Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress
Abstract
1. Introduction
2. Result
2.1. MGST3 Affects α-Syn and UBL3 Interaction by Split Gaussia Luciferase Complementation Assay
2.2. Silencing or Overexpression of MGST3 Has Not Significantly Altered the Expression of α-Syn and UBL3
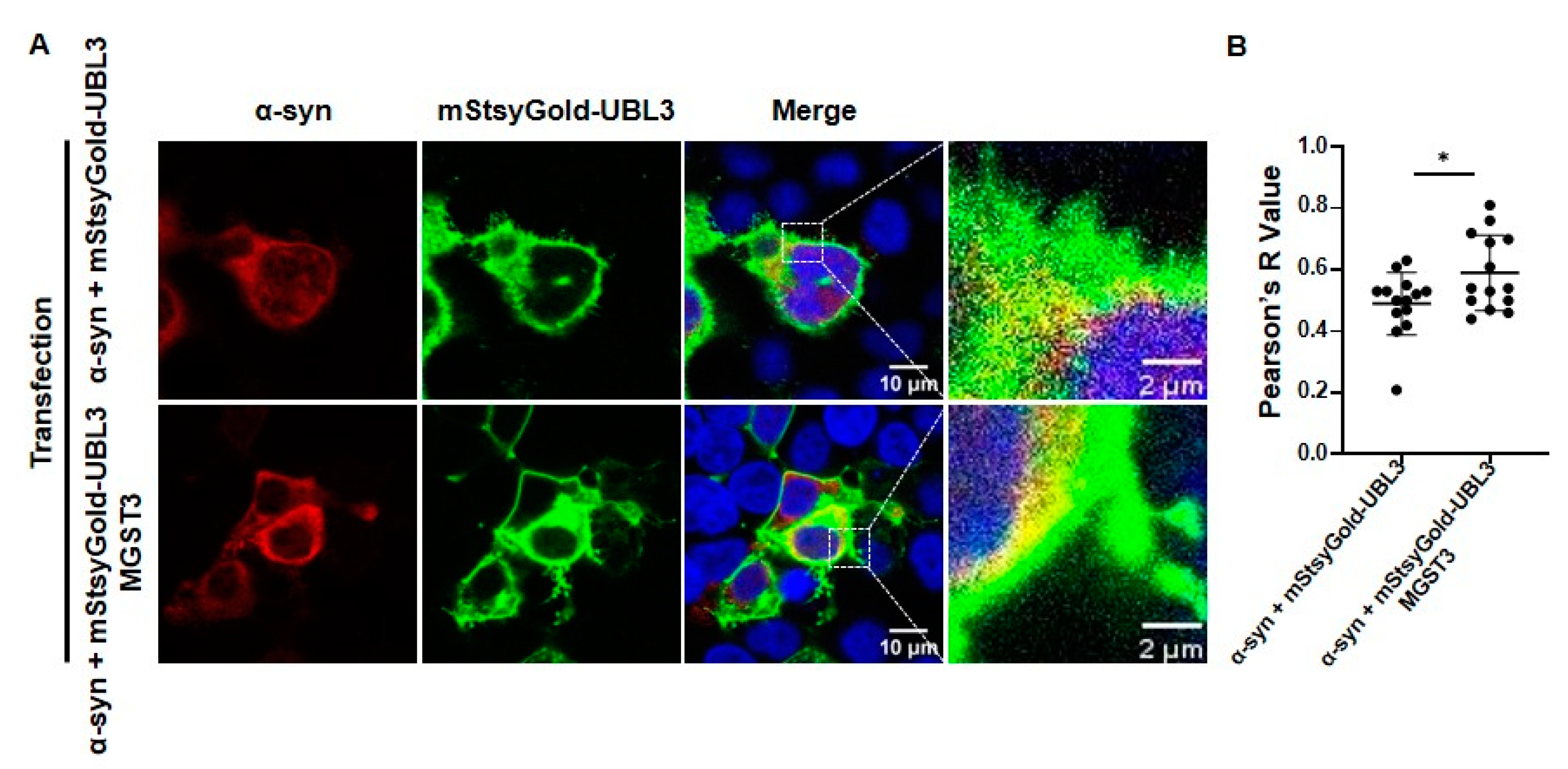
2.3. MGST3 Can Upregulate the Co-Localization of α-Syn and UBL3 in HEK293 Cells
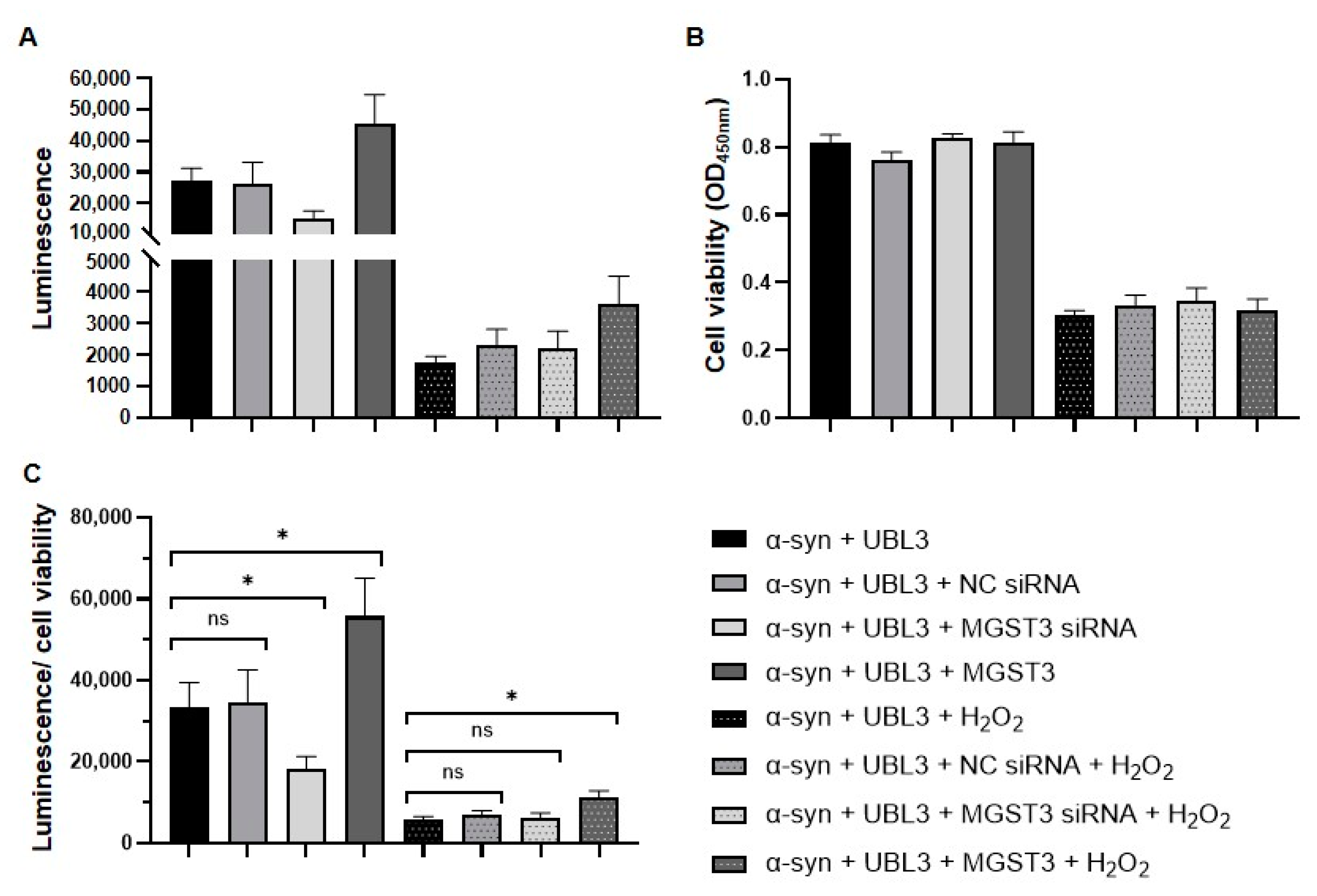
2.4. MGST3 Was Able to Attenuate the Effect of Oxidative Stress on the Interaction between α-Syn and UBL3
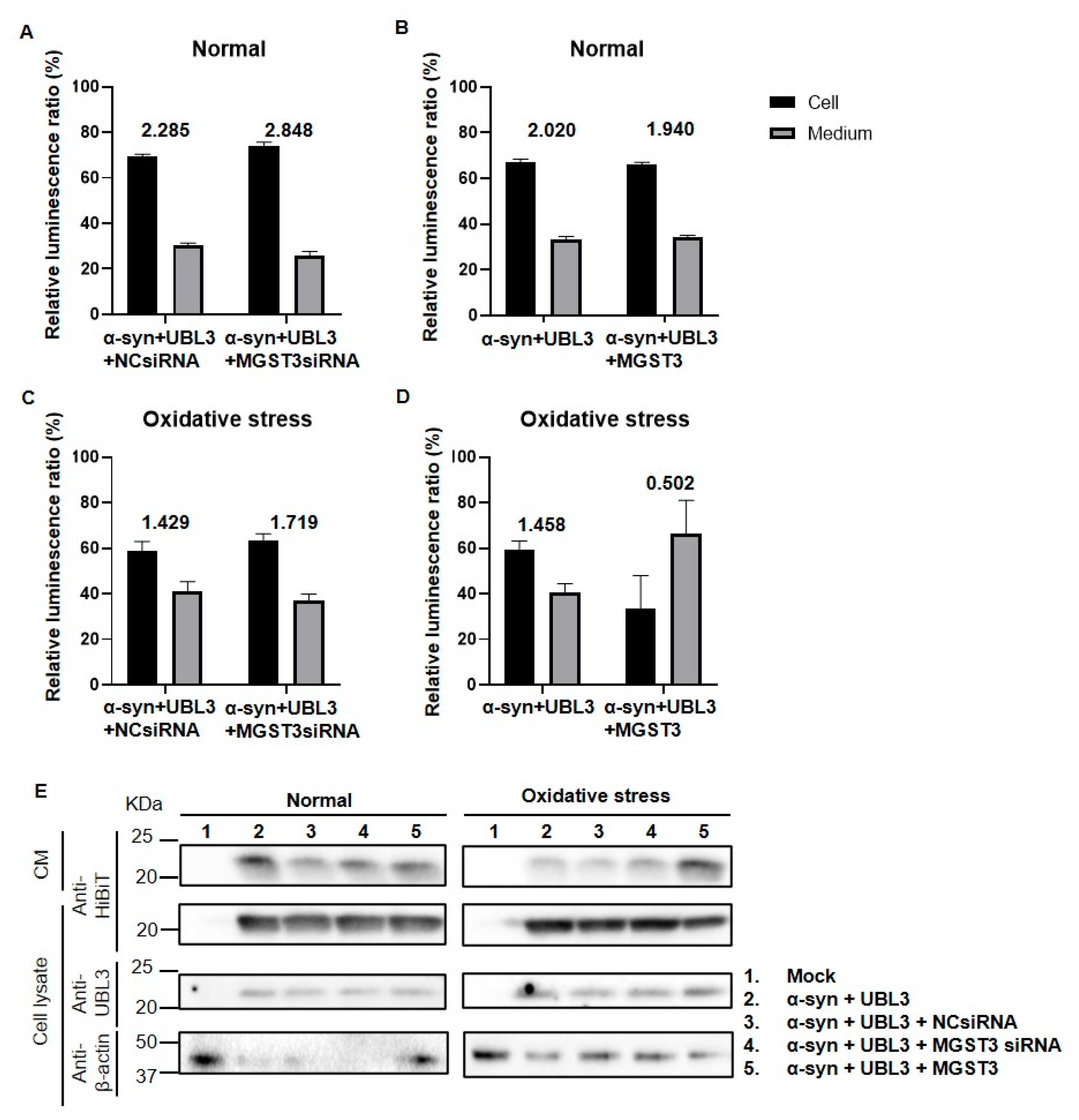
2.5. Overexpression of MGST3 Increases α-Syn Secretion into the Extracellular during Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Plasmid and siRNA
4.2. Cell Culture and Transfection
4.3. Luciferase Assay
4.4. Western Blotting (WB)
4.5. RT-qPCR
4.6. Immunocytochemistry
4.7. Oxidative Stress
4.8. HiBiT Bioluminescence Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goedert, M. Alpha-Synuclein and Neurodegenerative Diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons. Dis. 2017, 7, S53–S71. [Google Scholar] [CrossRef] [PubMed]
- Bieri, G.; Gitler, A.D.; Brahic, M. Internalization, Axonal Transport and Release of Fibrillar Forms of Alpha-Synuclein. Neurobiol. Dis. 2018, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Patel, S.; Lee, S.-J. Intravesicular Localization and Exocytosis of α-Synuclein and Its Aggregates. J. Neurosci. 2005, 25, 6016–6024. [Google Scholar] [CrossRef] [PubMed]
- Abounit, S.; Bousset, L.; Loria, F.; Zhu, S.; de Chaumont, F.; Pieri, L.; Olivo-Marin, J.; Melki, R.; Zurzolo, C. Tunneling Nanotubes Spread Fibrillar A-synuclein by Intercellular Trafficking of Lysosomes. EMBO J. 2016, 35, 2120–2138. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal Cell-to-Cell Transmission of Alpha Synuclein Oligomers. Mol. Neurodegener. 2012, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.T.; ALNasser, M.; Carter, W.G. Are Therapies That Target α-Synuclein Effective at Halting Parkinson’s Disease Progression? A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11022. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-Y.; Zhou, C.-J.; Zhou, Z.-R.; Hong, J.; Che, M.-X.; Fu, Q.-S.; Song, A.-X.; Lin, D.-H.; Hu, H.-Y. Interaction with Synphilin-1 Promotes Inclusion Formation of A-synuclein: Mechanistic Insights and Pathological Implication. FASEB J. 2010, 24, 196–205. [Google Scholar] [CrossRef]
- Schmid, A.W.; Fauvet, B.; Moniatte, M.; Lashuel, H.A. Alpha-Synuclein Post-Translational Modifications as Potential Biomarkers for Parkinson Disease and Other Synucleinopathies. Mol. Cell. Proteom. 2013, 12, 3543–3558. [Google Scholar] [CrossRef]
- Scudamore, O.; Ciossek, T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation in Vivo. J. Neuropathol. Exp. Neurol. 2018, 77, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Molecular Pathways of Neurodegeneration in Parkinson’s Disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef]
- Downes, B.P.; Saracco, S.A.; Lee, S.S.; Crowell, D.N.; Vierstra, R.D. MUBs, a Family of Ubiquitin-Fold Proteins That Are Plasma Membrane-Anchored by Prenylation. J. Biol. Chem. 2006, 281, 27145–27157. [Google Scholar] [CrossRef]
- Ageta, H.; Tsuchida, K. Post-Translational Modification and Protein Sorting to Small Extracellular Vesicles Including Exosomes by Ubiquitin and UBLs. Cell. Mol. Life Sci. 2019, 76, 4829–4848. [Google Scholar] [CrossRef]
