Interactions with DNA Models of the Oxaliplatin Analog (cis-1,3-DACH)PtCl2 †
Abstract
1. Introduction
2. Results and Discussion
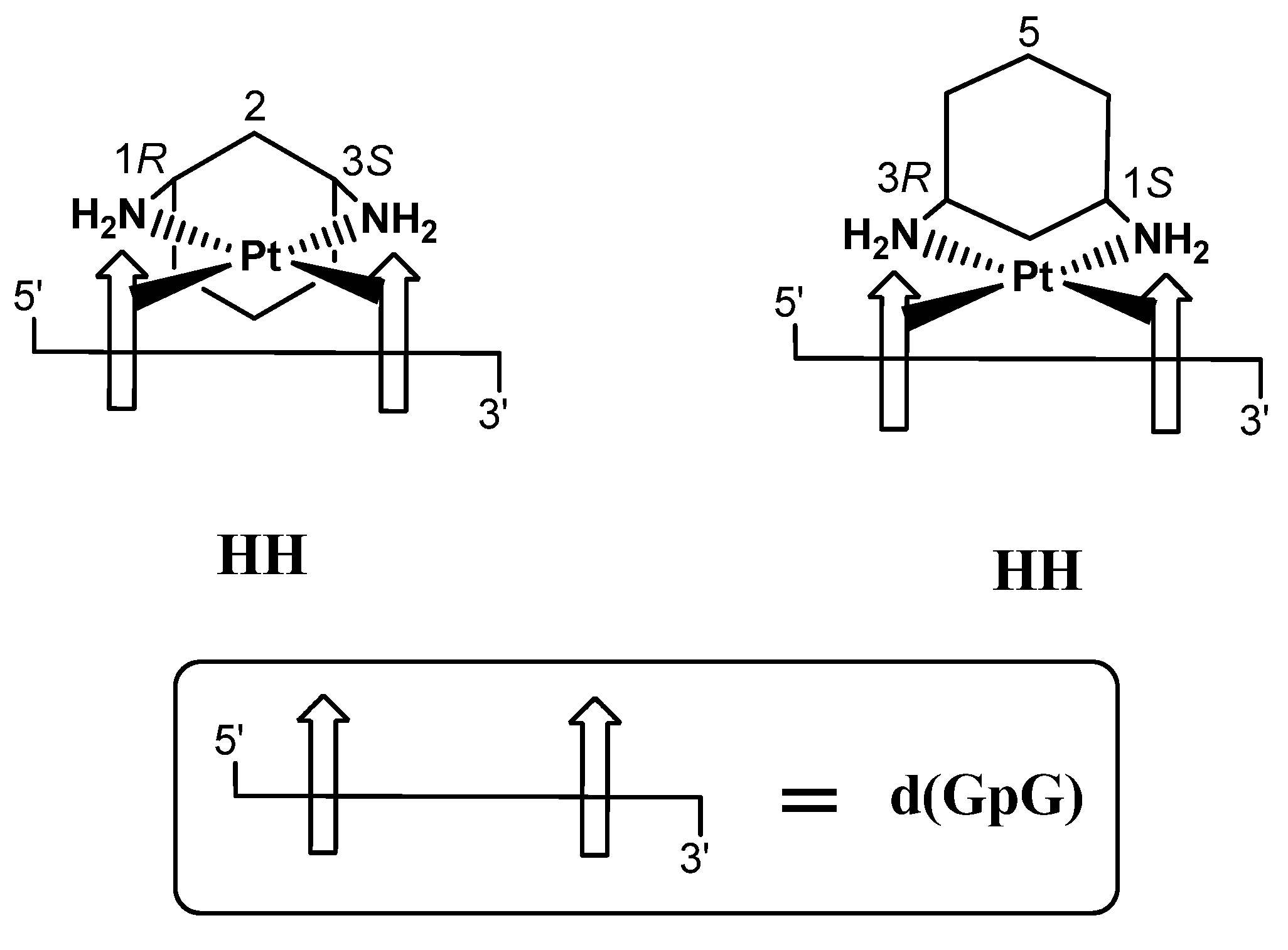
2.1. Synthesis and Characterization of [PtCl2(cis-1,3-DACH)]
2.2. Reaction with G Nucleotides
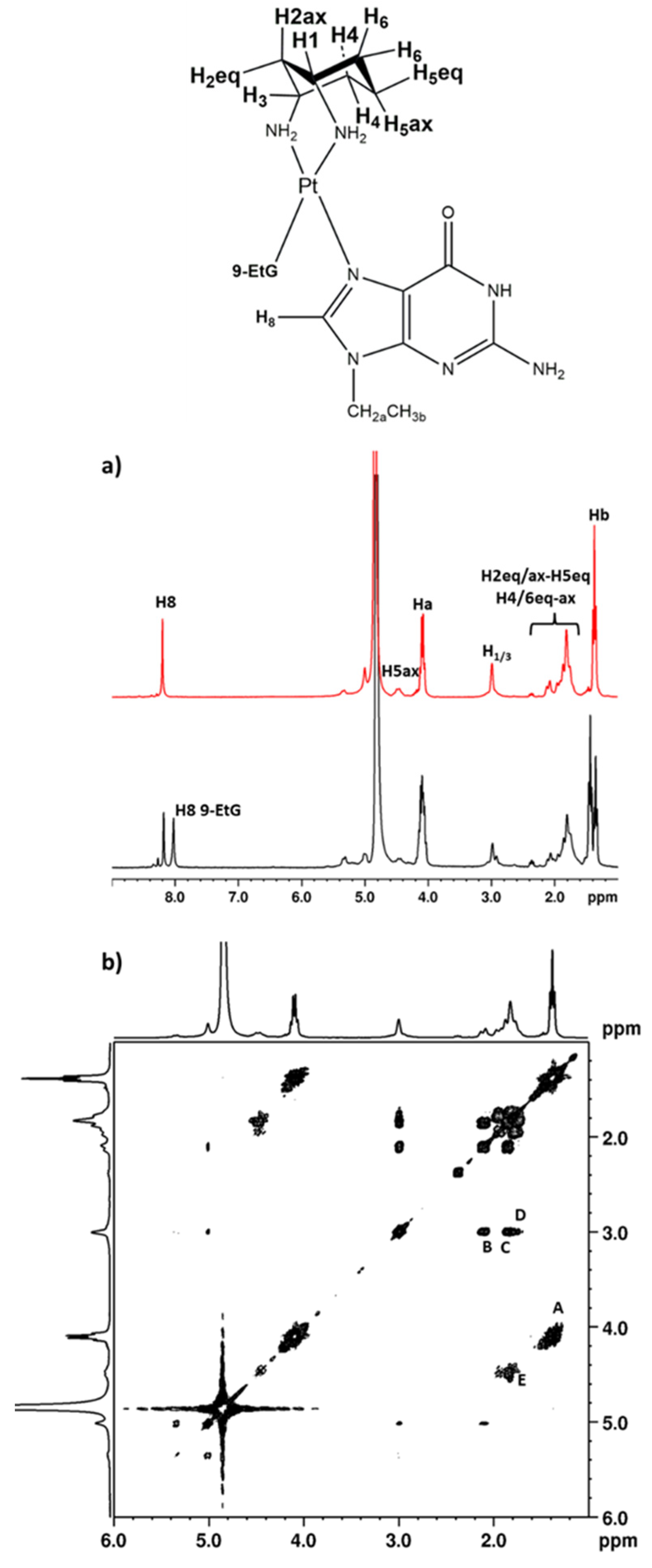
2.2.1. 9-EthylGuanine (9-EtG)
2.2.2. Guanosine (Guo)
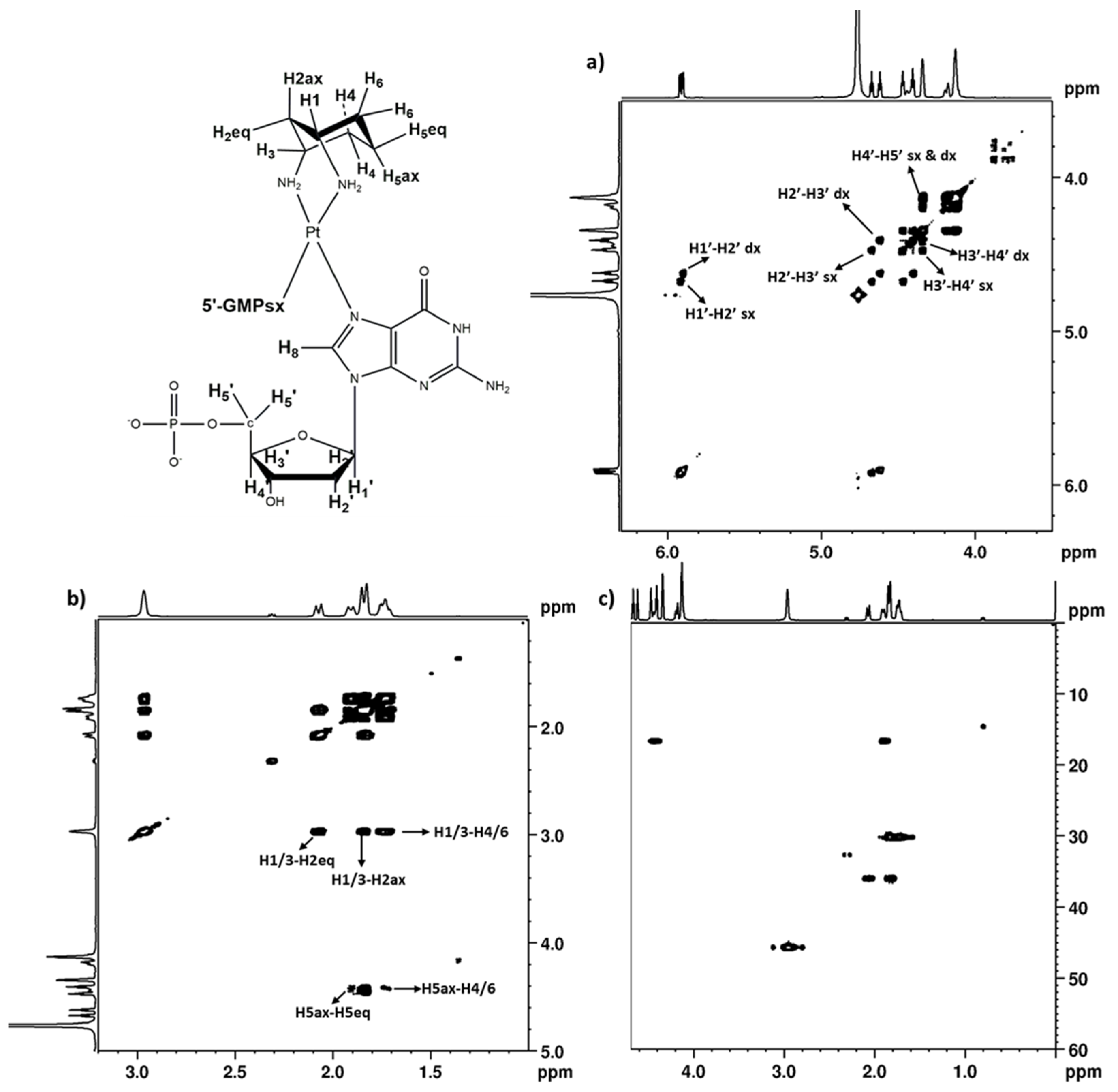
2.2.3. 5′GMP
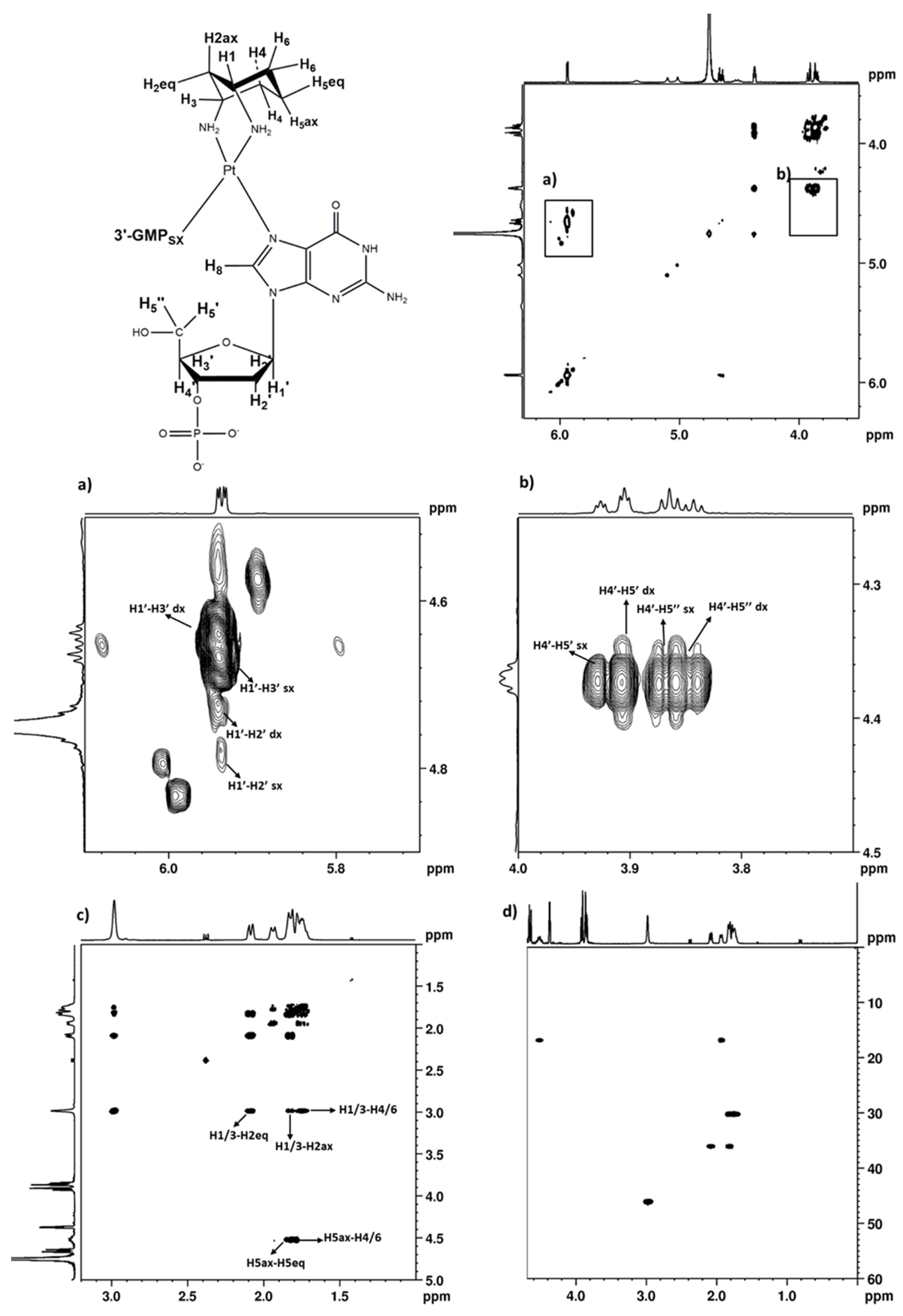
2.2.4. 3′GMP
2.2.5. Variable Temperature NMR Experiments on (cis-1,3-DACH)Pt(5′GMP)2 and (cis-1,3-DACH)Pt(3′GMP)2
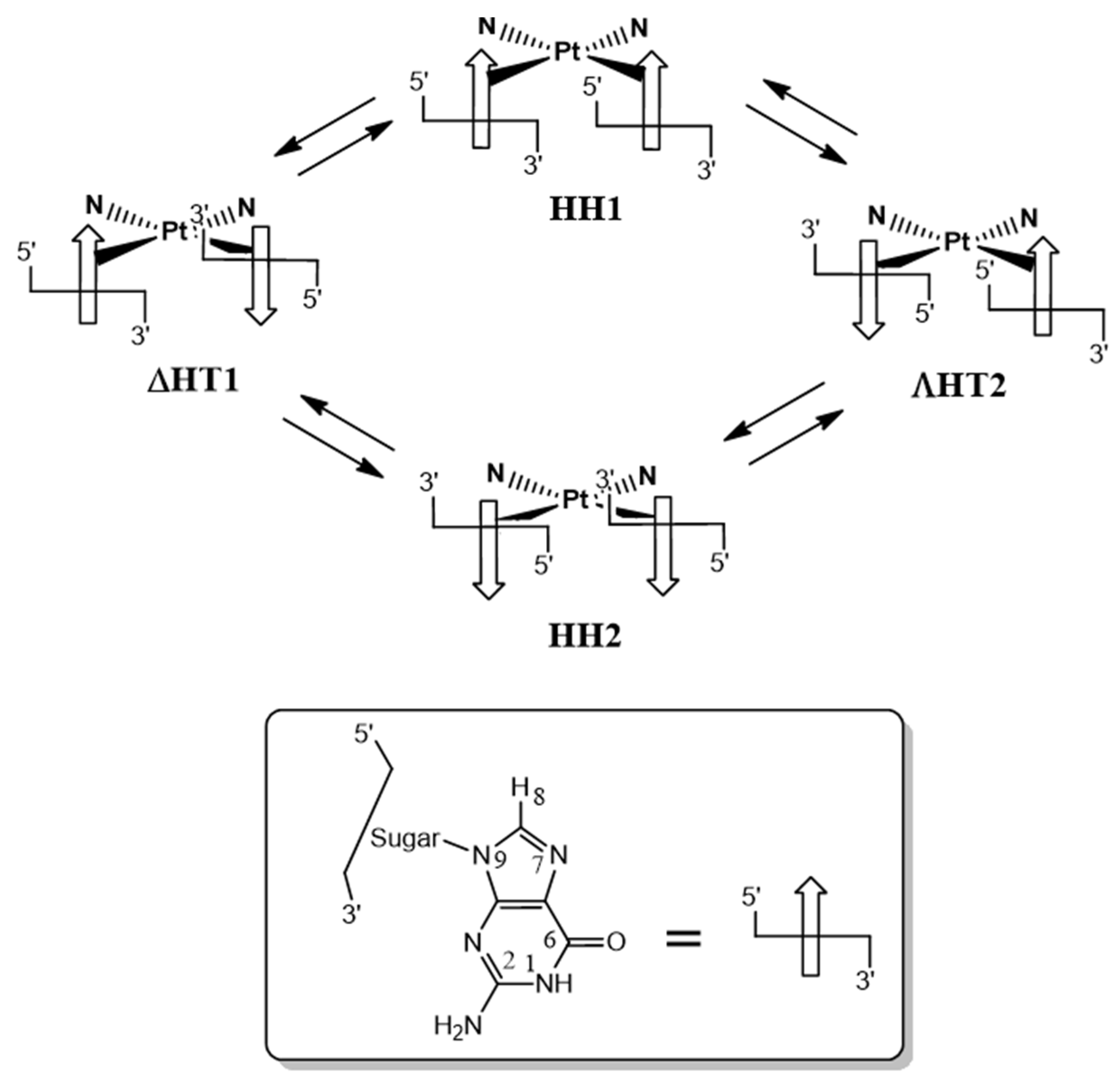
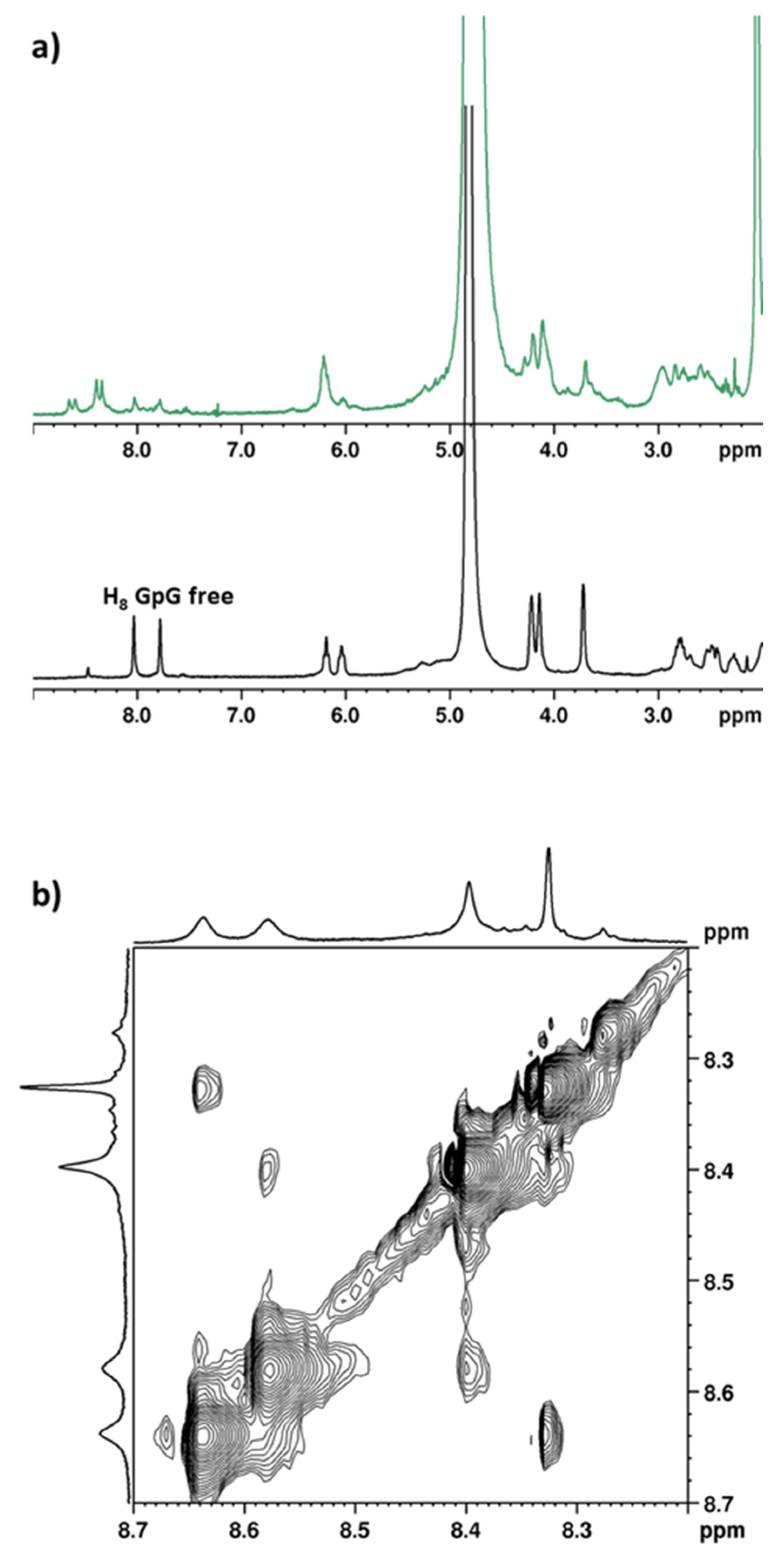
2.2.6. Deoxyguanosil(3′-5′)deoxyguanosine (d(GpG))
3. Materials and Methods
3.1. Synthesis of the Complexes
3.1.1. [PtCl2(cis-1,3-DACH)]
3.1.2. (cis-1,3-DACH)PtG2 Adducts (G = 9-EthylGuanine, Guanosine, 3′-GMP, and 5′-GMP)
3.1.3. (cis-1,3-DACH)Pt(d(GpG)) Adduct (d(GpG) = Deoxyguanosil(3′-5′)deoxyguanosine)
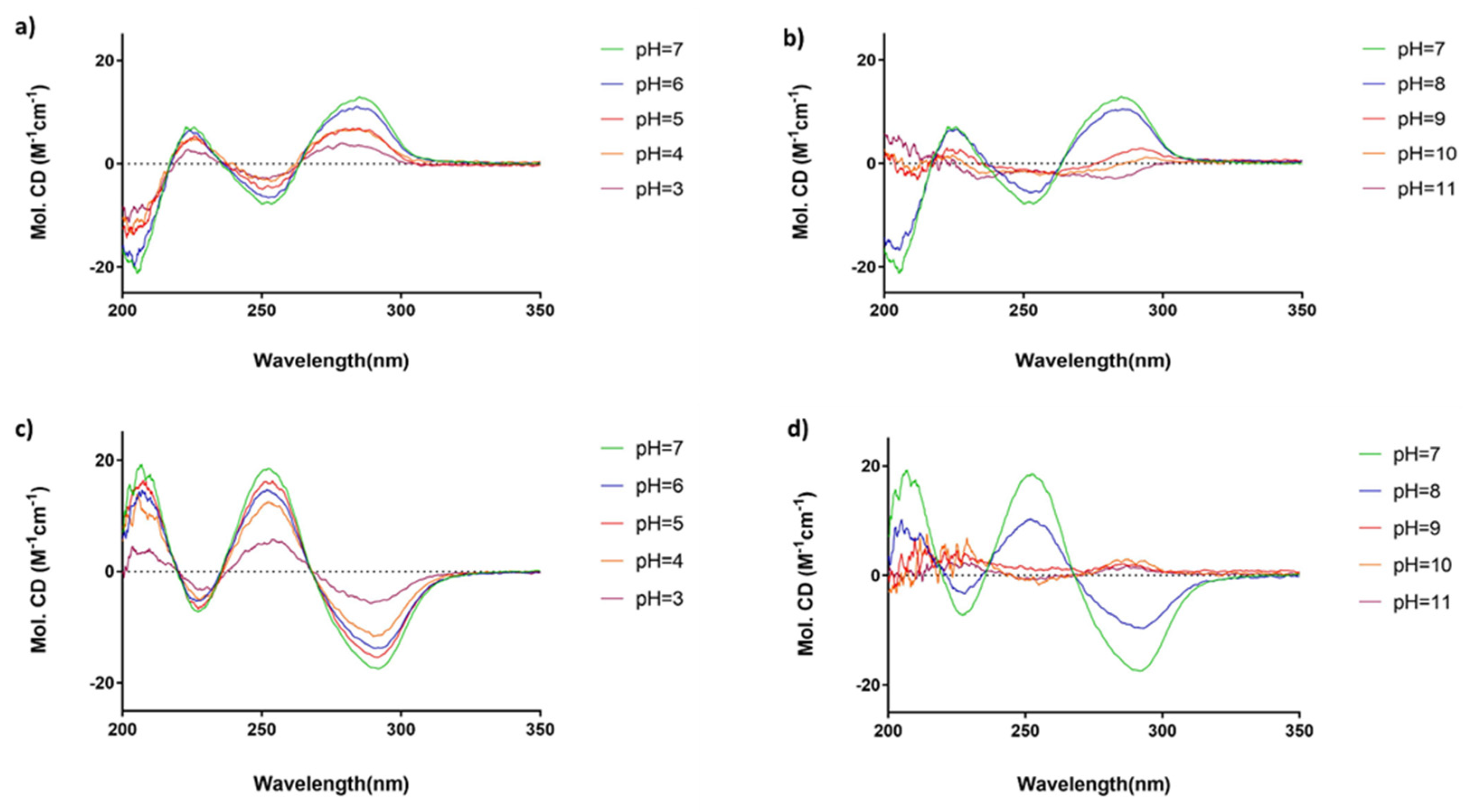
3.2. Solutions for Circular Dichroism (CD) Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A Chemical Perspective on the Clinical Use of Platinum-Based Anticancer Drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef]
- Todd, R.C.; Lippard, S.J. Inhibition of Transcription by Platinum Antitumor Compounds. Metallomics 2009, 1, 280. [Google Scholar] [CrossRef]
- Lebwohl, D.; Canetta, R. Clinical Development of Platinum Complexes in Cancer Therapy: An Historical Perspective and an Update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Hambley, T.W. The Influence of Structure on the Activity and Toxicity of Pt Anti-Cancer Drugs. Coord. Chem. Rev. 1997, 166, 181–223. [Google Scholar] [CrossRef]
