Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico—Chemical Properties
Abstract
:1. Introduction
2. Results and Discussion
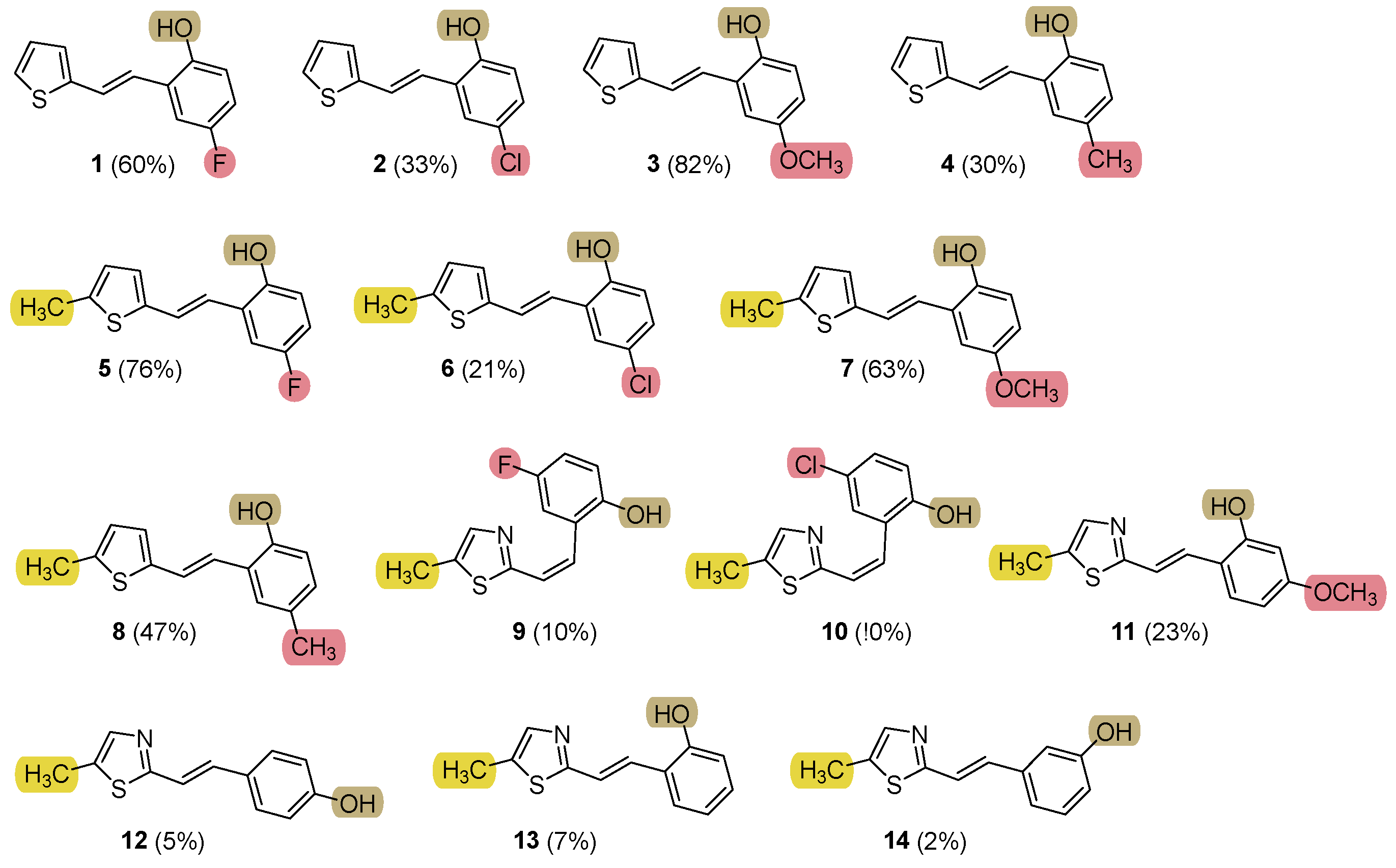
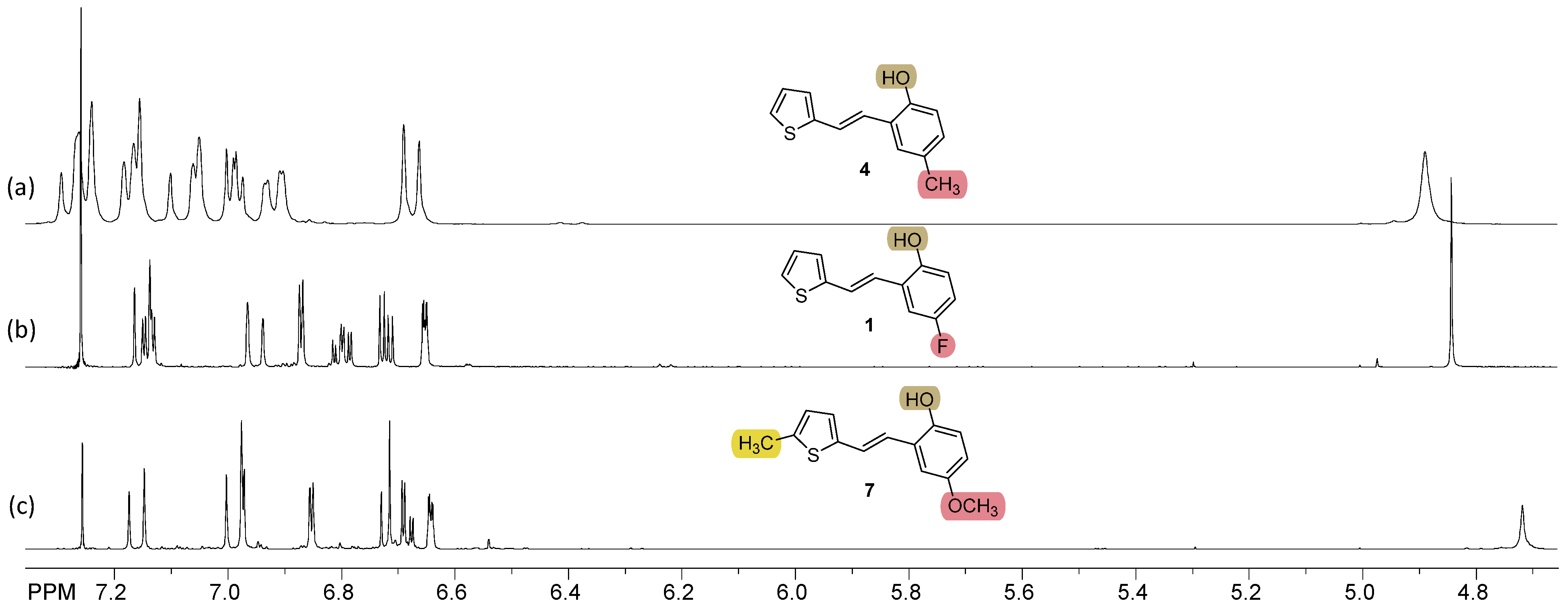