- Takanashi, Y.; Kahyo, T.; Kamamoto, S.; Zhang, H.; Chen, B.; Ping, Y.; Mizuno, K.; Kawase, A.; Koizumi, K.; Satou, M.; et al. Ubiquitin-like 3 as a New Protein-Sorting Factor for Small Extracellular Vesicles. Cell Struct. Funct. 2022, 47, 21078. [Google Scholar] [CrossRef]
- Ageta, H.; Ageta-Ishihara, N.; Hitachi, K.; Karayel, O.; Onouchi, T.; Yamaguchi, H.; Kahyo, T.; Hatanaka, K.; Ikegami, K.; Yoshioka, Y.; et al. UBL3 Modification Influences Protein Sorting to Small Extracellular Vesicles. Nat. Commun. 2018, 9, 3936. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, 640. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hasan, M.M.; Zhang, H.; Zhai, Q.; Waliullah, A.S.M.; Ping, Y.; Zhang, C.; Oyama, S.; Mimi, M.A.; Tomochika, Y.; et al. UBL3 Interacts with Alpha-Synuclein in Cells and the Interaction Is Downregulated by the EGFR Pathway Inhibitor Osimertinib. Biomedicines 2023, 11, 1685. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, H.; Tomochika, Y.; Chen, B.; Ping, Y.; Islam, M.S.; Aramaki, S.; Sato, T.; Nagashima, Y.; Nakamura, T.; et al. UBL3 Interaction with α-Synuclein Is Downregulated by Silencing MGST3. Biomedicines 2023, 11, 2491. [Google Scholar] [CrossRef]
- Higgins, L.G.; Hayes, J.D. Mechanisms of Induction of Cytosolic and Microsomal Glutathione Transferase (GST) Genes by Xenobiotics and pro-Inflammatory Agents. Drug Metab. Rev. 2011, 43, 92–137. [Google Scholar] [CrossRef]
- Jakobsson, P.-J.; Morgenstern, R.; Mancini, J.; Ford-Hutchinson, A.; Persson, B. Membrane-Associated Proteins in Eicosanoid and Glutathione Metabolism (MAPEG). Am. J. Respir. Crit. Care Med. 2000, 161, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Schröder, O.; Sjöström, M.; Qiu, H.; Jakobsson, P.-J.; Haeggström, J.Z. Microsomal Glutathione S-Transferases: Selective up-Regulation of Leukotriene C4 Synthase during Lipopolysaccharide-Induced Pyresis. Cell. Mol. Life Sci. 2005, 62, 87–94. [Google Scholar] [CrossRef]
