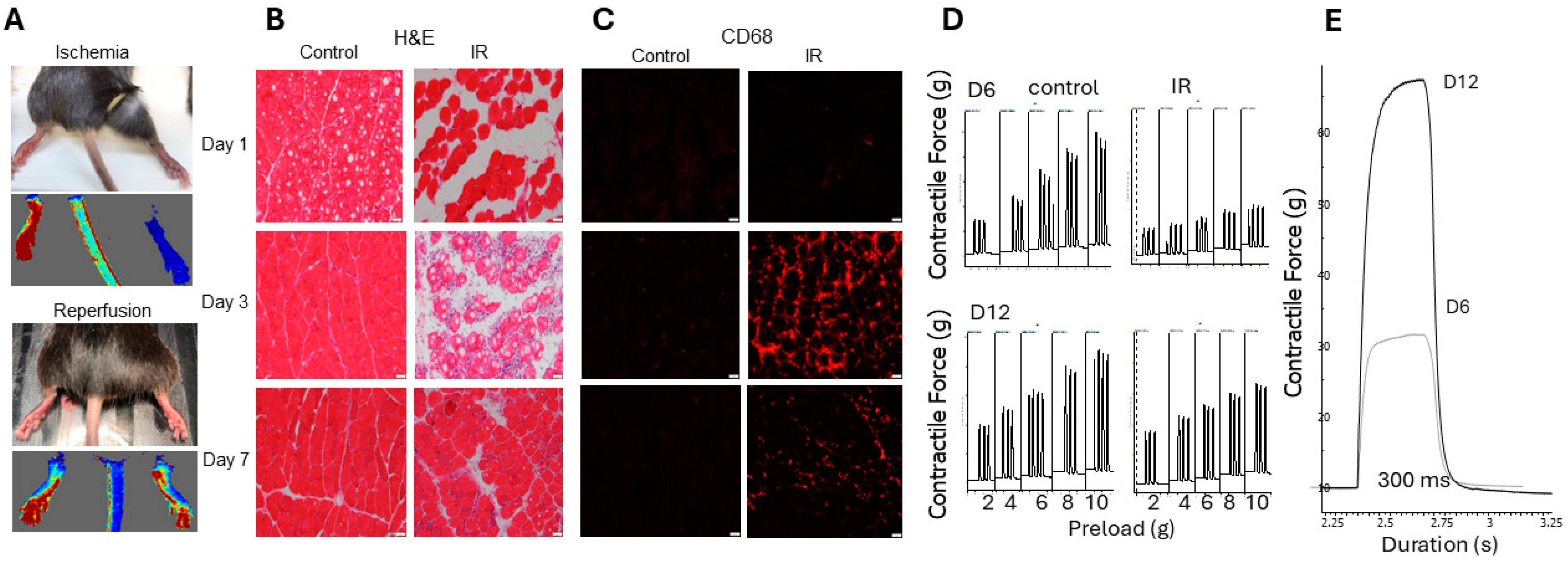
2.3. Non-Invasive Model of Hindlimb Ischemia-Reperfusion Injury
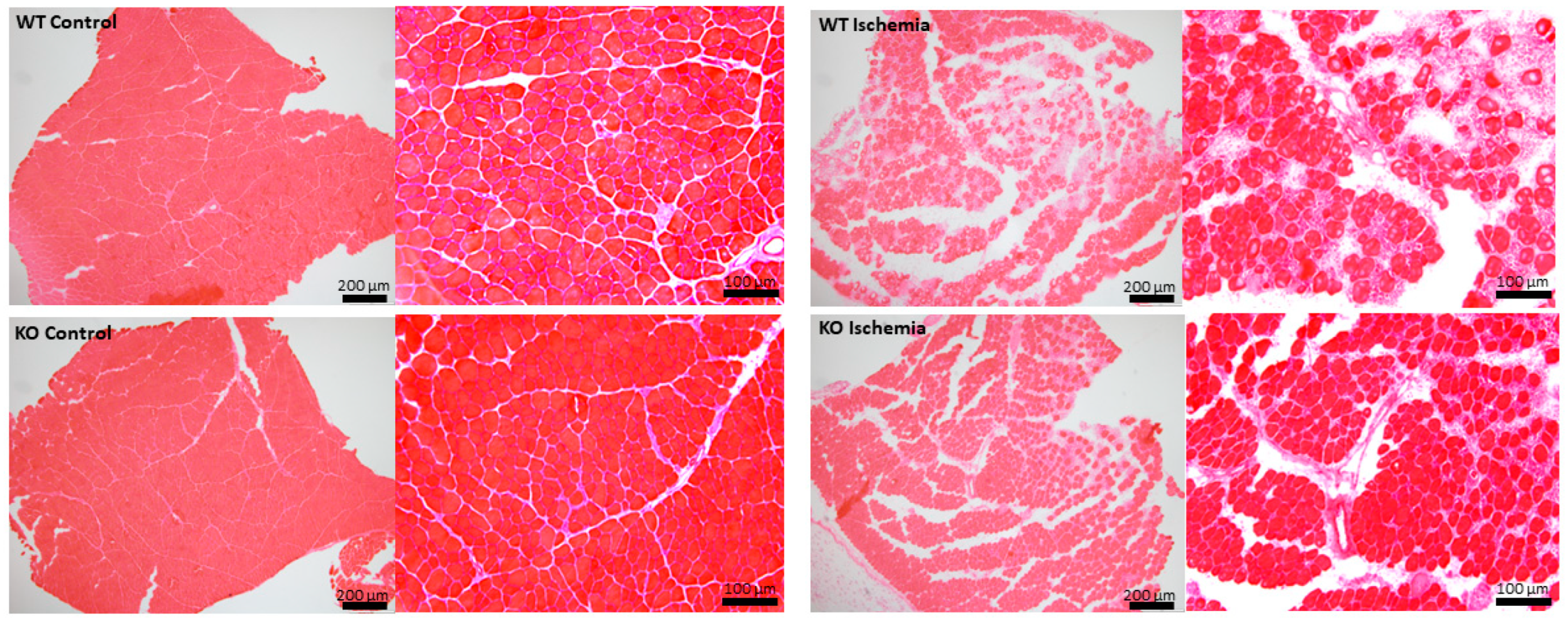
2.4. Effects of UCHL1 Knockout during Injury
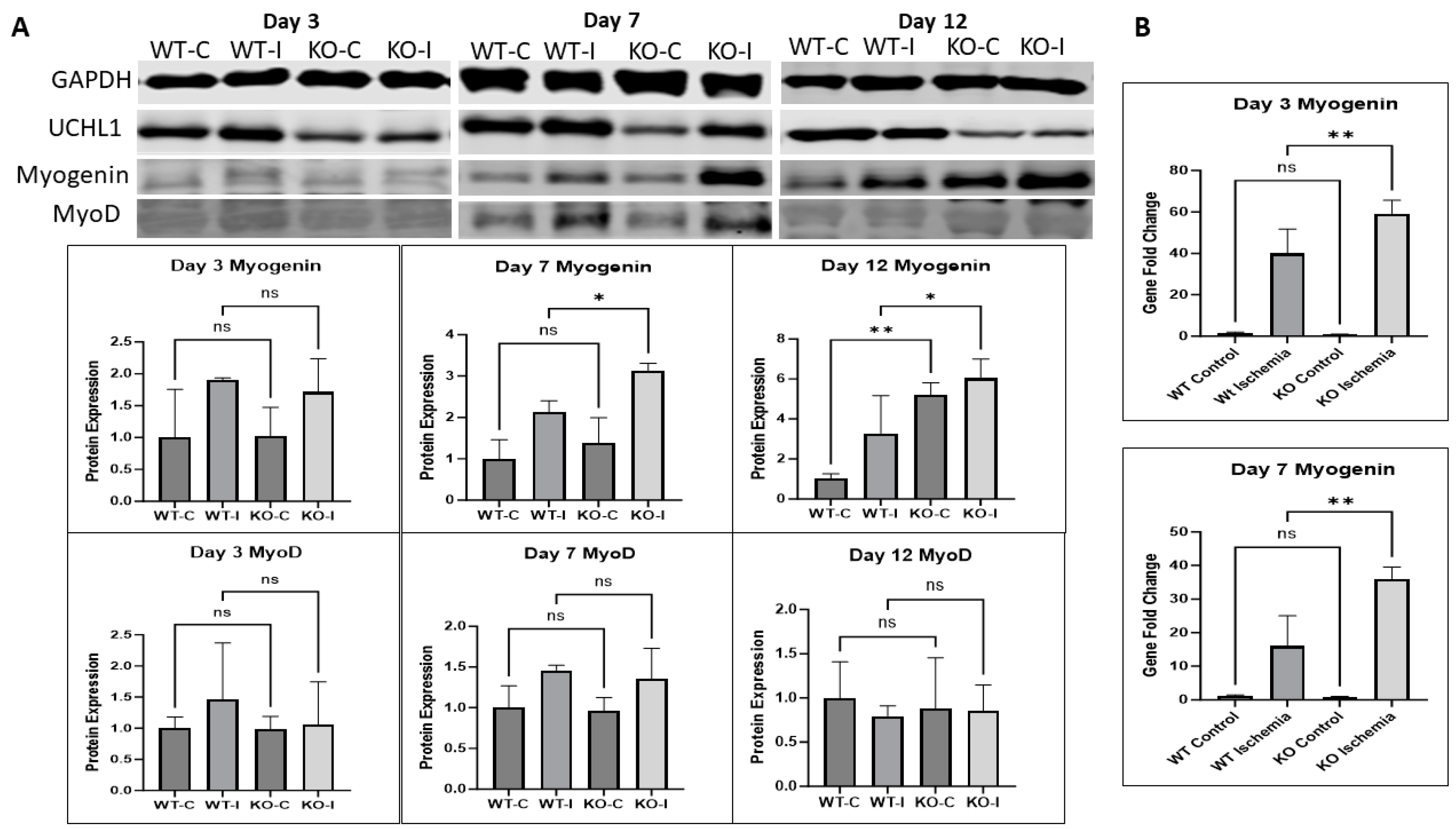
2.5. UCHL1 smKO Mice Exhibit Upregulated Myogenic Factors in Injured Muscle
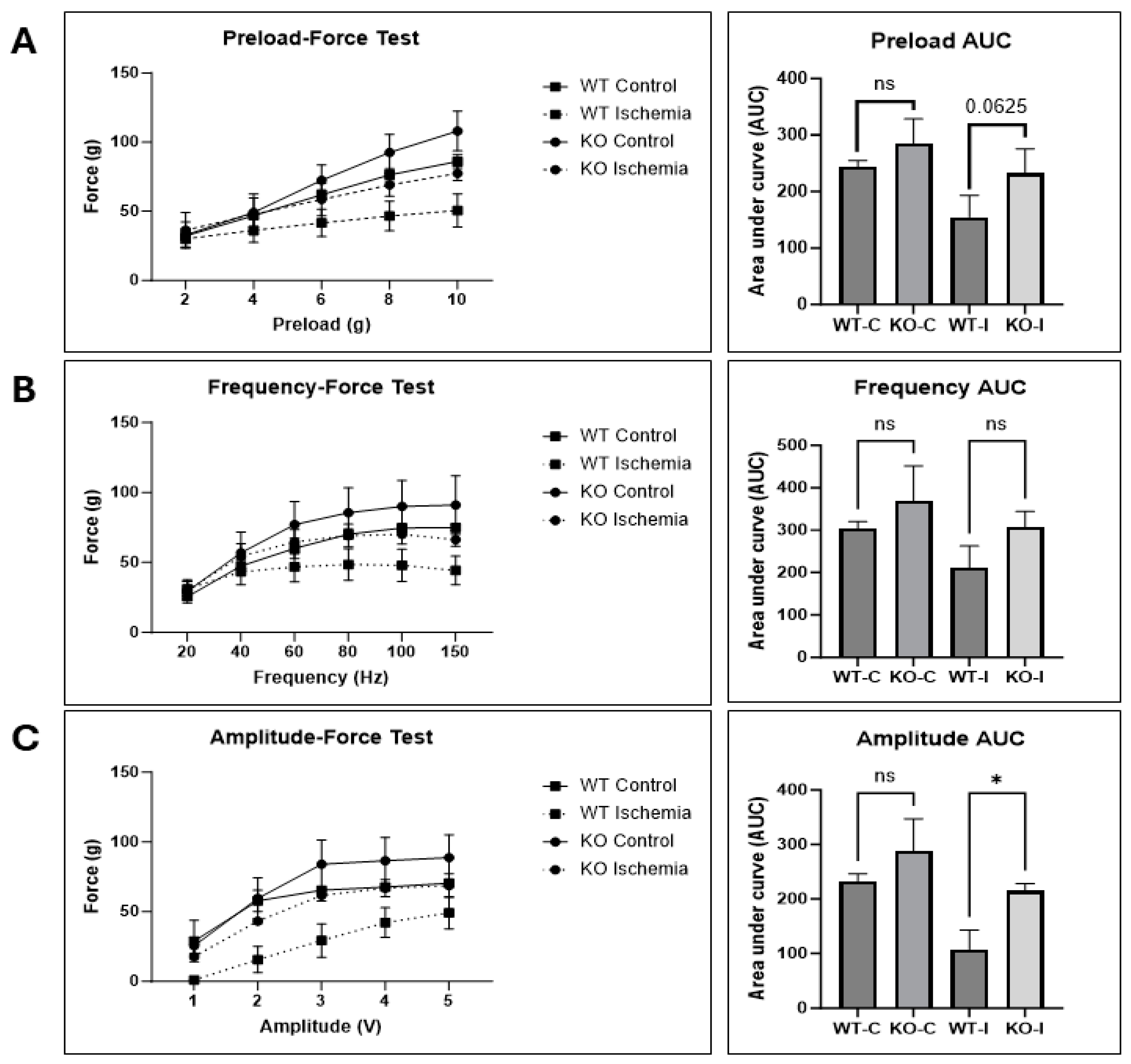
2.6. UCHL1 smKO Muscle Has Improved Functional Recovery Following Injury
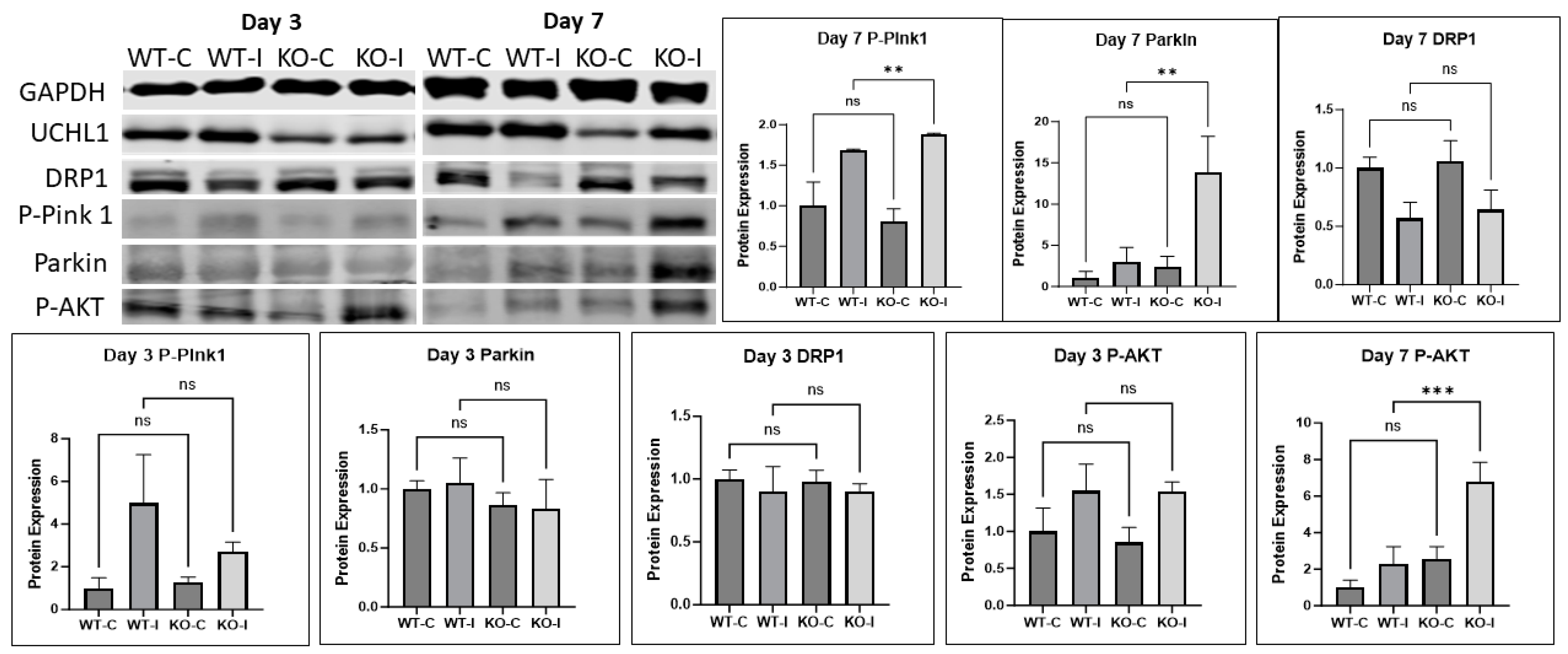
2.7. UCHL1 Regulates Mitophagy Signals
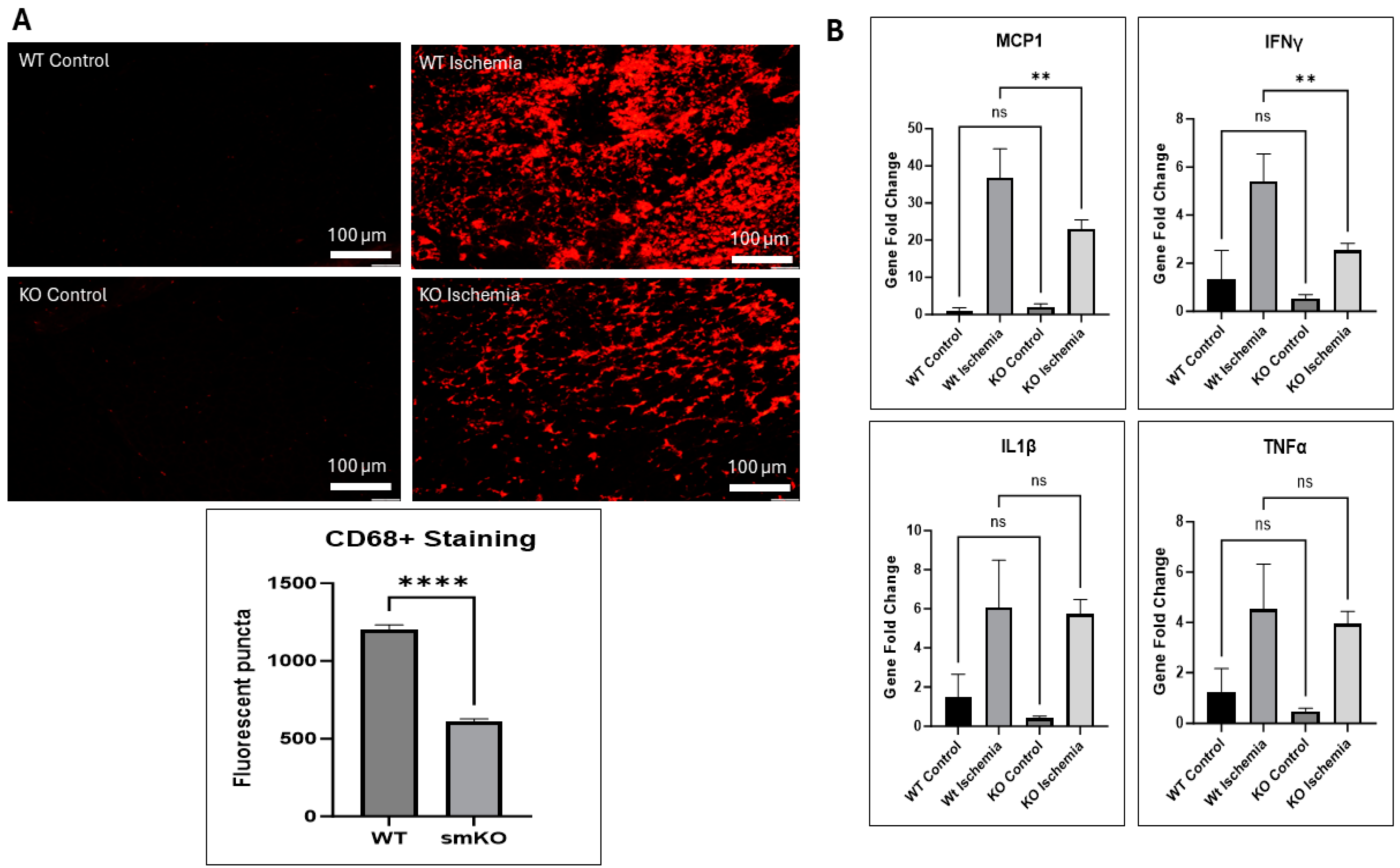
2.8. UCHL1 smKO Alters Inflammatory Markers
3. Discussion
4. Methods and Materials
4.1. Cell Culture
4.2. Animals
4.2.1. Non-Invasive Hindlimb Ischemia-Reperfusion Model
4.2.2. In Situ Muscle Contraction
4.3. Tissue Collection
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Tissue Immunostaining
4.6. Quantitative PCR (qPCR)
4.7. Western Blot
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Rose, A.J.; Richter, E.A. Skeletal muscle glucose uptake during exercise: How is it regulated? Physiol. (Bethesda) 2005, 20, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Hussain, S.N.; Barreiro, E.; Gouspillou, G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Péault, B.; Rudnicki, M.; Torrente, Y.; Cossu, G.; Tremblay, J.P.; Partridge, T.; Gussoni, E.; Kunkel, L.M.; Huard, J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007, 15, 867–877. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gillani, S.; Cao, J.; Suzuki, T.; Hak, D.J. The effect of ischemia reperfusion injury on skeletal muscle. Injury 2012, 43, 670–675. [Google Scholar] [CrossRef]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- D’Oria, M.; Mani, K.; Rodriguez Lorenzo, A. Microsurgical Salvage of Acute Lower Limb Ischemia after Iatrogenic Femoral Injury during Orthopedic Surgery in a Pediatric Patient. Ann. Vasc. Surg. 2020, 69, 452.e5–452.e11. [Google Scholar] [CrossRef]
- Magan, A.A.; Dunseath, O.; Armonis, P.; Fontalis, A.; Kayani, B.; Haddad, F.S. Tourniquet use in total knee arthroplasty and the risk of infection: A meta-analysis of randomised controlled trials. J. Exp. Orthop. 2022, 9, 62. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2293–2302. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; VanDenburgh, H.H.; Soslowsky, L.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Nichenko, A.S.; Southern, W.M.; Atuan, M.; Luan, J.; Peissig, K.B.; Foltz, S.J.; Beedle, A.M.; Warren, G.L.; Call, J.A. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am. J. Physiol. -Cell Physiol. 2016, 311, C190–C200. [Google Scholar] [CrossRef]
- Wilkinson, K.D.; Lee, K.M.; Deshpande, S.; Duerksen-Hughes, P.; Boss, J.M.; Pohl, J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 1989, 246, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Brackeva, B.; De Punt, V.; Kramer, G.; Costa, O.; Verhaeghen, K.; Stangé, G.; Sadones, J.; Xavier, C.; Aerts, J.; Gorus, F.; et al. Potential of UCHL1 as biomarker for destruction of pancreatic beta cells. J. Proteom. 2015, 117, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Vasu, V.T.; Ott, S.; Hobson, B.; Rashidi, V.; Oommen, S.; Cross, C.E.; Gohil, K. Sarcolipin and ubiquitin carboxy-terminal hydrolase 1 mRNAs are over-expressed in skeletal muscles of α-tocopherol deficient mice. Free. Radic. Res. 2009, 43, 106–116. [Google Scholar] [CrossRef]
