Cyclo(l-Pro-l-Tyr) Isolated from the Human Skin Commensal Corynebacterium tuberculostearicum Inhibits Tyrosinase
Abstract
:1. Introduction
2. Results
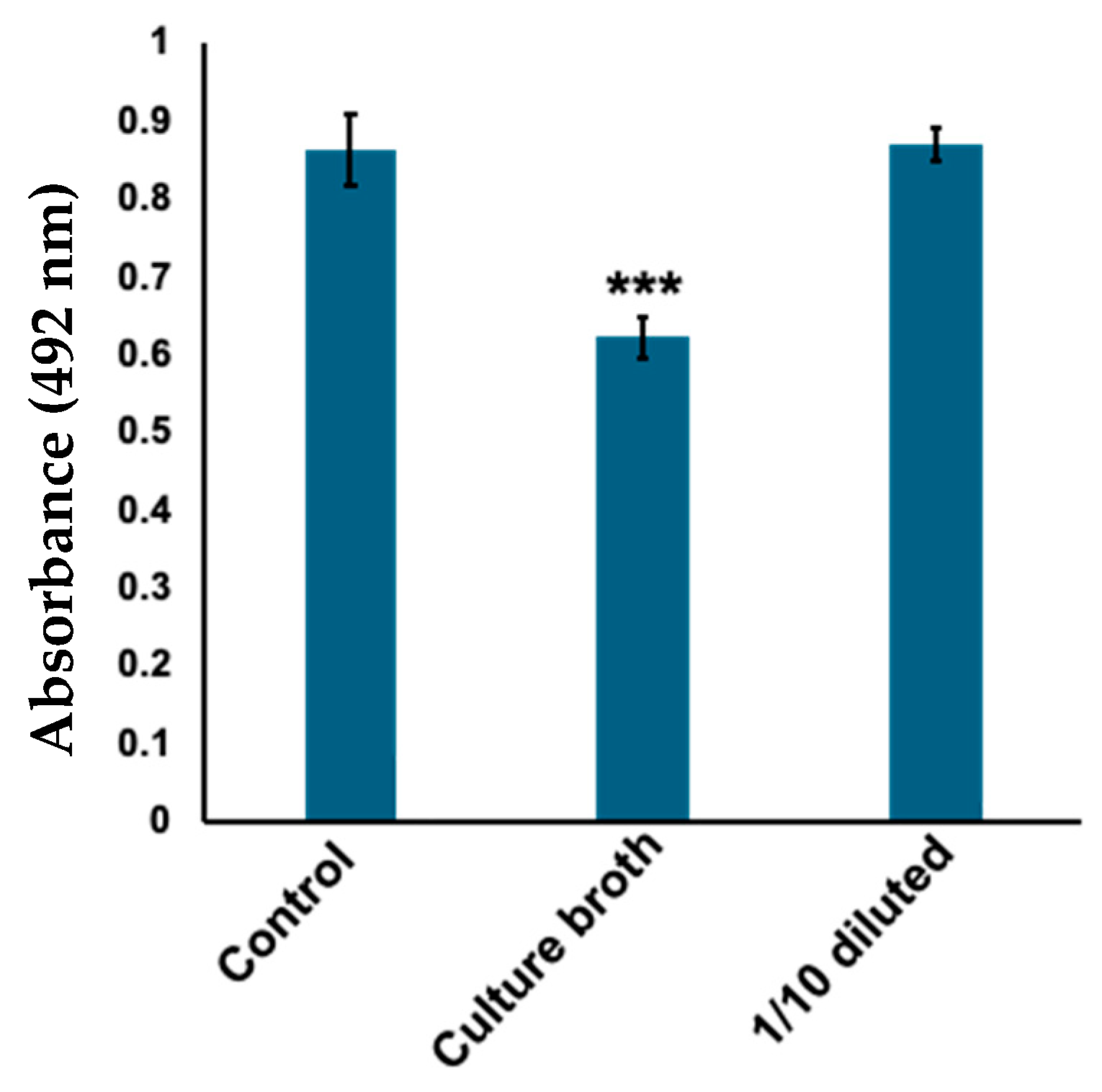
2.1. Corynebacterium tuberculostearicum Culture Broth Inhibited Mushroom Tyrosinase
2.2. Ethyl Acetate Extract Exhibited Tyrosinase Inhibitory Activity
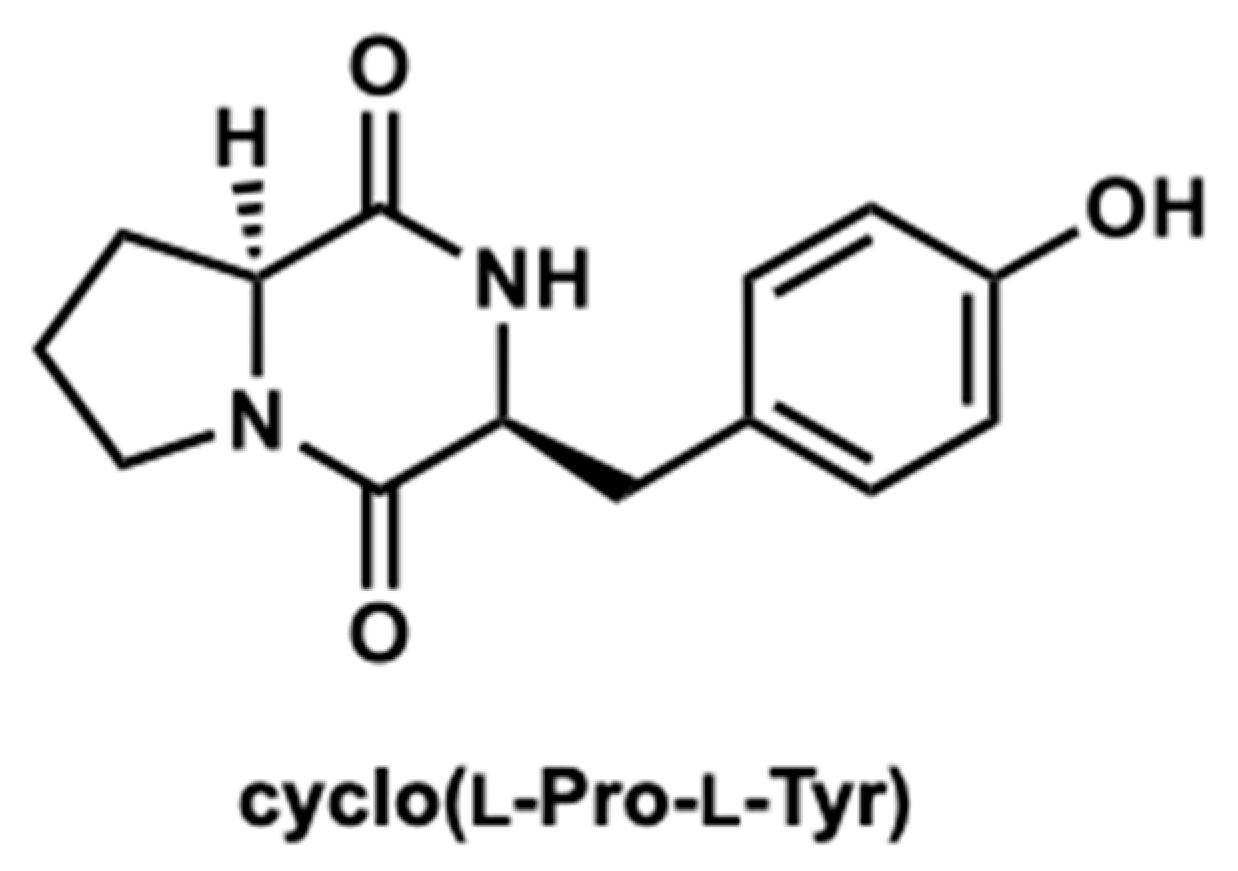
2.3. Purification of the Active Compound from C. tuberculostearicum Culture Broth
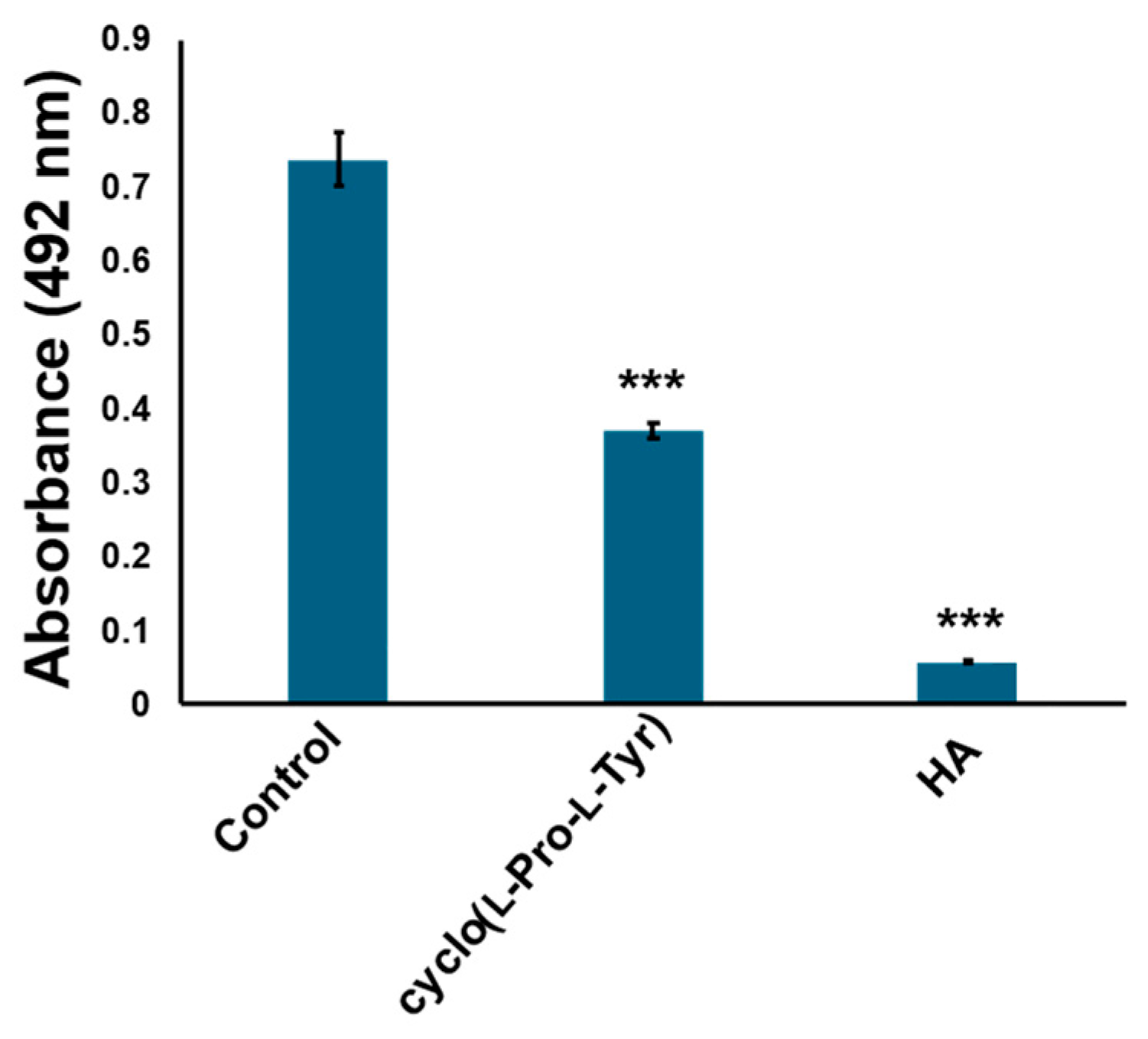
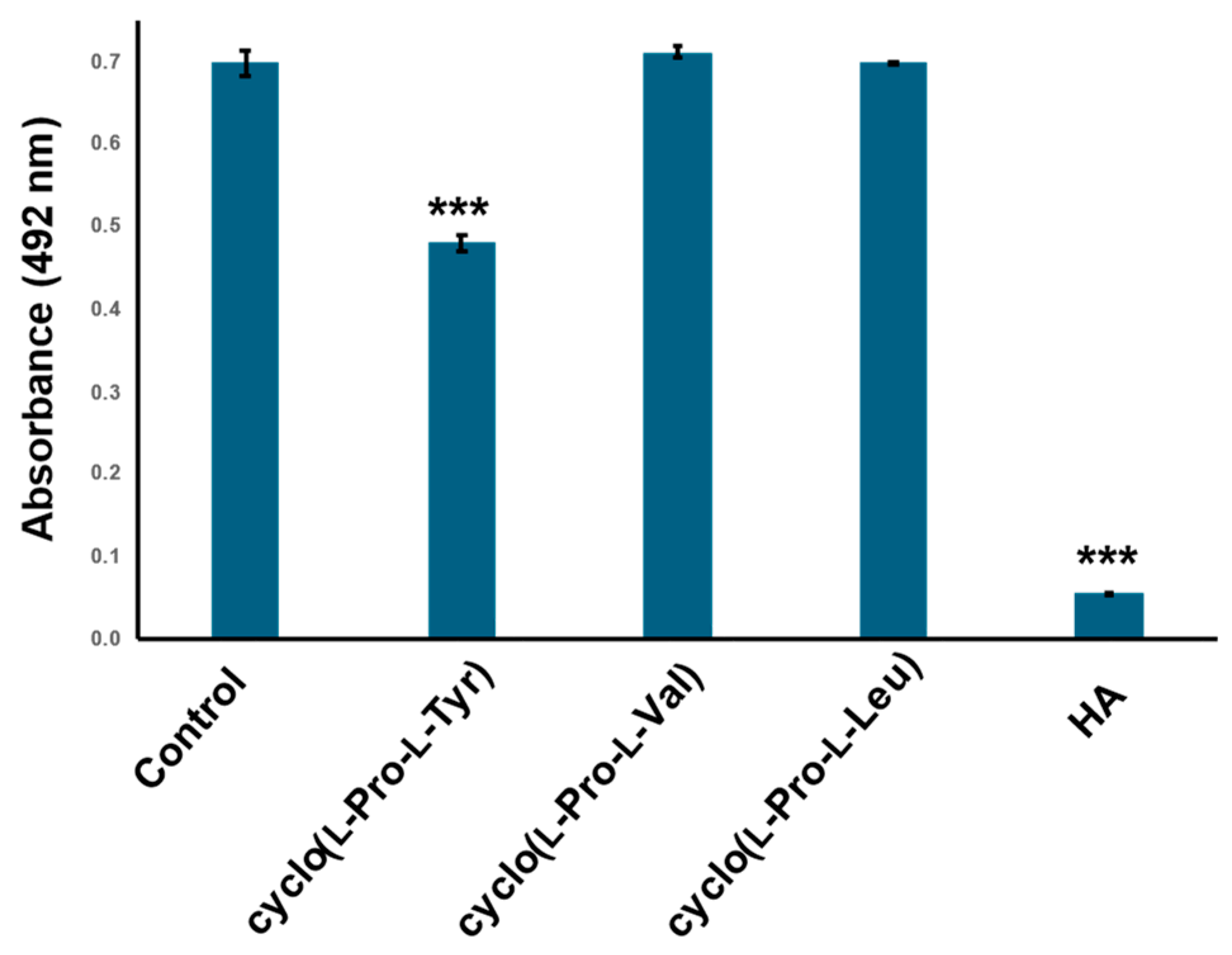
2.4. Cyclo(l-Pro-l-Val) and Cyclo(l-Pro-l-Leu) Dose Not Inhibit Tyrosinase Activity
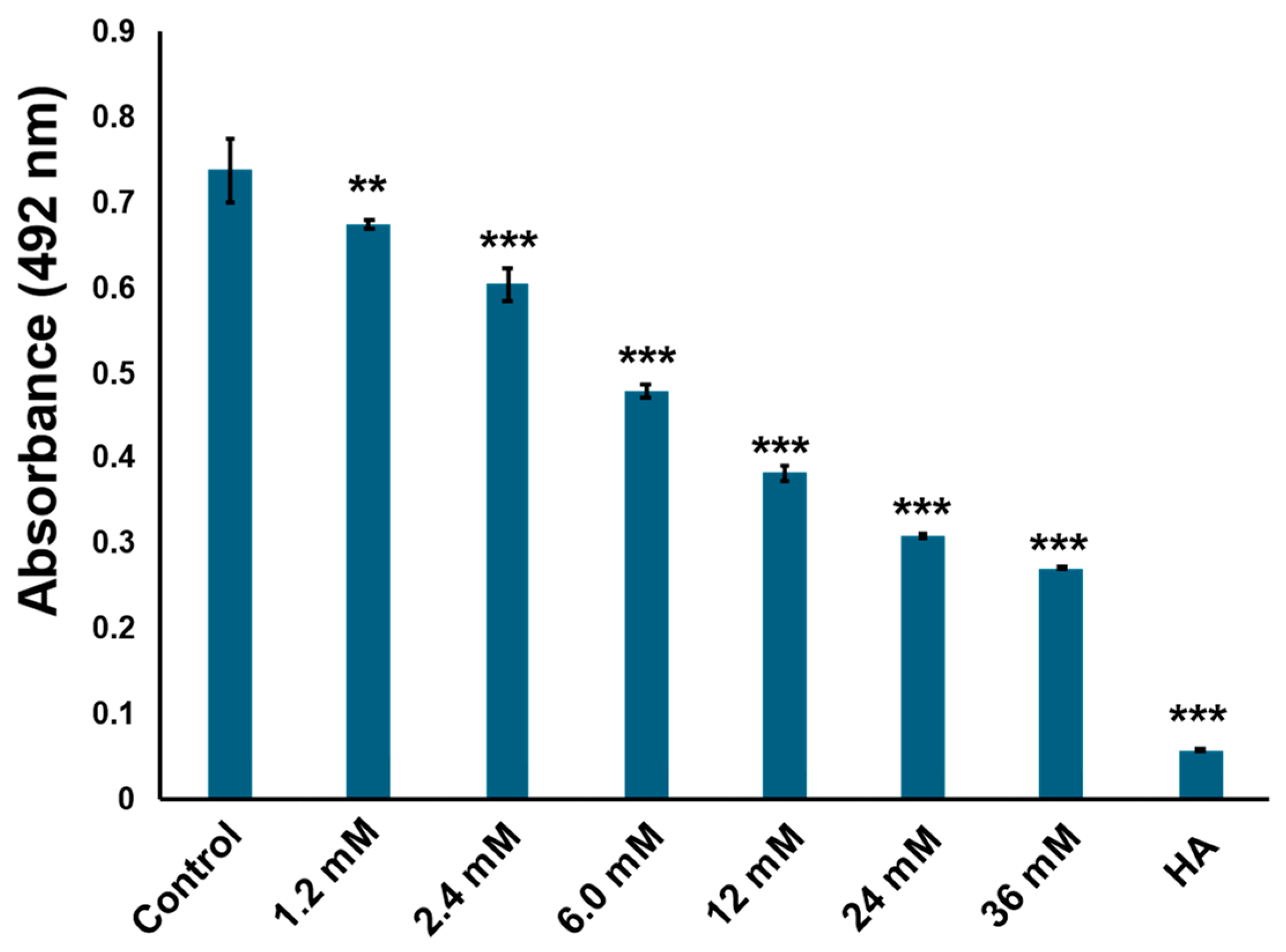
2.5. Cyclo (l-Pro-l-Tyr) Bound to Tyrosinase Substrate-Binding Site and Competitively Inhibited Its Enzymatic Activity
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Skin Bacteria
4.2. Identification of the Active Strain
4.3. Tyrosinase Inhibitory Assay
4.4. Kinetic Analysis of Tyrosinase Inhibition
4.5. Extraction, Purification, and Structural Identification of Cyclo(l-Pro-l-Tyr)
4.6. Molecular Docking Simulation
