Lack of VEGFA/KDR Signaling in Conventional Renal Cell Carcinoma Explains the Low Efficacy of Target Therapy and Frequent Adverse Events
Abstract
1. Introduction
2. Results
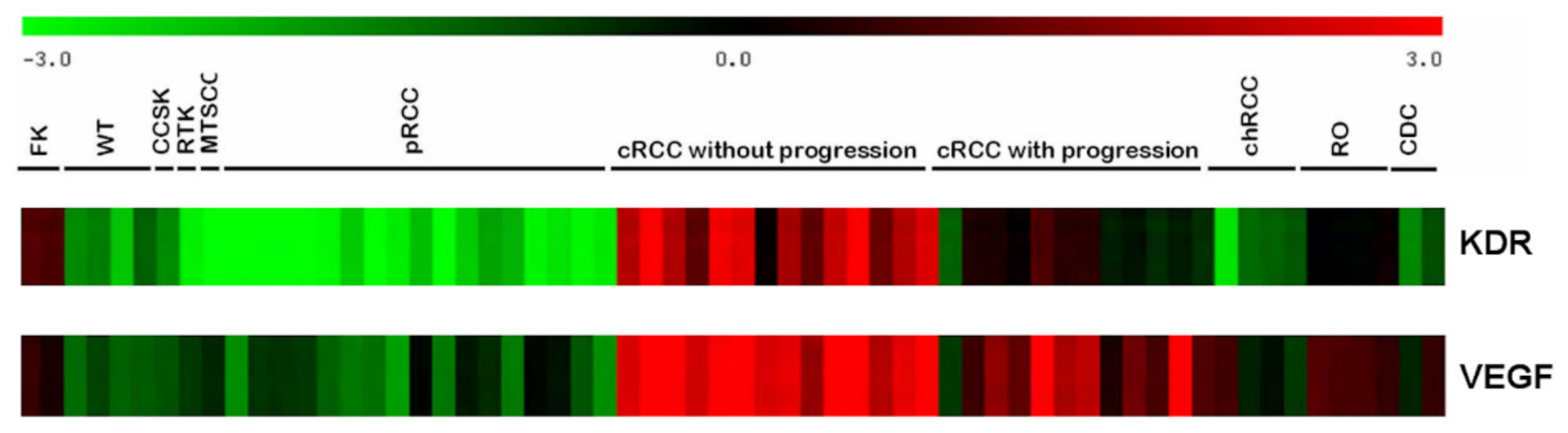
2.1. RNA Expression Profile
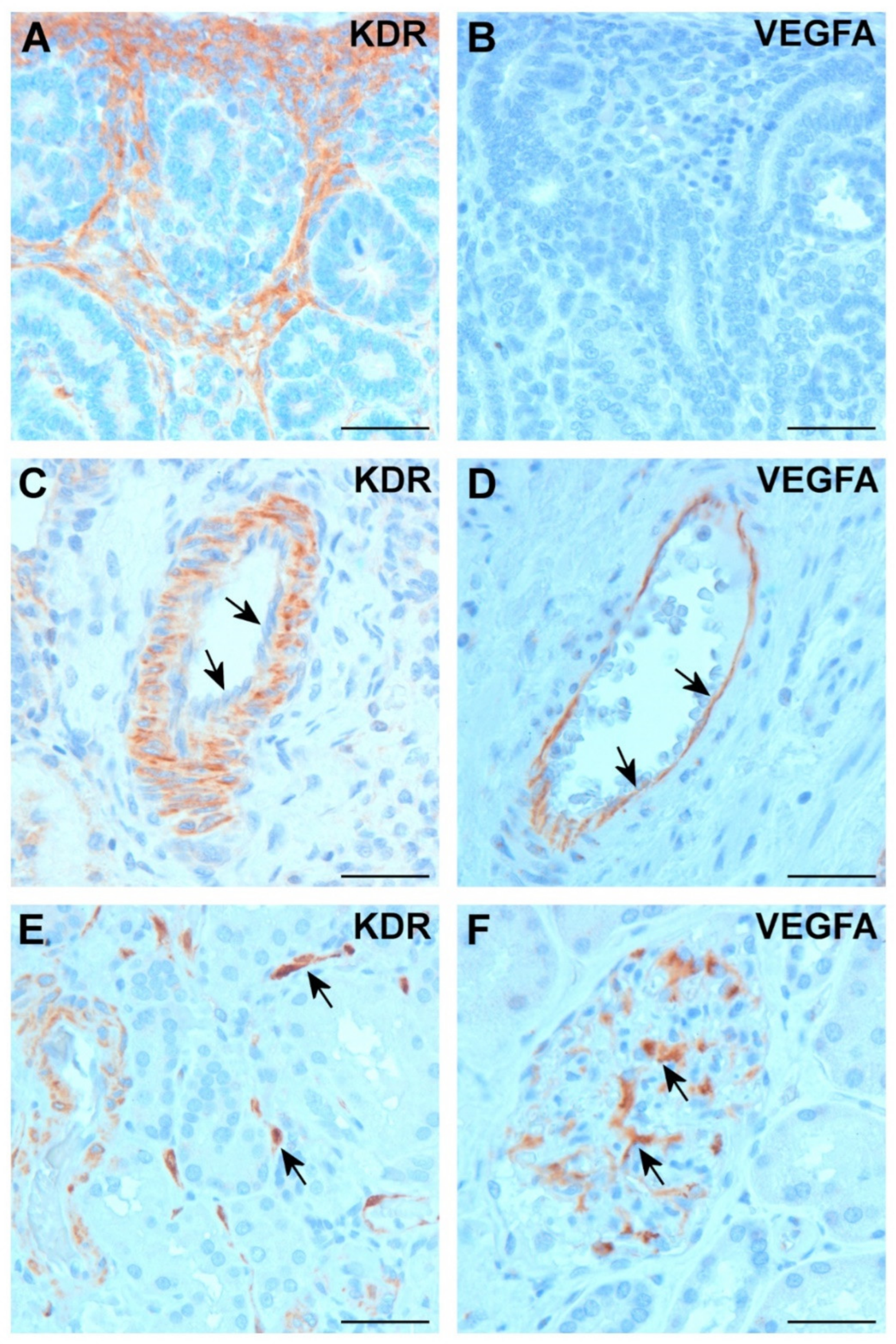
2.2. Expression of VEGFA and KDR in Normal Kidney
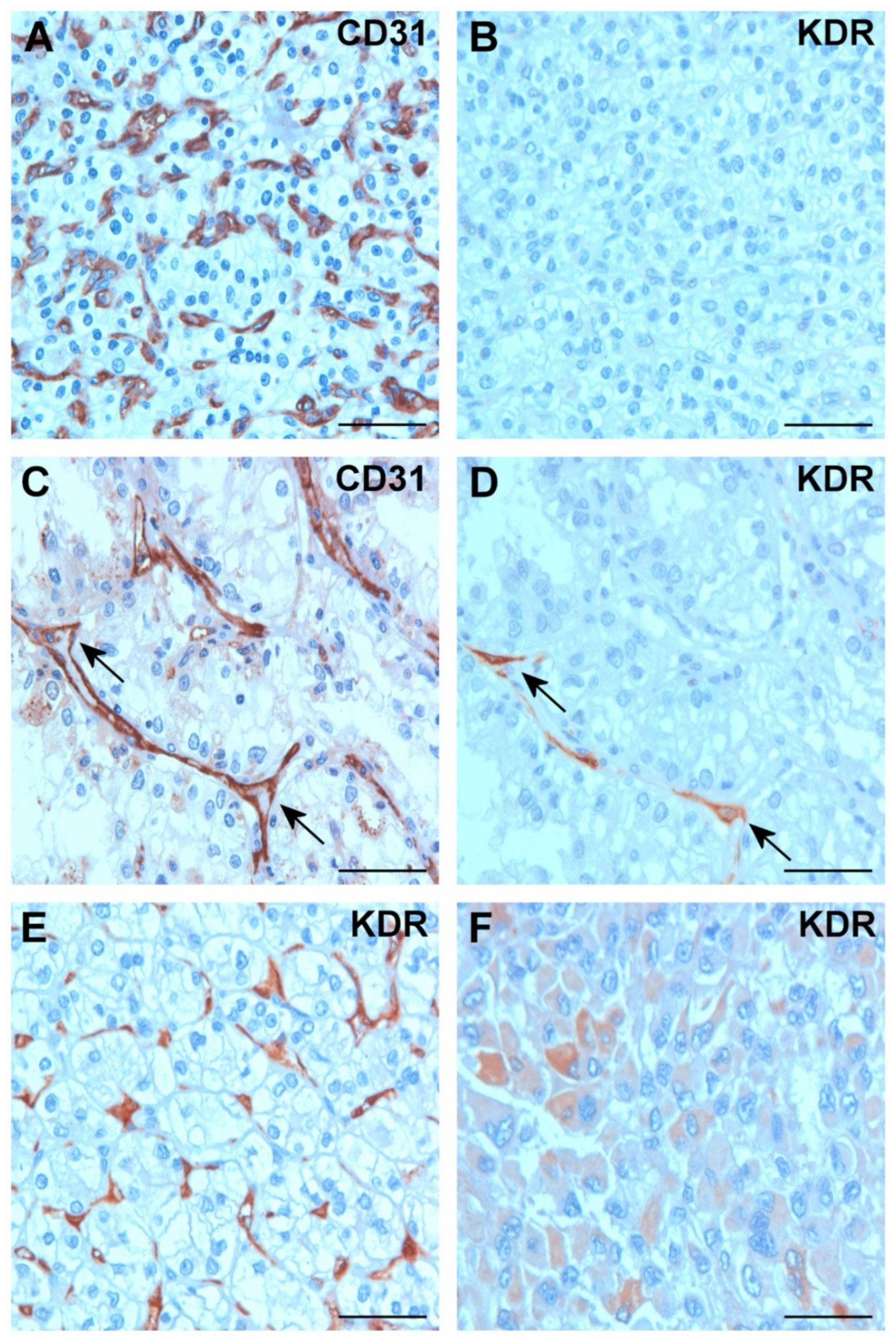
2.3. Expression of VEGFA and KDR in Tumor Tissues
2.4. Expression of KDR in Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Microarray-Based Gene Expression Analysis
4.2. Patients and Tissue Samples
4.3. Immunohistochemistry
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, M.H. Improving our resolution of kidney morphogenesis across time and space. Curr. Opin. Genet. Dev. 2015, 32, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. Tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin. Oncol. 2002, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.G.; Nanda, S.; Longo, S. Anti-angiogenic therapy in renal cell carcinoma. Recent Pat. Anticancer Drug Discov. 2010, 5, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Atkins, M.B. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009, 10, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Ravaud, A.; Patard, J.-J.; Pandha, H.S.; George, D.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: Subgroup analyses and updated overall survival results. Eur. Urol. 2018, 73, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin) in cancer treatment: A review of 15 years of clinical experience and outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Imai, A.; Krishnamurthy, S.; Zhang, Z.; Zeitlin, B.D.; Nör, J.E. Xenograft tumors vascularized with murine blood vessels may overestimate the effect of anti-tumor drugs: A pilot study. PLoS ONE 2013, 8, e84236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanz, L.; Cuesta, M.; Salas, C.; Corbacho, C.; Bellas, C.; Álvarez-Vallina, L. Differential transplantability of human endothelial cells in colorectal cancer and renal cell carcinoma primary xenografts. Lab. Investig. 2008, 89, 91–97. [Google Scholar] [CrossRef]