- Reedijk, J. Improved Understanding in Platinium Antitumour Chemistry. Chem. Commun. 1996, 801–806. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Marzilli, L.G.; Saad, J.S.; Kuklenyik, Z.; Keating, K.A.; Xu, Y. Relationship of Solution and Protein-Bound Structures of DNA Duplexes with the Major Intrastrand Cross-Link Lesions Formed on Cisplatin Binding to DNA. J. Am. Chem. Soc. 2001, 123, 2764–2770. [Google Scholar] [CrossRef]
- Chottard, J.C.; Girault, J.P.; Chottard, G.; Lallemand, J.Y.; Mansuy, D. Interaction of Cis-Diaquodiammineplatinum Dinitrate with Ribose Dinucleoside Monophosphates. J. Am. Chem. Soc. 1980, 102, 5565–5572. [Google Scholar] [CrossRef]
- den Hartog, J.H.; Altona, C.; Chottard, J.C.; Girault, J.P.; Lallemand, J.Y.; de Leeuw, F.A.; Marcelis, A.T.; Reedijk, J. Conformational Analysis of the Adduct Cis-[Pt(NH3)2 d(GpG)]+ in Aqueous Solution. A High Field (500–300 MHz) Nuclear Magnetic Resonance Investigation. Nucleic Acids Res. 1982, 10, 4715–4730. [Google Scholar] [CrossRef][Green Version]
- Girault, J.P.; Chottard, G.; Lallemand, J.Y.; Chottard, J.C. Interaction of Cis-[Pt(NH3)2(H2O)2](NO3)2 with Ribose Deoxyribose Diguanosine Phosphates. Biochemistry 1982, 21, 1352–1356. [Google Scholar] [CrossRef]
- Kozelka, J.; Fouchet, M.H.; Chottard, J.C. H8 Chemical Shifts in Oligonucleotide Cross-Linked at a GpG Sequence by Cis-Pt(NH3)22+: A Clue to the Adduct Structure. Eur. J. Biochem. 1992, 205, 895–906. [Google Scholar] [CrossRef]