- Bresell, A.; Weinander, R.; Lundqvist, G.; Raza, H.; Shimoji, M.; Sun, T.; Balk, L.; Wiklund, R.; Eriksson, J.; Jansson, C.; et al. Bioinformatic and Enzymatic Characterization of the MAPEG Superfamily. FEBS J. 2005, 272, 1688–1703. [Google Scholar] [CrossRef]
- Fetissov, S.; Schröder, O.; Jakobsson, P.-J.; Samuelsson, B.; Haeggström, J.; Hökfelt, T. Expression of Microsomal Glutathione S-Transferase Type 3 MRNA in the Rat Nervous System. Neuroscience 2002, 115, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, D.G.; Williams, R.W.; Lu, L.; Stein, J.L.; Hibar, D.P.; Nichols, T.E.; Medland, S.E.; Thompson, P.M.; Hager, R. Joint Genetic Analysis of Hippocampal Size in Mouse and Human Identifies a Novel Gene Linked to Neurodegenerative Disease. BMC Genom. 2014, 15, 850. [Google Scholar] [CrossRef]
- Jakobsson, P.-J.; Mancini, J.A.; Riendeau, D.; Ford-Hutchinson, A.W. Identification and Characterization of a Novel Microsomal Enzyme with Glutathione-Dependent Transferase and Peroxidase Activities. J. Biol. Chem. 1997, 272, 22934–22939. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, S.; Deng, Y.; Du, X.; Yu, Z. Cloning of a Novel Glutathione S-Transferase 3 (GST3) Gene and Expressionanalysis in Pearl Oyster, Pinctada Martensii. Fish Shellfish Immunol. 2011, 31, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Cellular Glutathione and Thiols Metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Ziegler, D.M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu. Rev. Biochem. 1985, 54, 305–329. [Google Scholar] [CrossRef]
- Ketterer, B. The Role of Nonenzymatic Reactions of Glutathione in Xenobiotic Metabolism. Drug Metab. Rev. 1982, 13, 161–187. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Arrigo, A.-P. Gene Expression and Thiol Redox State. In Methods in Enzymology; Elsevier Science Inc.: New Yrok, NY, USA, 2002; Volume 348, pp. 200–215. [Google Scholar]