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, H. Skin Whitening Products and Their Effects. J. Jpn. Cosmet. Sci. Soc. 2019, 43, 39–43. [Google Scholar]
- Fernandes, M.S.; Kerkar, S. Microorganisms as a Source of Tyrosinase Inhibitors: A Review. Ann. Microbiol. 2017, 67, 343–358. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Deliencourt-Godefroy, G.; Lopes, L. An Effective Hydroquinone Alternative for Topical Skin Lightening. J. Cosmet. Dermatol. 2020, 19, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Villarama, C.D.; Maibach, H.I. Glutathione as a Depigmenting Agent: An Overview. Int. J. Cosmet. Sci. 2005, 27, 147–153. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin Lightening Preparations and the Hydroquinone Controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the Skin Microbiota in Health and Disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of Tyrosinase Substrate-Binding Modes Reveals Mechanistic Differences between Type-3 Copper Proteins. Nat. Commun. 2014, 5, 4505. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.; Lameira, J.; Kruger, H.G.; Alves, C.N.; Silva, J.R.A. Evaluating the Performance of a Non-bonded Cu2+ Model Including Jahn−Teller Effect into the Binding of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2020, 21, 4783. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.A.; Araújo, R.S.A.; Silva, F.N.D.; Cytarska, J.; Łączkowski, K.Z.; Cardoso, S.H.; Mendonça-Júnior, F.J.B.; Silva-Júnior, E.F.D. Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and in Silico Approaches. Int. J. Mol. Sci. 2023, 24, 5216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, X.; Hyun, C.-G. Isolation, Characterization, Genome Annotation, and Evaluation of Tyrosinase Inhibitory Activity in Secondary Metabolites of Paenibacillus sp. JNUCC32: A Comprehensive Analysis Through Molecular Docking and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2024, 25, 2213. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Robertson, D.H.; Brooks, C.L.; Vieth, M. Detailed Analysis of Grid-Based Molecular Docking: A Case Study of CDOCKER–A CHARMm-Based MD Docking Algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Ahmed, N.; Joglekar, P.; Deming, C.; NISC