- Yuen, J.S.P.; Sim, M.Y.; Siml, H.G.; Chong, T.W.; O Lau, W.K.; Cheng, C.W.S.; Huynh, H. Inhibition of angiogenic and non-angiogenic targets by sorafenib in renal cell carcinoma (RCC) in a RCC xenograft model. Br. J. Cancer 2011, 104, 941–947. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B. Changing end points in breast cancer drug approval—The Avastin story. N. Eng. J. Med. 2011, 365, e2. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A. FDA Revokes Approval of Avastin for Use as Breast Cancer Drug; New York Times: New York, NY, USA, 2011. [Google Scholar]
- Quintanilha, J.C.F.; Wang, J.; Sibley, A.B.; Jiang, C.; Etheridge, A.S.; Shen, F. Bevacizumab-induced hypertension and proteinuria: A genome-wide study of more than 1000 patients. Br. J. Cancer 2020, 126, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, K.; Duyster, J. Anti-Angiogenis: Current situation and future perspectives. Oncol. Res. Treat. 2018, 41, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Garcia, J.A. Overcoming VEGF resistance in renal cancer: Biologic and therapeutic implications. Clin. Investig. 2012, 2, 615–621. [Google Scholar] [CrossRef]
- Wachhani, P.; George, S. VEGF inhibitors in renal cell carcinoma. Clin. Adv. Hematol. Oncol. 2016, 14, 1016–1028. [Google Scholar] [CrossRef]
- Mantia, C.M.; McDermott, D.F. Vascular endothelial growth factor and programmed death-1 pathway inhibitors in renal cell carcinoma. Cancer 2019, 125, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Gannon, A.; Figlin, R.A. Sunitinib: Ten years successful clinical use and a study in advanced renal cell