2.1. Synthesis and Characterization of New Heteroaromatic Resveratrol Analogs 1–14
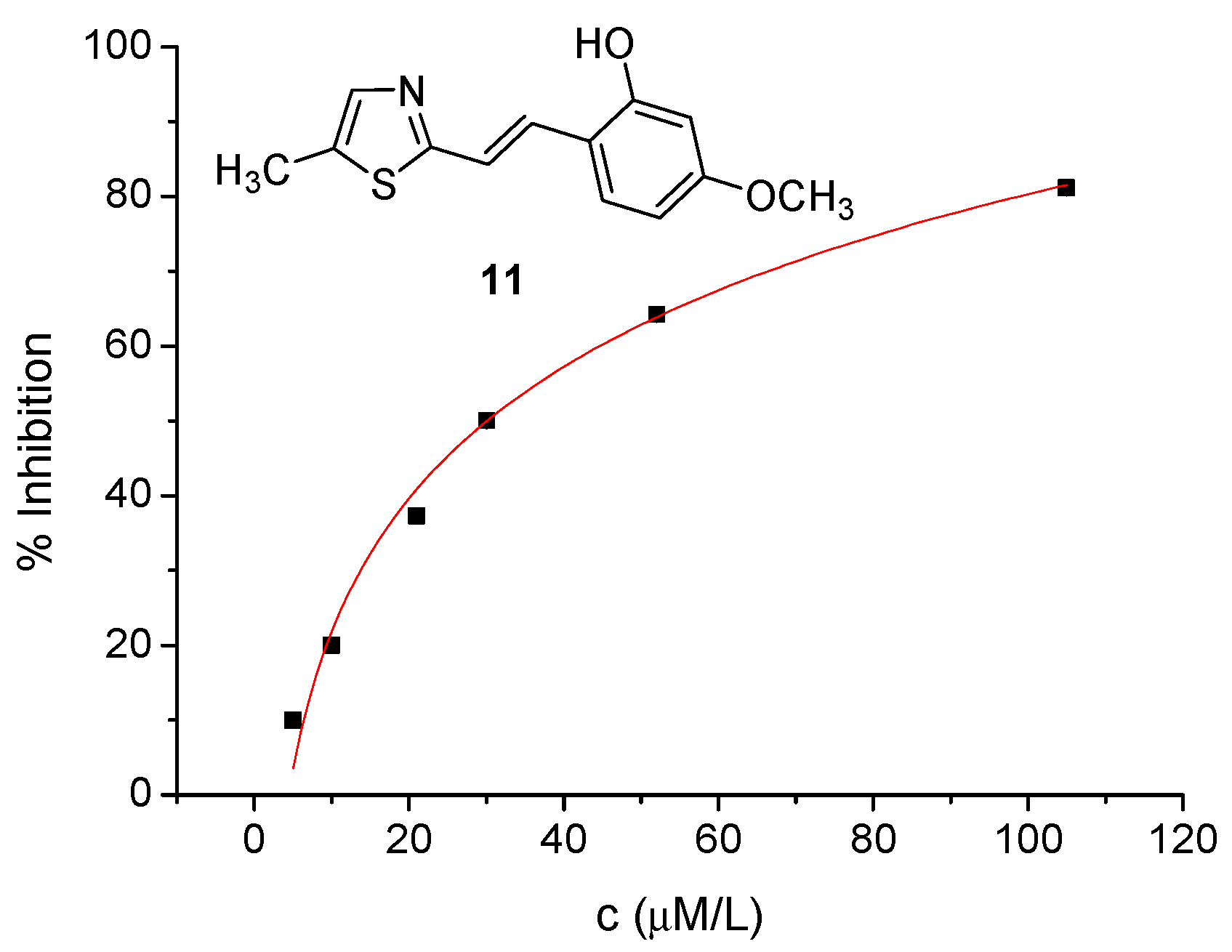
2.2. Cholinesterase Inhibition and Antioxidative Potential of Resveratrol Analogs 1–14
2.3. ADME Properties of Resveratrol Analogs 1–14