Comparative Sequencing Program; Lemon, K.P.; Kong, H.H.; Segre, J.A.; Conlan, S. Genomic Characterization of the C. tuberculostearicum Species Complex, a Prominent Member of the Human Skin Microbiome. mSystems 2023, 8, e0063223. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Karanam, G.; Arumugam, M.K.; Sirpu Natesh, N. Anticancer Effect of Marine Sponge-Associated Bacillus pumilus AMK1 Derived Dipeptide Cyclo (-Pro–Tyr) in Human Liver Cancer Cell Line Through Apoptosis and G2/M Phase Arrest. Int. J. Pept. Res. Ther. 2020, 26, 445–457. [Google Scholar] [CrossRef]
- Paudel, B.; Maharjan, R.; Rajbhandari, P.; Aryal, N.; Aziz, S.; Bhattarai, K.; Baral, B.; Malla, R.; Bhattarai, H.D. Maculosin, a Non-toxic Antioxidant Compound Isolated from Streptomyces sp. KTM18. Pharm. Biol. 2021, 59, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a Host-Specific Phytotoxin for Spotted Knapweed from Alternaria alternata. Proc. Natl Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Puopolo, G.; Perazzolli, M.; Andolfi, A.; Melck, D.; Pertot, I.; Evidente, A. Cyclo(L-Pro-L-TYR), the Fungicide Isolated from Lysobacter capsici AZ78: A Structure–Activity Relationship Study. Chem. Heterocycl. Compd. 