carcinoma. Oncologist 2017, 22, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Rikala, M.S.; Rysz, J.; Lasota, J.; Wang, Z.F. Vascular endothelial growth factor receptor 2 (VEGFR2) as a marker for malignant vascular tumours and mesothelioma—Immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumours. Am. J. Surg. Pathol. 2012, 36, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Bellmunt, J.; Négrier, S.; Bajetta, E.; Melichar, B.; Bracarda, S.; Ravaud, A.; Golding, S.; Jethwa, S.; Sneller, V. Phase III trial of bevacizumab plus interferon alpha-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of survival. J. Clin. Oncol. 2010, 28, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Kamba, T.; McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 96, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.B.; Budolfsen, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M.; Magnusson, N.E. Drug-induced hypertension caused by multikinase inhibitors (Sorafenib, Sunitinib, Lenvatinib and Axitinib) in renal cell carcinoma treatment. Int. J. Mol. Sci. 2019, 20, 4712. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.R.; Rivard, A.; van der Zee, R.; Hariawala, M.; Sheriff, D.D.; Esakof, D.D.; Chaudhry, G.M.; Symes, J.F.; Isner, J.M. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Eremina, V.; Sood, M.; Haigh, J.; Nagy, A.; Lajoie, G.; Ferrara, N.; Gerber, H.-P.; Kikkawa, Y.; Miner, J.H.; Quaggin, S.E. Glomerular specific alteratios of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 2003, 111, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kamba, T.; Tam, B.Y.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C.; et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef] [PubMed]