- Sherman, S.E.; Gibson, D.; Wang, A.H.J.; Lippard, S.J. Crystal and Molecular Structure of Cis-[Pt(NH3)2[d(PGpG)]], the Principal Adduct Formed by Cis-Diamminedichloroplatinum(II) with DNA. J. Am. Chem. Soc. 1988, 110, 7368–7381. [Google Scholar] [CrossRef]
- Yang, D.; van Boom, S.S.; Reedijk, J.; van Boom, J.H.; Wang, A.H. Structure and Isomerization of an Intrastrand Cisplatin-Cross-Linked Octamer DNA Duplex by NMR Analysis. Biochemistry 1995, 34, 12912–12920. [Google Scholar] [CrossRef]
- Natile, G.; Marzilli, L.G. Non-Covalent Interactions in Adducts of Platinum Drugs with Nucleobases in Nucleotides and DNA as Revealed by Using Chiral Substrates. Coord. Chem. Rev. 2006, 250, 1315–1331. [Google Scholar] [CrossRef]
- Lippert, B. (Ed.) Cisplatin; Wiley: Hoboken, NJ, USA, 1999; ISBN 9783906390208. [Google Scholar]
- Coste, F.; Malinge, J.M.; Serre, L.; Shepard, W.; Roth, M.; Leng, M.; Zelwer, C. Crystal Structure of a Double-Stranded DNA Containing a Cisplatin Interstrand Cross-Link at 1.63 A Resolution: Hydration at the Platinated Site. Nucleic Acids Res. 1999, 27, 1837–1846. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, L.; Reid, B.R.; Drobny, G.P.; Hopkins, P.B. Solution Structure of a Cisplatin-Induced DNA Interstrand Cross-Link. Science 1995, 270, 1842–1845. [Google Scholar] [CrossRef]
