Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen
Abstract
1. Introduction
2. Results
2.1. BP LMC Increase Barrier Integrity in Bronchial Epithelial Cells
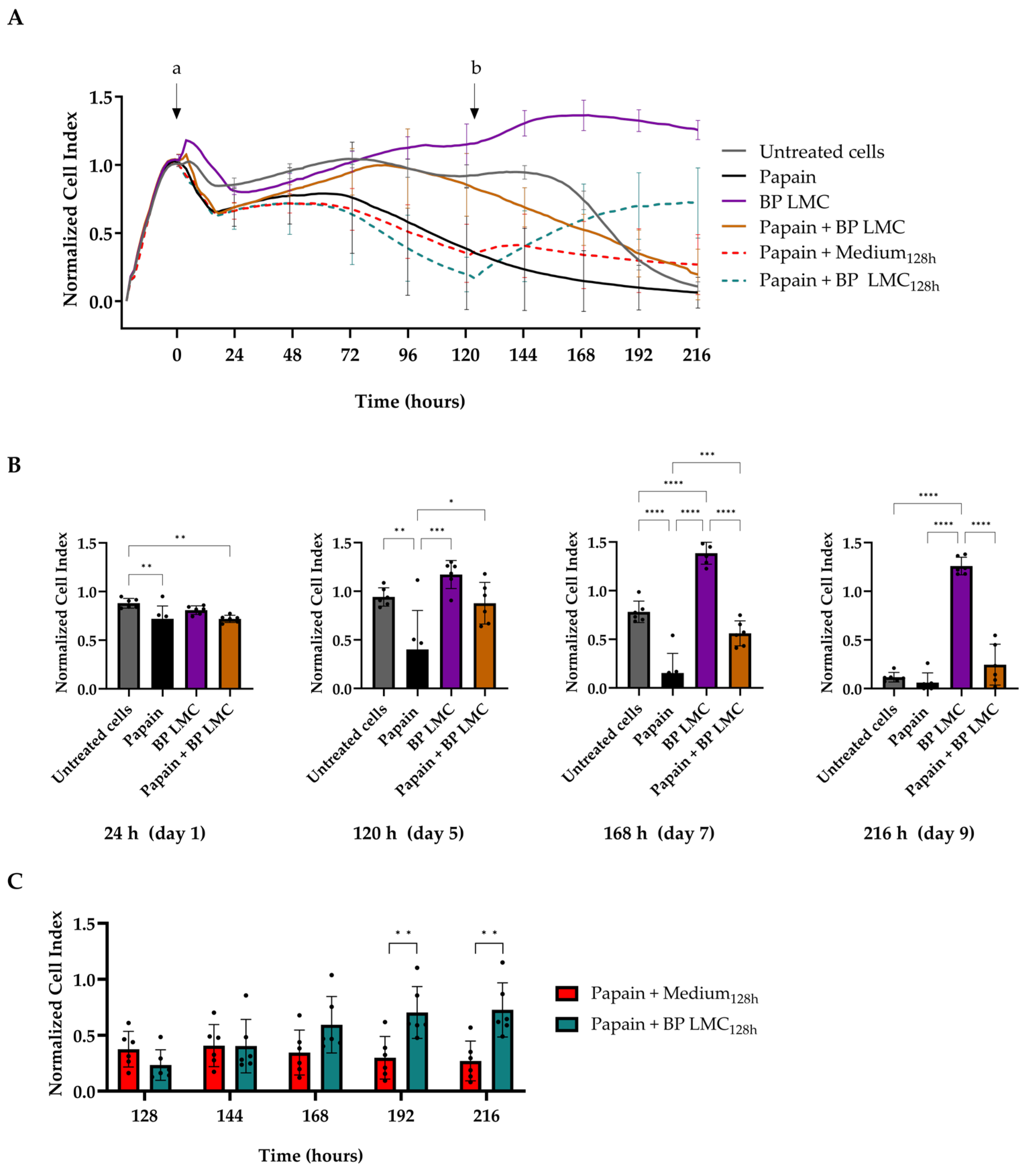
2.2. Effect of BP LMC on Papain-Damaged Epithelial Barrier
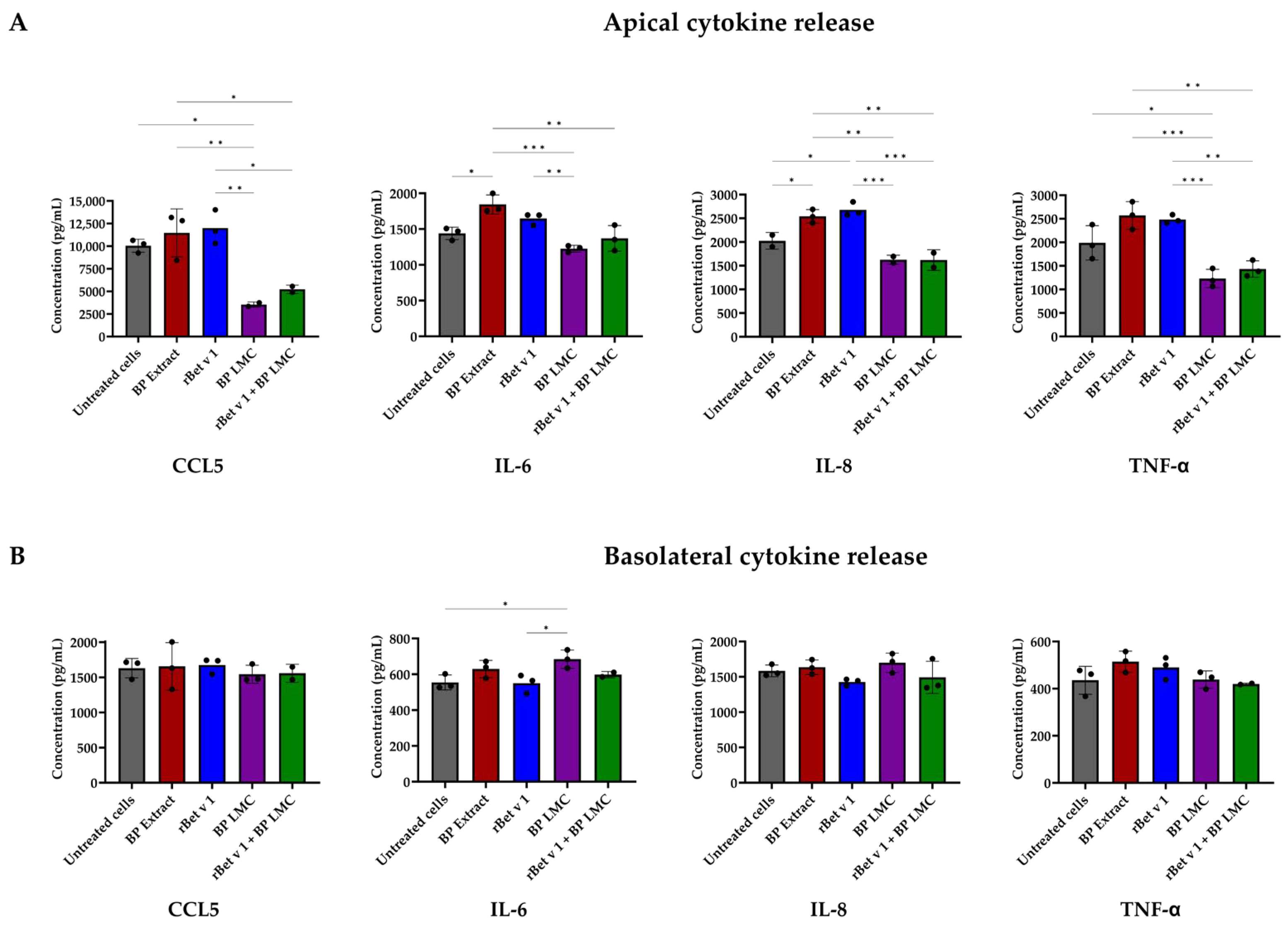
2.3. BP LMC Influence Polarized Release of Cytokines
3. Discussion
4. Materials and Methods
4.1. Recombinant Bet v 1 Production and Purification
4.2. Birch Pollen Aqueous Extract and Isolation of Low Molecular Weight Components from Birch Pollen
4.3. Bronchial Epithelial Cell Culture
4.4. Cell Viability Assay
4.5. Transepithelial Electrical Resistance Measurement
4.6. Monitoring Epithelial Properties Using xCELLigence System
4.7. Measurement of Polarized Release of Cytokines
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salazar, F.; Ghaemmaghami, A.M. Allergen recognition by innate immune cells: Critical role of dendritic and epithelial cells. Front. Immunol. 2013, 4, 356. [Google Scholar] [CrossRef]
- Gandhi, V.D.; Vliagoftis, H. Airway epithelium interactions with aeroallergens: Role of secreted cytokines and chemokines in innate immunity. Front. Immunol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Pointner, L.; Bethanis, A.; Thaler, M.; Traidl-Hoffmann, C.; Gilles, S.; Ferreira, F.; Aglas, L. Initiating pollen sensitization—Complex source, complex mechanisms. Clin. Transl. Allergy 2020, 10, 020–00341. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Bohle, B.; Ovchinnikova, T.V. How Do Pollen Allergens Sensitize? Front. Mol. Biosci. 2022, 9, 900533. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, 13854. [Google Scholar] [CrossRef]
- Breiteneder, H.; Kraft, D. The History and Science of the Major Birch Pollen Allergen Bet v 1. Biomolecules 2023, 13, 1151. [Google Scholar] [CrossRef]
- Bublin, M.; Eiwegger, T.; Breiteneder, H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014, 134, 521–529. [Google Scholar] [CrossRef]