- Paret, C.; Schön, Z.; Szponar, A.; Kovacs, G. Inflammatory protein serum amyloid A1 marks a subset of conventional renal cell carcinomas with fatal outcome. Eur. Urol. 2010, 57, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Akhtar, M.; Beckwith, B.J.; Bugert, P.; Cooper, C.S.; Delahunt, B.; Eble, J.N.; Fleming, S.; Ljungberg, B.; Medeiros, L.J.; et al. The Heidelberg classification of renal cell tumours. J. Pathol. 1997, 183, 131–133. [Google Scholar] [CrossRef]
- Domonkos, L.; Yjusenko, M.; Kovacs, G.; Peterfi, L. Impact of cellular morphology and three-tiered nuclear grade on progression of conventioonal renal cell carcinoma. J. Clin. Pathol. 2024, 77, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) The TNM Classification of Malignant Tumors, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterfi, L.; Yusenko, M.V.; Kovacs, G.; Beothe, T. Lack of VEGFA/KDR Signaling in Conventional Renal Cell Carcinoma Explains the Low Efficacy of Target Therapy and Frequent Adverse Events. Int. J. Mol. Sci. 2024, 25, 7359. https://doi.org/10.3390/ijms25137359
Peterfi L, Yusenko MV, Kovacs G, Beothe T. Lack of VEGFA/KDR Signaling in Conventional Renal Cell Carcinoma Explains the Low Efficacy of Target Therapy and Frequent Adverse Events. International Journal of Molecular Sciences. 2024; 25(13):7359. https://doi.org/10.3390/ijms25137359
Chicago/Turabian StylePeterfi, Lehel, Maria V. Yusenko, Gyula Kovacs, and Tamas Beothe. 2024. "Lack of VEGFA/KDR Signaling in Conventional Renal Cell Carcinoma Explains the Low Efficacy of Target Therapy and Frequent Adverse Events" International Journal of Molecular Sciences 25, no. 13: 7359. https://doi.org/10.3390/ijms25137359
APA StylePeterfi, L., Yusenko, M. V., Kovacs, G., & Beothe, T. (2024). Lack of VEGFA/KDR Signaling in Conventional Renal Cell Carcinoma Explains the Low Efficacy of Target Therapy and Frequent Adverse Events. International Journal of Molecular Sciences, 25(13), 7359. https://doi.org/10.3390/ijms25137359