- Paquet, F.; Pérez, C.; Leng, M.; Lancelot, G.; Malinge, J.M. NMR Solution Structure of a DNA Decamer Containing an Interstrand Cross-Link of the Antitumor Drug Cis-Diamminedichloroplatinum (II). J. Biomol. Struct. Dyn. 1996, 14, 67–77. [Google Scholar] [CrossRef]
- Pinto, A.L.; Lippard, S.J. Binding of the Antitumor Drug Cis-Diamminedichloroplatinum(II) (Cisplatin) to DNA. Biochim. Biophys. Acta 1985, 780, 167–180. [Google Scholar] [CrossRef]
- Hacker, M.P.; Douple, E.B.; Krakoff, I.H. (Eds.) Platinum Coordination Complexes in Cancer Chemotherapy; Springer: Boston, MA, USA, 1984; ISBN 978-0-89838-619-6. [Google Scholar]
- Wong, E.; Giandomenico, C.M. Current Status of Platinum-Based Antitumor Drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef]
- Wu, Y.; Bhattacharyya, D.; King, C.L.; Baskerville-Abraham, I.; Huh, S.-H.; Boysen, G.; Swenberg, J.A.; Temple, B.; Campbell, S.L.; Chaney, S.G. Solution Structures of a DNA Dodecamer Duplex with and without a Cisplatin 1,2-d(GG) Intrastrand Cross-Link: Comparison with the Same DNA Duplex Containing an Oxaliplatin 1,2-d(GG) Intrastrand Cross-Link. Biochemistry 2007, 46, 6477–6487. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Ramachandran, S.; Sharma, S.; Pathmasiri, W.; King, C.L.; Baskerville-Abraham, I.; Boysen, G.; Swenberg, J.A.; Campbell, S.L.; Dokholyan, N.V.; et al. Flanking Bases Influence the Nature of DNA Distortion by Platinum 1,2-Intrastrand (GG) Cross-Links. PLoS ONE 2011, 6, e23582. [Google Scholar] [CrossRef]
