Intestinal Epithelial Co-Culture Sensitivity to Pro-Inflammatory Stimuli and Polyphenols Is Medium-Independent
Abstract
:1. Introduction
2. Results
2.1. Time-Dependent Effects of the Pro-Inflammatory Cytokine Cocktail Stimulation on the Caco-2/HT29-MTX Co-Culture Are Medium-Dependent
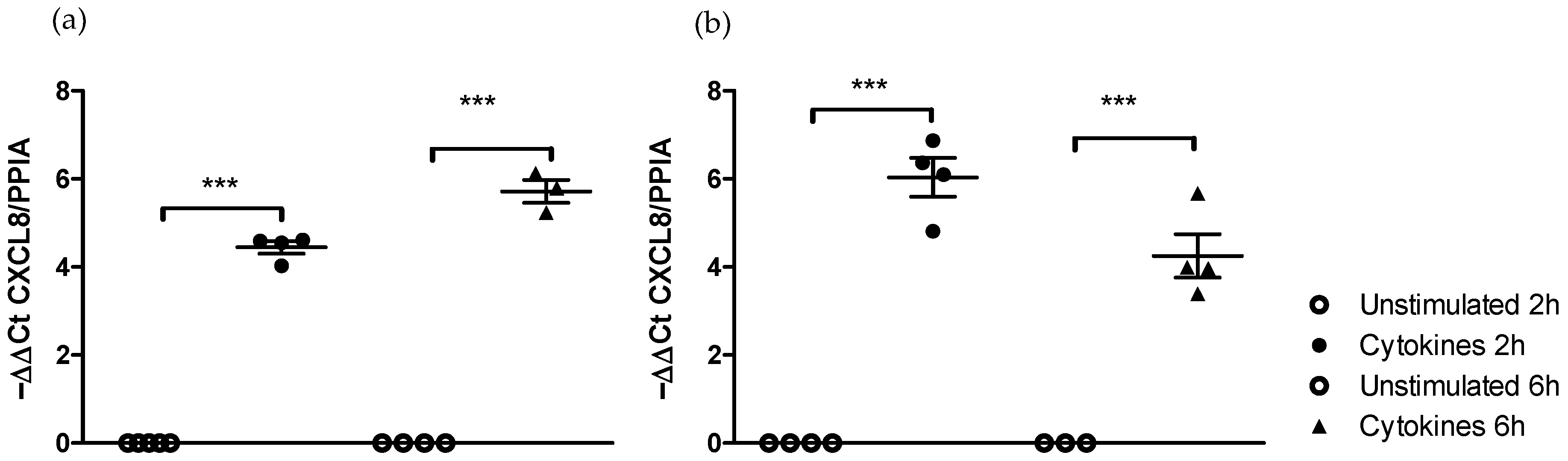
2.1.1. Effect of Medium on the Kinetics of Pro-Inflammatory Gene Stimulation
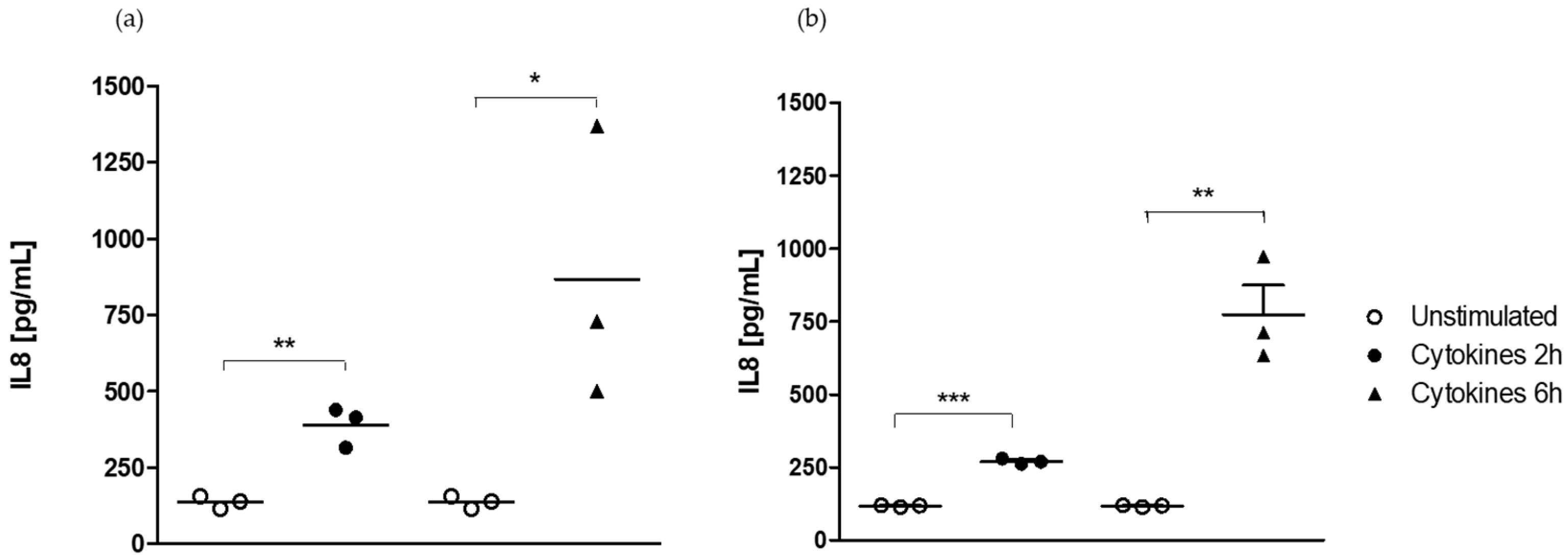
2.1.2. Effect of Medium on the Kinetics of IL-8 Secretion
2.2. Epithelial Cell Response Is Dependent on the Stimulus but Not on the Medium
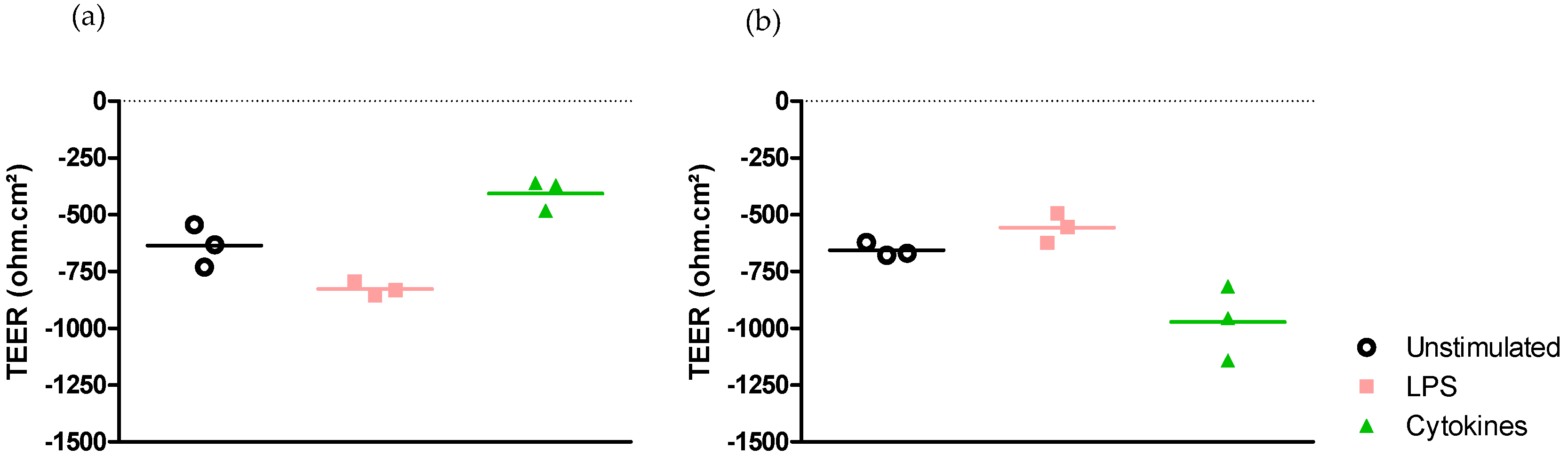
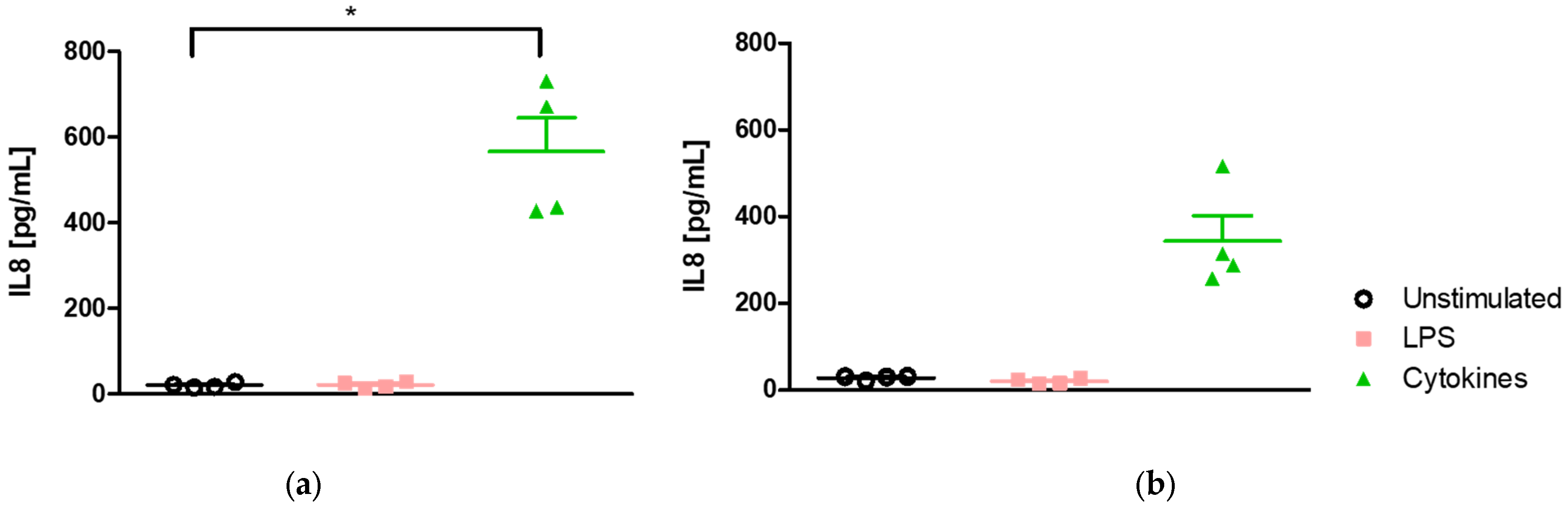
2.2.1. Medium-Independent Sensitivity of Epithelial Cell in Response to Pro-Inflammatory Stimuli
- Effect on epithelial permeability.
- Effect on CXCL8 and NF-κB pro-inflammatory gene expressions.
- Effect of medium on IL-8-induced secretion
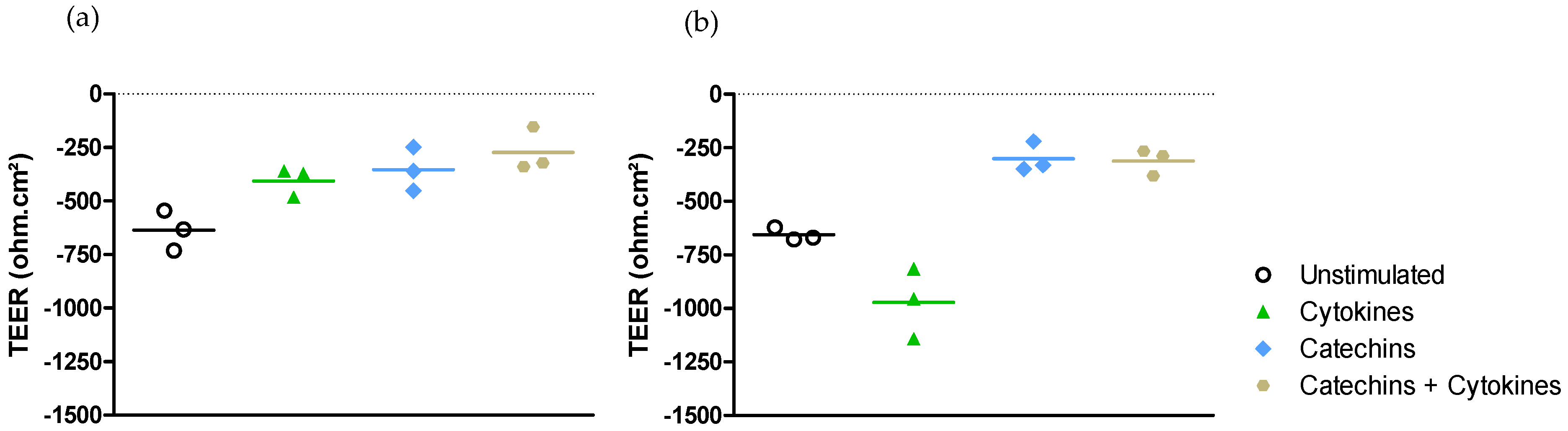
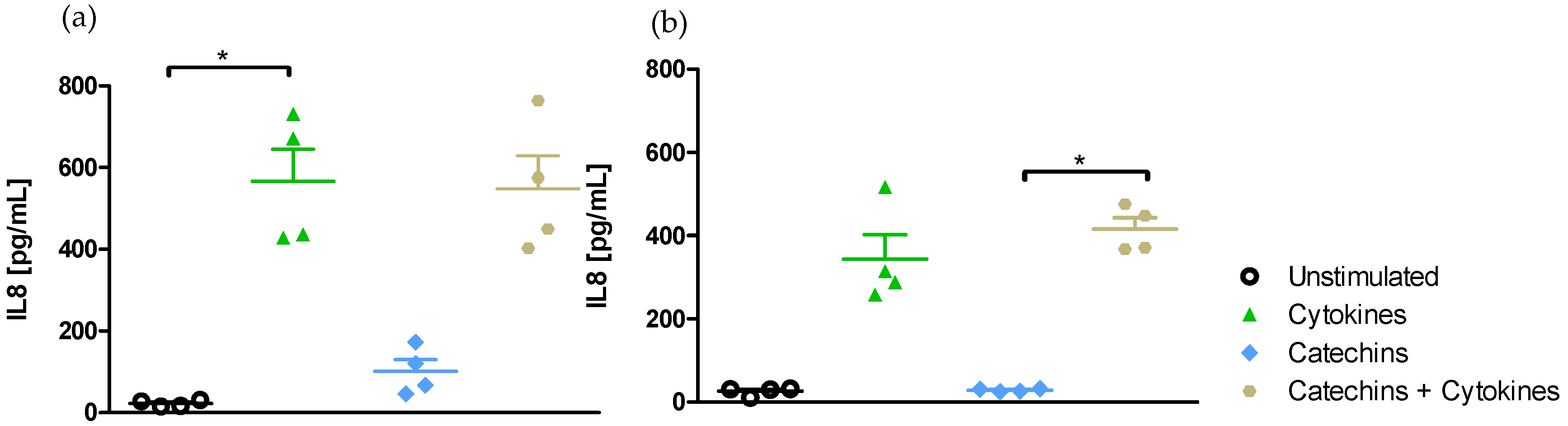
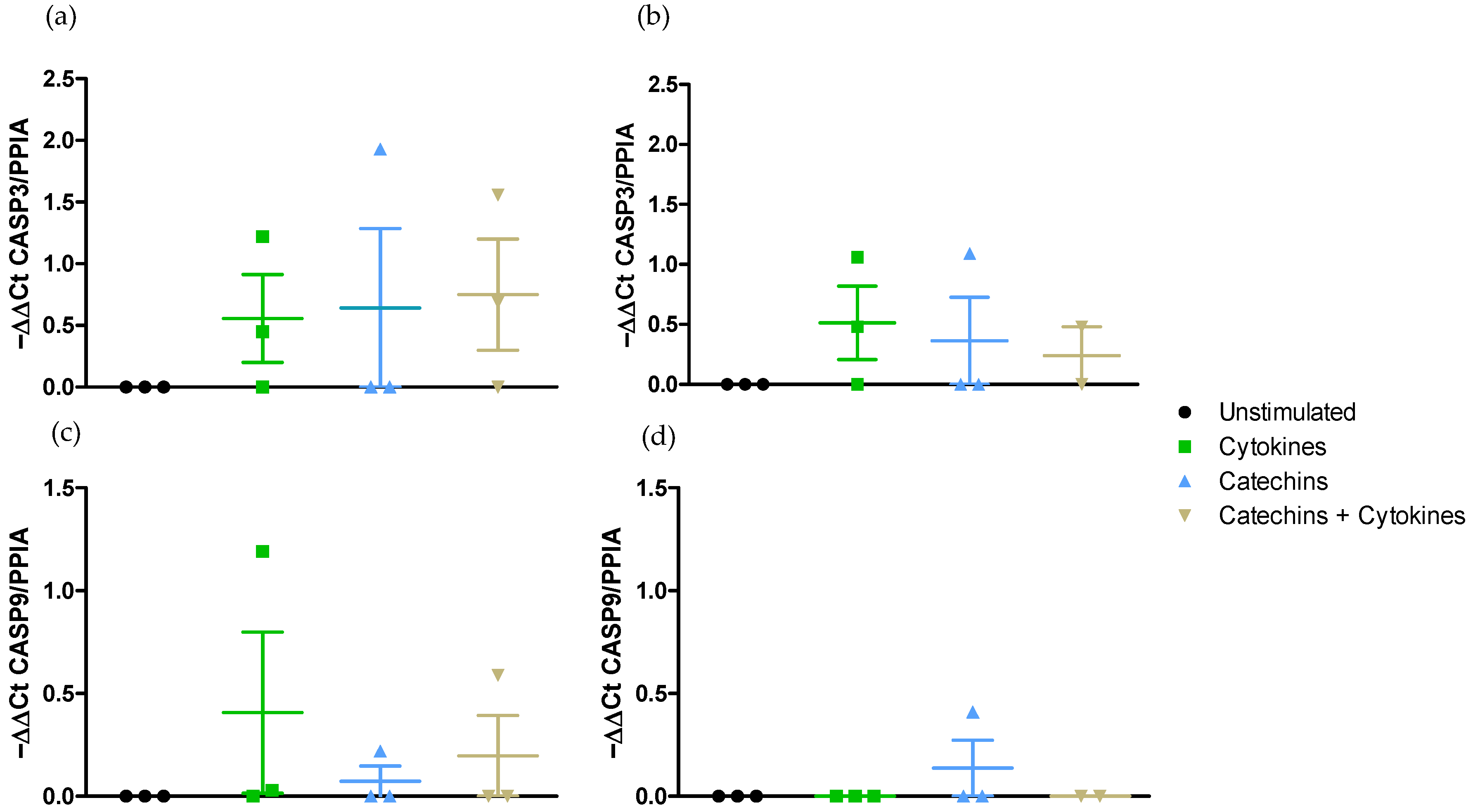
2.2.2. Medium-Independent Sensitivity of Epithelial Cells Exposed to Polyphenols before Their Stimulation by a Pro-Inflammatory Cytokine
- Effect on epithelial permeability.
- Effect on pro-inflammatory NF-κΒ and CXCL8 gene expressions.
- Effect of medium on IL-8-induced secretion
- Effect on pro-apoptotic pathway CASP3 and CASP9 gene expressions.
3. Discussion