- Powis, R.A.; Mutsaers, C.A.; Wishart, T.M.; Hunter, G.; Wirth, B.; Gillingwater, T.H. Increased levels of UCHL 1 are a compensatory response to disrupted ubiquitin homeostasis in spinal muscular atrophy and do not represent a viable therapeutic target. Neuropathol. Appl. Neurobiol. 2014, 40, 873–887. [Google Scholar] [CrossRef]
- Gao, H.; Antony, R.; Srinivasan, R.; Wu, P.; Wang, X.; Li, Y. UCHL1 regulates oxidative activity in skeletal muscle. PLoS ONE 2020, 15, e0241716. [Google Scholar] [CrossRef]
- Gao, H.; Freeling, J.; Wu, P.; Liang, A.P.; Wang, X.; Li, Y. UCHL1 regulates muscle fibers and mTORC1 activity in skeletal muscle. Life Sci. 2019, 233, 116699. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hartnett, S.; Li, Y. Ubiquitin C-Terminal Hydrolase L1 regulates myoblast proliferation and differentiation. Biochem. Biophys. Res. Commun. 2017, 492, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.S.; Hashmi, F.F.; Jones, J.E.; Albadawi, H.; McCormack, M.; Eberlin, K.; Entabi, F.; Atkins, M.D.; Conrad, M.F.; Austen, W.G., Jr.; et al. A novel model of acute murine hindlimb ischemia. Am. J. Physiol. -Heart Circ. Physiol. 2007, 292, H830–H837. [Google Scholar] [CrossRef] [PubMed]