- Ranaldo, R.; Margiotta, N.; Intini, F.P.; Pacifico, C.; Natile, G. Conformer Distribution in (Cis-1,4-DACH)Bis(Guanosine-5′-Phosphate)Platinum(II) Adducts: A Reliable Model for DNA Adducts of Antitumoral Cisplatin. Inorg. Chem. 2008, 47, 2820–2830. [Google Scholar] [CrossRef]
- Kasparkova, J.; Suchankova, T.; Halamikova, A.; Zerzankova, L.; Vrana, O.; Margiotta, N.; Natile, G.; Brabec, V. Cytotoxicity, Cellular Uptake, Glutathione and DNA Interactions of an Antitumor Large-Ring Pt II Chelate Complex Incorporating the Cis-1,4-Diaminocyclohexane Carrier Ligand. Biochem. Pharmacol. 2010, 79, 552–564. [Google Scholar] [CrossRef]
- Margiotta, N.; Marzano, C.; Gandin, V.; Osella, D.; Ravera, M.; Gabano, E.; Platts, J.A.; Petruzzella, E.; Hoeschele, J.D.; Natile, G. Revisiting [PtCl2(Cis-1,4-DACH)]: An Underestimated Antitumor Drug with Potential Application to the Treatment of Oxaliplatin-Refractory Colorectal Cancer. J. Med. Chem. 2012, 55, 7182–7192. [Google Scholar] [CrossRef]
- Brabec, V.; Malina, J.; Margiotta, N.; Natile, G.; Kasparkova, J. Thermodynamic and Mechanistic Insights into Translesion DNA Synthesis Catalyzed by Y-Family DNA Polymerase across a Bulky Double-Base Lesion of an Antitumor Platinum Drug. Chemistry 2012, 18, 15439–15448. [Google Scholar] [CrossRef]
- Margiotta, N.; Ranaldo, R.; Intini, F.P.; Natile, G. Cationic Intermediates in Oxidative Addition Reactions of Cl2 to [PtCl2(Cis-1,4-DACH)]. Dalton Trans. 2011, 40, 12877–12885. [Google Scholar] [CrossRef]
- Petruzzella, E.; Margiotta, N.; Ravera, M.; Natile, G. NMR Investigation of the Spontaneous Thermal- and/or Photoinduced Reduction of Trans Dihydroxido Pt(IV) Derivatives. Inorg. Chem. 2013, 52, 2393–2403. [Google Scholar] [CrossRef]