4. Materials and Methods
4.1. Cell Culture
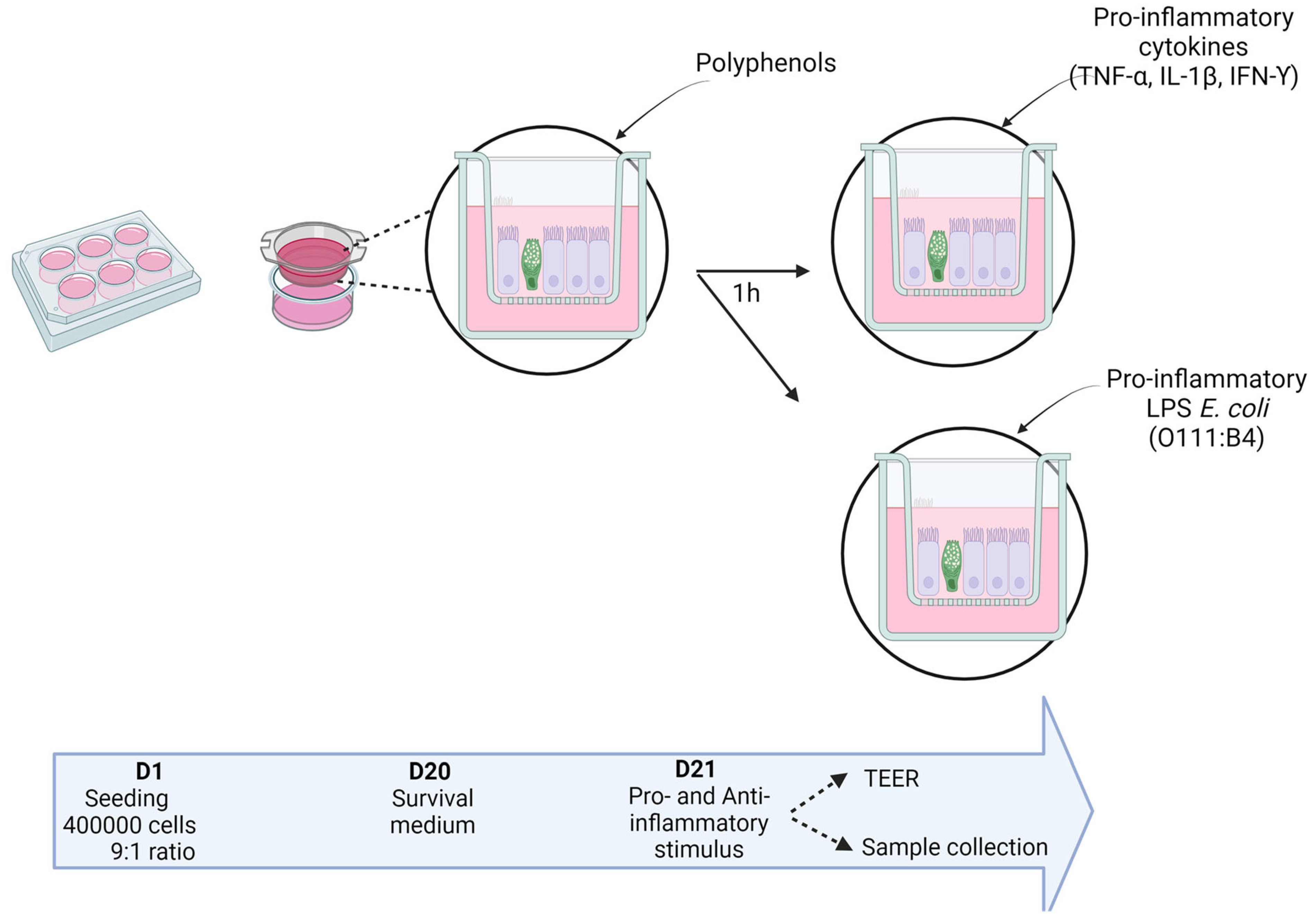
4.2. Co-Culture Stimulation
4.2.1. Time-Dependent Effect of the Growth Medium on the Co-Culture Response to the Pro-Inflammatory Cytokine Cocktail
4.2.2. Incidence of Medium on the Co-Culture Response to Pro- and Anti-Inflammatory Stimuli
4.3. Measurement of Membrane Permeability Alteration by TEER
4.4. Real-Time PCR for Gene Expression of Cell Mediators
4.5. Evaluation of Il-8 Secretion in Media by ELISA
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soderholm, A.T.; Pedicord, V.A. Intestinal Epithelial Cells: At the Interface of the Microbiota and Mucosal Immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; Le Maitre, C.L. Long-Term in Vitro {3D} Hydrogel Co-Culture Model of Inflammatory Bowel Disease. Sci. Rep. 2019, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, M.; Cendra, M.d.M.; Alonso-Roman, R.; Urdániz, M.; Torrents, E.; Martínez, E. Mimicking the Intestinal Host–Pathogen Interactions in a 3D In Vitro Model: The Role of the Mucus Layer. Pharmaceutics 2022, 14, 1552. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.W.; Graham, A.E.; Re, N.A.; Carr, I.M.; Robinson, J.I.; Mackie, S.L.; Morgan, A.W. Standardized Protocols for Differentiation of THP-1 Cells to Macrophages with Distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) Phenotypes. J. Immunol. Methods 2020, 478, 112721. [Google Scholar] [CrossRef]