- Blume, C.; Swindle, E.J.; Gilles, S.; Traidl-Hoffmann, C.; Davies, D.E. Low molecular weight components of pollen alter bronchial epithelial barrier functions. Tissue Barriers 2015, 3, e1062316. [Google Scholar] [CrossRef]
- Kalic, T.; Ellinger, I.; Kamath, S.D.; Palladino, C.; Mayr, V.; Tscheppe, A.; Ruethers, T.; Waltl, E.E.; Niederberger, V.; Lengger, N.; et al. Fish-derived low molecular weight components modify bronchial epithelial barrier properties and release of pro-inflammatory cytokines. Mol. Immunol. 2019, 112, 140–150. [Google Scholar] [CrossRef]
- Gilles, S.; Fekete, A.; Zhang, X.; Beck, I.; Blume, C.; Ring, J.; Schmidt-Weber, C.; Behrendt, H.; Schmitt-Kopplin, P.; Traidl-Hoffmann, C. Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J. Allergy Clin. Immunol. 2011, 127, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.; Alessandrini, F.; Gilles, S.; Frank, U.; Oeder, S.; Hauser, M.; Ring, J.; Ferreira, F.; Ernst, D.; Winkler, J.B.; et al. Pollen-derived adenosine is a necessary cofactor for ragweed allergy. Allergy 2015, 70, 944–954. [Google Scholar] [CrossRef]
- Gilles-Stein, S.; Beck, I.; Chaker, A.; Bas, M.; McIntyre, M.; Cifuentes, L.; Petersen, A.; Gutermuth, J.; Schmidt-Weber, C.; Behrendt, H.; et al. Pollen derived low molecular compounds enhance the human allergen specific immune response in vivo. Clin. Exp. Allergy 2016, 46, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- González Roldán, N.; Engel, R.; Düpow, S.; Jakob, K.; Koops, F.; Orinska, Z.; Vigor, C.; Oger, C.; Galano, J.M.; Durand, T.; et al. Lipid Mediators From Timothy Grass Pollen Contribute to the Effector Phase of Allergy and Prime Dendritic Cells for Glycolipid Presentation. Front. Immunol. 2019, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Traidl-Hoffmann, C.; Kasche, A.; Jakob, T.; Huger, M.; Plötz, S.; Feussner, I.; Ring, J.; Behrendt, H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J. Allergy Clin. Immunol. 