- Margiotta, N.; Petruzzella, E.; Platts, J.A.; Mutter, S.T.; Deeth, R.J.; Ranaldo, R.; Papadia, P.; Marzilli, P.A.; Marzilli, L.G.; Hoeschele, J.D.; et al. DNA Fragment Conformations in Adducts with Kiteplatin. Dalton Trans. 2015, 44, 3544–3556. [Google Scholar] [CrossRef]
- Mutter, S.T.; Margiotta, N.; Papadia, P.; Platts, J.A. Computational Evidence for Structural Consequences of Kiteplatin Damage on DNA. JBIC J. Biol. Inorg. Chem. 2015, 20, 35–48. [Google Scholar] [CrossRef]
- Cham, S.T.; Diakos, C.I.; Ellis, L.T.; Fenton, R.R.; Munk, V.P.; Messerle, B.A.; Hambley, T.W. Isomer Formation in the Binding of [PtCl2(Cis-Cyclohexane-1,3-Diamine)] to Oligonucleotides and the X-Ray Crystal Structure of [PtCl2(Cis-Cyclohexane-1,3-Diamine)]·dimethylformamide†. J. Chem. Soc. Dalt. Trans. 2001, 2769–2774. [Google Scholar] [CrossRef]
- Ellis, L.T.; Hambley, T.W. Dichloro(Ethylenediamine)Platinum(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 1888–1889. [Google Scholar] [CrossRef]
- Lock, C.J.L.; Pilon, P. Tris[Cis-Dichloro(1,2-Diaminocyclohexane)-Platinum(II)] Hydrate and Cis-Dibromo(1,2-Diaminocyclohexane)Platinum(II). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1981, 37, 45–49. [Google Scholar] [CrossRef]
- Hoeschele, J.D.; Kasparkova, J.; Kostrhunova, H.; Novakova, O.; Pracharova, J.; Pineau, P.; Brabec, V. Synthesis, Antiproliferative Activity in Cancer Cells and DNA Interaction Studies of [Pt(Cis-1,3-Diaminocycloalkane)Cl2] Analogs. JBIC J. Biol. Inorg. Chem. 2020, 25, 913–914. [Google Scholar] [CrossRef]