- Haddad, M.J.; Sztupecki, W.; Delayre-Orthez, C.; Rhazi, L.; Barbezier, N.; Depeint, F.; Anton, P.M. Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach. Int. J. Mol. Sci. 2023, 24, 3595. [Google Scholar] [CrossRef]
- Guibourdenche, M.; Haug, J.; Chevalier, N.; Spatz, M.; Barbezier, N.; Gay-Quéheillard, J.; Anton, P.M. Food Contaminants Effects on an In Vitro Model of Human Intestinal Epithelium. Toxics 2021, 9, 135. [Google Scholar] [CrossRef]
- Wei, C.-X.; Wu, J.-H.; Huang, Y.-H.; Wang, X.-Z.; Li, J.-Y. Lactobacillus plantarum Improves LPS-Induced Caco2 Cell Line Intestinal Barrier Damage via Cyclic AMP-PKA Signaling. PLoS ONE 2022, 17, e0267831. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Boonyong, C.; Vardhanabhuti, N.; Jianmongkol, S. Natural Polyphenols Prevent Indomethacin-Induced and Diclofenac-Induced Caco-2 Cell Death by Reducing Endoplasmic Reticulum Stress Regardless of Their Direct Reactive Oxygen Species Scavenging Capacity. J. Pharm. Pharmacol. 2020, 72, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Kukita, T.; Ishikawa, E.; Nakashima, S.; Masuda, S.; Kanda, T.; Akiyama, H.; Teshima, R.; Nakamura, S. Apple Polyphenols Suppress Antigen Presentation of Ovalbumin by THP-1-Derived Dendritic Cells. Food Chem. 2013, 138, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Brighenti, F.; Del