2014, 50, 290–295. [Google Scholar] [CrossRef]
- Castaldi, S.; Cimmino, A.; Masi, M.; Evidente, A. Bacterial Lipodepsipeptides and Some of Their Derivatives and Cyclic Dipeptides as Potential Agents for Biocontrol of Pathogenic Bacteria and Fungi of Agrarian Plants. J. Agric. Food Chem. 2022, 70, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.P.; Lee, S.-H.; Choi, J.-H.; Kashima, A.; Nakamura, G.; Suzuki, T. Crystal Structure and Spectroscopic Properties of Cyclic Dipeptide: A Racemic Mixture of Cyclo(ᴅ-Prolyl-ʟ-Tyrosyl) and Cyclo(ʟ-Prolyl-ᴅ-Tyrosyl). Bull. Korean Chem. Soc. 2014, 35, 2299–2303. [Google Scholar] [CrossRef]
- Bock, V.D.; Perciaccante, R.; Jansen, T.P.; Hiemstra, H.; van Maarseveen, J.H. Click Chemistry as a Route to Cyclic Tetrapeptide Analogues: Synthesis of Cyclo-[Pro–Val-ψ(triazole)-Pro-Tyr]. Org. Lett. 2006, 8, 919–922. [Google Scholar] [CrossRef]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First Structures