- Inagaki, K.; Sawaki, K. Characterization of Isomers Which Are Produced by Reactions of (1R,3S-Cyclohexanediamine)Platinum(II) with Nucleotide d(GpG). Bull. Chem. Soc. Jpn. 1993, 66, 1822–1825. [Google Scholar] [CrossRef]
- Dhara, S.C. A Rapid Method for the Synthesis of Cis-[Pt(NH3)2Cl2]. Indian J. Chem. 1970, 8, 193–194. [Google Scholar]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A Subset of Platinum-Containing Chemotherapeutic Agents Kills Cells by Inducing Ribosome Biogenesis Stress. Nat. Med. 2017, 23, 461–471. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- O’Dowd, P.D.; Sutcliffe, D.F.; Griffith, D.M. Oxaliplatin and Its Derivatives—An Overview. Coord. Chem. Rev. 2023, 497, 215439. [Google Scholar] [CrossRef]
- Reedijk, J. Platinum Anticancer Coordination Compounds: Study of DNA Binding Inspires New Drug Design. Eur. J. Inorg. Chem. 2009, 2009, 1303–1312. [Google Scholar] [CrossRef]
- Feltham, R.D.; Hayter, R.G. 875. The Electrolyte Type of Ionized Complexes. J. Chem. Soc. 1964, 4587–4591. [Google Scholar] [CrossRef]

| Adduct | G sx/dx | cis-1,3-DACH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H8 | CH2a | CH3b | H1′ | H2′ | H3′ | H4′ | H5′ | H5″ | H1/3 | H2ax | H2eq | H5ax | H5eq | H4/6 | |
| 9-EtG | 8.21 | 4.10 | 1.38 | - | - | - | - | - | - | 2.99 | 1.87 | 2.10 | 4.49 | 1.95 | 1.81 |
| Guanosine | 8.44 | - | - | 5.89 | 4.60 | 4.33 | 4.23 | 3.84 | 3.77 | 2.97 | 1.80 | 2.08 | 4.46 | 1.92 | 1.74 |