- Kim, H.; Ha, S.; Lee, H.Y.; Lee, K. ROSics: Chemistry and Proteomics of Cysteine Modifications in Redox Biology. Mass Spectrom. Rev. 2015, 34, 184–208. [Google Scholar] [CrossRef] [PubMed]
- González-Castillo, C.; Ortuño-Sahagún, D.; Guzmán-Brambila, C.; Márquez-Aguirre, A.L.; Raisman-Vozari, R.; Pallás, M.; Rojas-Mayorquín, A.E. The Absence of Pleiotrophin Modulates Gene Expression in the Hippocampus in Vivo and in Cerebellar Granule Cells in Vitro. Mol. Cell. Neurosci. 2016, 75, 113–121. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Dibra, D.; Xiong, S.; Moyer, S.M.; El-Naggar, A.K.; Qi, Y.; Su, X.; Kong, E.K.; Korkut, A.; Lozano, G. Mutant P53 Protects Triple-Negative Breast Adenocarcinomas from Ferroptosis in Vivo. Sci. Adv. 2024, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sin, O.; Nollen, E.A.A. Regulation of Protein Homeostasis in Neurodegenerative Diseases: The Role of Coding and Non-Coding Genes. Cell. Mol. Life Sci. 2015, 72, 4027–4047. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bidram, E.; Łos, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting Autophagy, Oxidative Stress, and ER Stress for Neurodegenerative Disease Treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef] [PubMed]
- Ayemele, A.G.; Tilahun, M.; Lingling, S.; Elsaadawy, S.A.; Guo, Z.; Zhao, G.; Xu, J.; Bu, D. Oxidative Stress in Dairy Cows: Insights into the Mechanistic Mode of Actions and Mitigating Strategies. Antioxidants 2021, 10, 1918. [Google Scholar] [CrossRef] [PubMed]