Rio, D.; Mena, P.; Bussolati, O. Catechin and Procyanidin B2 Modulate the Expression of Tight Junction Proteins but Do Not Protect from Inflammation-Induced Changes in Permeability in Human Intestinal Cell Monolayers. Nutrients 2019, 11, 2271. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in Vitro Co-Culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Calvello, R.; Aresta, A.; Trapani, A.; Zambonin, C.; Cianciulli, A.; Salvatore, R.; Clodoveo, M.L.; Corbo, F.; Franchini, C.; Panaro, M.A. Bovine and Soybean Milk Bioactive Compounds: Effects on Inflammatory Response of Human Intestinal Caco-2 Cells. Food Chem. 2016, 210, 276–285. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Lee, T.-C.; Yang, H.-Y.; Chen, C.-Y.; Au, Y.-C.; Lu, Y.-Z.; Wu, L.-L.; Wei, S.-C.; Ni, Y.-H.; Lin, B.-R.; et al. LPS Receptor Subunits Have Antagonistic Roles in Epithelial Apoptosis and Colonic Carcinogenesis. Cell Death Differ. 2015, 22, 1590–1604. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Laboisse, C.; Darmaun, D. Glutamine Preserves Protein Synthesis and Paracellular Permeability in Caco-2 Cells Submitted to “luminal Fasting”. Am. J. Physiol. Liver Physiol. 2003, 285, G128–G136. [Google Scholar] [CrossRef]