2002, 109, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Plötz, S.G.; Traidl-Hoffmann, C.; Feussner, I.; Kasche, A.; Feser, A.; Ring, J.; Jakob, T.; Behrendt, H. Chemotaxis and activation of human peripheral blood eosinophils induced by pollen-associated lipid mediators. J. Allergy Clin. Immunol. 2004, 113, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Stremnitzer, C.; Manzano-Szalai, K.; Willensdorfer, A.; Starkl, P.; Pieper, M.; König, P.; Mildner, M.; Tschachler, E.; Reichart, U.; Jensen-Jarolim, E. Papain Degrades Tight Junction Proteins of Human Keratinocytes In Vitro and Sensitizes C57BL/6 Mice via the Skin Independent of its Enzymatic Activity or TLR4 Activation. J. Investig. Dermatol. 2015, 135, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Maillet, I.; Mackowiak, C.; Le Bérichel, J.; Philippeau, A.; Panek, C.; Boury, M.; Oswald, E.; Saoudi, A.; Erard, F.; et al. The probiotic strain Escherichia coli Nissle 1917 prevents papain-induced respiratory barrier injury and severe allergic inflammation in mice. Sci. Rep. 2018, 8, 11245. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Mueller, M.J.; Jakob, T.; Behrendt, H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 2005, 201, 627–636. [Google Scholar] [CrossRef]
- Oeder, S.; Alessandrini, F.; Wirz, O.F.; Braun, A.; Wimmer, M.; Frank, U.; Hauser, M.; Durner, J.; Ferreira, F.; Ernst, D.; et al. Pollen-derived nonallergenic substances enhance Th2-induced IgE production in B cells. Allergy 2015, 70, 1450–1460. [Google Scholar] [CrossRef]