- Soutar, M.P.M.; Kempthorne, L.; Miyakawa, S.; Annuario, E.; Melandri, D.; Harley, J.; O’Sullivan, G.A.; Wray, S.; Hancock, D.C.; Cookson, M.R.; et al. AKT signalling selectively regulates PINK1 mitophagy in SHSY5Y cells and human iPSC-derived neurons. Sci. Rep. 2018, 8, 8855. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2009, 28, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of sterile inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Day, I.N.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Briata, P.; Lin, W.-J.; Giovarelli, M.; Pasero, M.; Chou, C.-F.; Trabucchi, M.; Rosenfeld, M.G.; Chen, C.-Y.; Gherzi, R. PI3K/AKT signaling determines a dynamic switch between distinct KSRP functions favoring skeletal myogenesis. Cell Death Differ. 2012, 19, 478–487. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J. Biol. Chem. 2000, 275, 36750–36757. [Google Scholar] [CrossRef] [PubMed]
- Endo, T. Postnatal skeletal muscle myogenesis governed by signal transduction networks: MAPKs and PI3K–akt control multiple steps. Biochem. Biophys. Res. Commun. 2023, 682, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, S.; Giovarelli, M.; Perrotta, C.; Morisi, F.; Touvier, T.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Cervia, D.; Sandri, M.; et al. Autophagy controls neonatal myogenesis by regulating the GH-IGF1 system through a NFE2L2-and DDIT3-mediated mechanism. Autophagy 2019, 15, 58–77. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Singh, N.; Reyes-Ordonez, A.; Khanna, N.; Bao, Z.; Zhao, H.; Chen, J. ARHGEF3 regulates skeletal muscle regeneration and strength through autophagy. Cell Rep. 2021, 34, 108594. [Google Scholar] [CrossRef] [PubMed]
- Fortini, P.; Ferretti, C.; Iorio, E.; Cagnin, M.; Garribba, L.; Pietraforte, D.; Falchi, M.; Pascucci, B.; Baccarini, S.; Morani, F.; et al. The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. Cell Death Dis. 2016, 7, e2168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatzinikita, E.; Maridaki, M.; Palikaras, K.; Koutsilieris, M.; Philippou, A. The Role of Mitophagy in Skeletal Muscle Damage and Regeneration. Cells 2023, 12, 716. [Google Scholar] [CrossRef]