- Zeller, P.; Bricks, T.; Vidal, G.; Jacques, S.; Anton, P.M.; Leclerc, E. Multiparametric Temporal Analysis of the Caco-2/TC7 Demonstrated Functional and Differentiated Monolayers as Early as 14 Days of Culture. Eur. J. Pharm. Sci. 2015, 72, 1–11. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-Induced Macrophage Activation and Signal Transduction in the Absence of Src-Family Kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.-Y. Lipopolysaccharides Modulate Intestinal Epithelial Permeability and Inflammation in a Species-Specific Manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Beamer, M.A.; Zamora, C.; Nestor-Kalinoski, A.L.; Fernando, V.; Sharma, V.; Furuta, S. Novel 3D Flipwell System That Models Gut Mucosal Microenvironment for Studying Interactions between Gut Microbiota, Epithelia and Immunity. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Kassab, G.S. Glutathione: A Samsonian Life-Sustaining Small Molecule That Protects against Oxidative Stress, Ageing and Damaging Inflammation. Front. Nutr. 2022, 9, 1007816. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Vallee, S.; Laforest, S.; Fouchier, F.; Montero, M.P.; Penel, C.; Champion, S. Cytokine-Induced Upregulation of NF-ΚB, IL-8, and ICAM-1 Is Dependent on Colonic Cell Polarity: Implication for PKCδ. Exp. Cell Res. 2004, 297, 165–185. [Google Scholar] [CrossRef]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J. Barrier Effects of Nutritional Factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Planchon, S.; Renaut, J.