| 5′GMP | 8.52 | - | - | 5.92 | 4.67 | 4.47 | 4.34 | 4.13 | 4.13 | 2.97 | 1.84 | 2.07 | 4.44 | 1.91 | 1.73 |
| 8.50 | - | - | 5.90 | 4.62 | 4.41 | 4.34 | 4.13 | 4.13 | |||||||
| 3′GMP | 8.49 | - | - | 5.94 | 4.64 | 4.37 | 3.93 | 3.84 | 3.84 | 2.98 | 1.83 | 2.08 | 4.51 | 1.94 | 1.75 |
| 8.47 | - | - | 5.94 | 4.67 | 4.37 | 3.91 | 3.86 | 3.86 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbanente, A.; Papadia, P.; Di Cosola, A.M.; Pacifico, C.; Natile, G.; Hoeschele, J.D.; Margiotta, N. Interactions with DNA Models of the Oxaliplatin Analog (cis-1,3-DACH)PtCl2 . Int. J. Mol. Sci. 2024, 25, 7392. https://doi.org/10.3390/ijms25137392
Barbanente A, Papadia P, Di Cosola AM, Pacifico C, Natile G, Hoeschele JD, Margiotta N. Interactions with DNA Models of the Oxaliplatin Analog (cis-1,3-DACH)PtCl2 . International Journal of Molecular Sciences. 2024; 25(13):7392. https://doi.org/10.3390/ijms25137392
Chicago/Turabian StyleBarbanente, Alessandra, Paride Papadia, Anna Maria Di Cosola, Concetta Pacifico, Giovanni Natile, James D. Hoeschele, and Nicola Margiotta. 2024. "Interactions with DNA Models of the Oxaliplatin Analog (cis-1,3-DACH)PtCl2 " International Journal of Molecular Sciences 25, no. 13: 7392. https://doi.org/10.3390/ijms25137392
APA StyleBarbanente, A., Papadia, P., Di Cosola, A. M., Pacifico, C., Natile, G., Hoeschele, J. D., & Margiotta, N. (2024). Interactions with DNA Models of the Oxaliplatin Analog (cis-1,3-DACH)PtCl2 . International Journal of Molecular Sciences, 25(13), 7392. https://doi.org/10.3390/ijms25137392