- Killackey, S.A.; Philpott, D.J.; Girardin, S.E. Mitophagy pathways in health and disease. J. Cell Biol. 2020, 219, e202004029. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation and skeletal muscle regeneration: Leave it to the macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation during skeletal muscle regeneration and tissue remodeling: Application to exercise-induced muscle damage management. Immunol. Cell Biol. 2016, 94, 140–145. [Google Scholar] [CrossRef]
- Kim, J.; Grotegut, C.A.; Wisler, J.W.; Mao, L.; Rosenberg, P.B.; Rockman, H.A.; Lefkowitz, R.J. The β-arrestin-biased β-adrenergic receptor blocker carvedilol enhances skeletal muscle contractility. Proc. Natl. Acad. Sci. USA 2020, 117, 12435–12443. [Google Scholar] [CrossRef]
- Washington University School of Medicine. Hematoxylin & Eosin (H & E) Stain Protocol. Available online: https://neuromuscular.wustl.edu/pathol/histol/HE.pdf (accessed on 26 December 2015).
- Aby, K.; Antony, R.; Li, Y. ProBDNF Upregulation in Murine Hind Limb Ischemia Reperfusion Injury: A Driver of Inflammation. Biology 2023, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Aby, K.; Antony, R.; Eichholz, M.; Srinivasan, R.; Li, Y. Enhanced pro-BDNF-p75NTR pathway activity in denervated skeletal muscle. Life Sci. 2021, 286, 120067. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antony, R.; Aby, K.; Montgomery, M.; Li, Y. Skeletal Muscle UCHL1 Negatively Regulates Muscle Development and Recovery after Muscle Injury. Int. J. Mol. Sci. 2024, 25, 7330. https://doi.org/10.3390/ijms25137330
Antony R, Aby K, Montgomery M, Li Y. Skeletal Muscle UCHL1 Negatively Regulates Muscle Development and Recovery after Muscle Injury. International Journal of Molecular Sciences. 2024; 25(13):7330. https://doi.org/10.3390/ijms25137330
Chicago/Turabian StyleAntony, Ryan, Katherine Aby, Morgan Montgomery, and Yifan Li. 2024. "Skeletal Muscle UCHL1 Negatively Regulates Muscle Development and Recovery after Muscle Injury" International Journal of Molecular Sciences 25, no. 13: 7330. https://doi.org/10.3390/ijms25137330
APA StyleAntony, R., Aby, K., Montgomery, M., & Li, Y. (2024). Skeletal Muscle UCHL1 Negatively Regulates Muscle Development and Recovery after Muscle Injury. International Journal of Molecular Sciences, 25(13), 7330. https://doi.org/10.3390/ijms25137330