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Proteomic Response of Inflammatory Stimulated Intestinal Epithelial Cells to in Vitro Digested Plums and Cabbages Rich in Carotenoids and Polyphenols. Food Funct. 2016, 7, 4388–4399. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The Role of Dietary Polyphenols in the Management of Inflammatory Bowel Disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, Anthocyanins, and Inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Rodríguez, H.; Acebrón, I.; Mancheño, J.M.; Rivas, B.D.L.; Muñoz, R. Production and Physicochemical Properties of Recombinant Lactobacillus plantarum Tannase. J. Agric. Food Chem. 2009, 57, 6224–6230. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M. Procyanidin B2 and a Cocoa Polyphenolic Extract Inhibit Acrylamide-Induced Apoptosis in Human Caco-2 Cells by Preventing Oxidative Stress and Activation of JNK Pathway. J. Nutr. Biochem. 2011, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, W.; Cao, Q.; Guo, Q.; Han, J.; Qin, Y. (Epi)Catechin Damage Effects on the Development of Mouse Intestinal Epithelial Structure through the PERK-EIF2α-ATF4-CHOP Pathway. Food Funct. 2023, 14, 6665–6677. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Song, D.; Cheng, L.; Zhang, X. Interactions of Tea Catechins with Intestinal Microbiota and Their Implication for Human Health. Food Sci. Biotechnol. 