- Fussi, N.; Höllerhage, M.; Chakroun, T.; Nykänen, N.-P.; Rösler, T.W.; Koeglsperger, T.; Wurst, W.; Behrends, C.; Höglinger, G.U. Exosomal Secretion of α-Synuclein as Protective Mechanism after Upstream Blockage of Macroautophagy. Cell Death Dis. 2018, 9, 757. [Google Scholar] [CrossRef]
- Ando, R.; Shimozono, S.; Ago, H.; Takagi, M.; Sugiyama, M.; Kurokawa, H.; Hirano, M.; Niino, Y.; Ueno, G.; Ishidate, F.; et al. StayGold Variants for Molecular Fusion and Membrane-Targeting Applications. Nat. Methods 2024, 21, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Yao, I.; Iida, J.; Nishimura, W.; Hata, Y. Synaptic and Nuclear Localization of Brain-Enriched Guanylate Kinase-Associated Protein. J. Neurosci. 2002, 22, 5354–5364. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Kahyo, T.; Zhang, H.; Ping, Y.; Zhang, C.; Jiang, S.; Ji, Q.; Ferdous, R.; Islam, M.S.; Oyama, S.; et al. Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 7353. https://doi.org/10.3390/ijms25137353
Yan J, Kahyo T, Zhang H, Ping Y, Zhang C, Jiang S, Ji Q, Ferdous R, Islam MS, Oyama S, et al. Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress. International Journal of Molecular Sciences. 2024; 25(13):7353. https://doi.org/10.3390/ijms25137353
Chicago/Turabian StyleYan, Jing, Tomoaki Kahyo, Hengsen Zhang, Yashuang Ping, Chi Zhang, Shuyun Jiang, Qianqing Ji, Rafia Ferdous, Md. Shoriful Islam, Soho Oyama, and et al. 2024. "Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress" International Journal of Molecular Sciences 25, no. 13: 7353. https://doi.org/10.3390/ijms25137353
APA StyleYan, J., Kahyo, T., Zhang, H., Ping, Y., Zhang, C., Jiang, S., Ji, Q., Ferdous, R., Islam, M. S., Oyama, S., Aramaki, S., Sato, T., Mimi, M. A., Hasan, M. M., & Setou, M. (2024). Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress. International Journal of Molecular Sciences, 25(13), 7353. https://doi.org/10.3390/ijms25137353