- Seutter von Loetzen, C.; Hoffmann, T.; Hartl, M.J.; Schweimer, K.; Schwab, W.; Rösch, P.; Hartl-Spiegelhauer, O. Secret of the major birch pollen allergen Bet v 1: Identification of the physiological ligand. Biochem. J. 2014, 457, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kerienė, I.; Šaulienė, I.; Šukienė, L.; Judžentienė, A.; Ligor, M.; Buszewski, B. Patterns of Phenolic Compounds in Betula and Pinus Pollen. Plants 2023, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Hardyman, M.A.; Wilkinson, E.; Martin, E.; Jayasekera, N.P.; Blume, C.; Swindle, E.J.; Gozzard, N.; Holgate, S.T.; Howarth, P.H.; Davies, D.E.; et al. TNF-α-mediated bronchial barrier disruption and regulation by src-family kinase activation. J. Allergy Clin. Immunol. 2013, 132, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Y.; Zhang, J.; Chang, R.; Li, J.; Huo, L. IL-9 promotes the pathogenesis of ulcerative colitis through STAT3/SOCS3 signaling. Biosci. Rep. 2018, 38, 21. [Google Scholar] [CrossRef]
- Gerlach, K.; McKenzie, A.N.; Neurath, M.F.; Weigmann, B. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tissue Barriers 2015, 3, e983777. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudharson, S.; Kalic, T.; Eckl-Dorna, J.; Lengger, N.; Breiteneder, H.; Hafner, C. Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen. Int. J. Mol. Sci. 2024, 25, 7374. https://doi.org/10.3390/ijms25137374
Sudharson S, Kalic T, Eckl-Dorna J, Lengger N, Breiteneder H, Hafner C. Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen. International Journal of Molecular Sciences. 2024; 25(13):7374. https://doi.org/10.3390/ijms25137374
Chicago/Turabian StyleSudharson, Srinidhi, Tanja Kalic, Julia Eckl-Dorna, Nina Lengger, Heimo Breiteneder, and Christine Hafner. 2024. "Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen" International Journal of Molecular Sciences 25, no. 13: 7374. https://doi.org/10.3390/ijms25137374
APA StyleSudharson, S., Kalic, T., Eckl-Dorna, J., Lengger, N., Breiteneder, H., & Hafner, C. (2024). Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen. International Journal of Molecular Sciences, 25(13), 7374. https://doi.org/10.3390/ijms25137374