2019, 28, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In Vivo Comparison of the Bioavailability of (+)-Catechin, (−)-Epicatechin and Their Mixture in Orally Administered Rats. J. Nutr. 2001, 131, 2885–2891. [Google Scholar] [CrossRef]
- Henning, S.M.; Choo, J.J.; Heber, D. Nongallated Compared with Gallated Flavan-3-Ols in Green and Black Tea Are More Bioavailable. J. Nutr. 2008, 138, 1529S–1534S. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced Uptake and Transport of (+)-Catechin and (-)-Epigallocatechin Gallate in Niosomal Formulation by Human Intestinal Caco-2 Cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Beroske, L.; Wyngaert, T.V.D.; Stroobants, S.; Van der Veken, P.; Elvas, F. Molecular Imaging of Apoptosis: The Case of Caspase-3 Radiotracers. Int. J. Mol. Sci. 2021, 22, 3948. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kunisawa, J.; Kiyono, H. Dietary and Microbial Metabolites in the Regulation of Host Immunity. Front. Microbiol. 2017, 8, 2171. [Google Scholar] [CrossRef] [PubMed]


| Function | Gene | Name | Sequences 5′-> 3′ or Reference | Su |
|---|---|---|---|---|
| Housekeeping Gene | PPIA | Peptidylprolyl Isomerase A | CCTATCCTAGAGGTGGCGGA TCATCGCAGAAGGAACCAGAC | Eurofins |
| Inflammatory Genes | CXCL8 | Interleukin 8 | AGAGTGATTGAGAGTGGACC ACTTCTCCACAACCCTCTG | Eurofins |