of an Active Bacterial Tyrosinase Reveal Copper Plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Kwon, K.S.; Babar, M.M.; Zaidi, N.U. Kinetic and In Silico Studies of Novel Hydroxy-Based Thymol Analogues as Inhibitors of Mushroom Tyrosinase. Eur. J. Med. Chem. 2015, 98, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F. New Insights Into the Active Site Structure and Catalytic Mechanism of Tyrosinase and Its Related Proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.; Sturm, S.; Ganzera, M.; Waltenberger, B.; Martens, S.; Schwaiger, S.; Schuster, D.; Stuppner, H. Mushroom Tyrosinase-Based Enzyme Inhibition Assays Are Not Suitable for Bioactivity-Guided Fractionation of Extracts. J. Nat. Prod. 2019, 82, 136–147. [Google Scholar] [CrossRef]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S. The Structural Differences Between Mushroom and Human Tyrosinase Cleared by Investigating the Inhibitory Activities of Stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase Inhibitors from Natural and Synthetic Sources as Skin-Lightening Agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Higa, T. Tyrosinase Activity Inhibitor, Bleaching Cosmetic, External Preparation for Skin, and Browning Inhibitor for Food. Patent No. JP2013133314A, 8 July 2013. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekino, Y.; Yamamoto, I.; Watanabe, M.; Kuramochi, K.; Furuyama, Y. Cyclo(l-Pro-l-Tyr) Isolated from the Human Skin Commensal Corynebacterium tuberculostearicum Inhibits Tyrosinase. Int. J. Mol. Sci. 2024, 25, 7365. https://doi.org/10.3390/ijms25137365
Sekino Y, Yamamoto I, Watanabe M, Kuramochi K, Furuyama Y. Cyclo(l-Pro-l-Tyr) Isolated from the Human Skin Commensal Corynebacterium tuberculostearicum Inhibits Tyrosinase. International Journal of Molecular Sciences. 2024; 25(13):7365. https://doi.org/10.3390/ijms25137365
Chicago/Turabian StyleSekino, Yuika, Ikuya Yamamoto, Masahiro Watanabe, Kouji Kuramochi, and Yuuki Furuyama. 2024. "Cyclo(l-Pro-l-Tyr) Isolated from the Human Skin Commensal Corynebacterium tuberculostearicum Inhibits Tyrosinase" International Journal of Molecular Sciences 25, no. 13: 7365. https://doi.org/10.3390/ijms25137365