| NF-κB | Nuclear Factor κB p65 subunit | GGGGGCATCAAACCTGAAGA GGAGAGAAGTCCCCAAAGGC | Eurofins | |
| Apoptosis Genes | CASP3 | Caspase 3 | QT00029162 | QIAGEN |
| CASP9 | Caspase 9 | QT00036267 | QIAGEN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, M.J.; Zuluaga-Arango, J.; Mathieu, H.; Barbezier, N.; Anton, P.M. Intestinal Epithelial Co-Culture Sensitivity to Pro-Inflammatory Stimuli and Polyphenols Is Medium-Independent. Int. J. Mol. Sci. 2024, 25, 7360. https://doi.org/10.3390/ijms25137360
Haddad MJ, Zuluaga-Arango J, Mathieu H, Barbezier N, Anton PM. Intestinal Epithelial Co-Culture Sensitivity to Pro-Inflammatory Stimuli and Polyphenols Is Medium-Independent. International Journal of Molecular Sciences. 2024; 25(13):7360. https://doi.org/10.3390/ijms25137360
Chicago/Turabian StyleHaddad, Michelle J., Juanita Zuluaga-Arango, Hugo Mathieu, Nicolas Barbezier, and Pauline M. Anton. 2024. "Intestinal Epithelial Co-Culture Sensitivity to Pro-Inflammatory Stimuli and Polyphenols Is Medium-Independent" International Journal of Molecular Sciences 25, no. 13: 7360. https://doi.org/10.3390/ijms25137360
APA StyleHaddad, M. J., Zuluaga-Arango, J., Mathieu, H., Barbezier, N., & Anton, P. M. (2024). Intestinal Epithelial Co-Culture Sensitivity to Pro-Inflammatory Stimuli and Polyphenols Is Medium-Independent. International Journal of Molecular Sciences, 25(13), 7360. https://doi.org/10.3390/ijms25137360