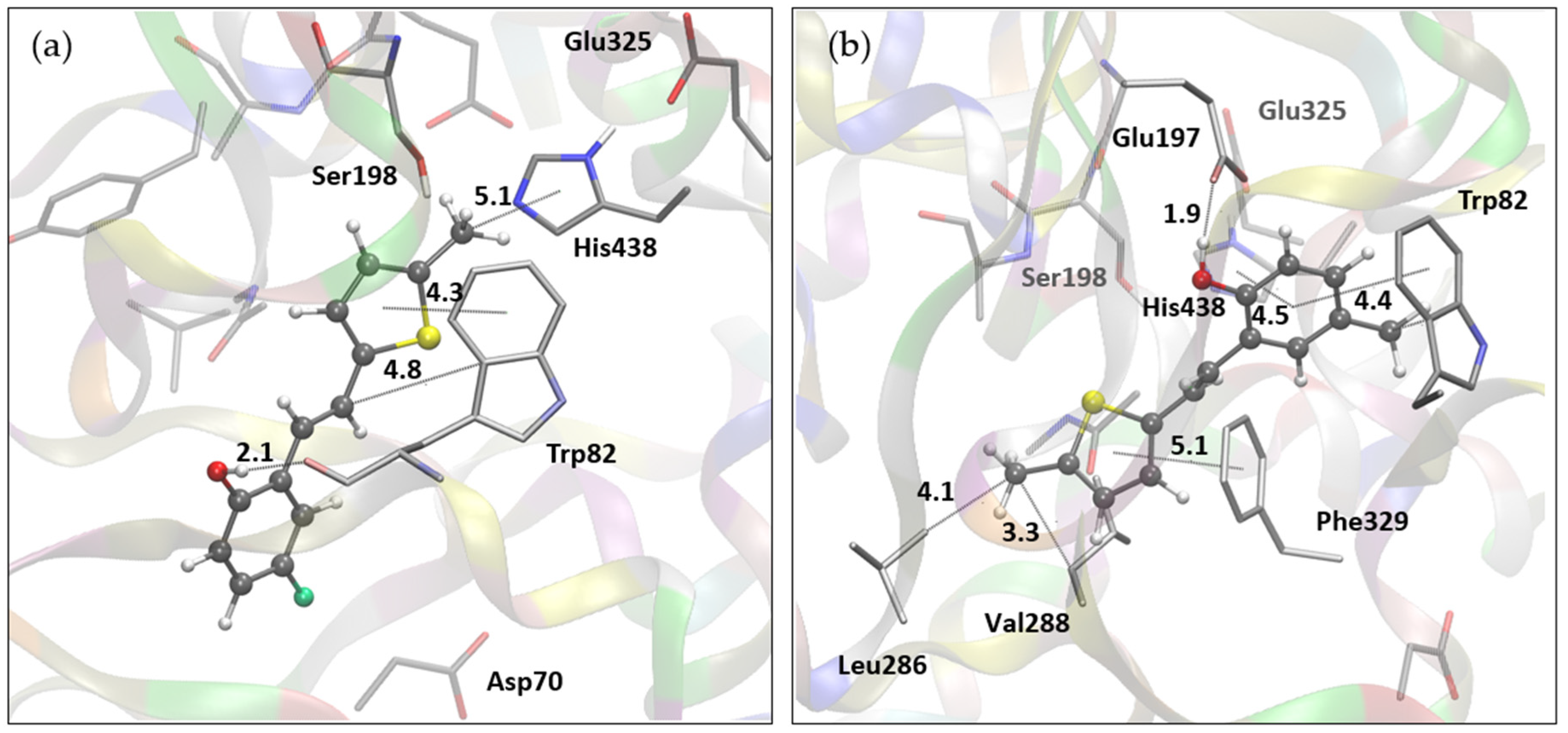
2.4. Molecular Docking Study of Biologically Active Resveratrol Analogs
2.5. Genotoxicity of 1–14
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Phosphonium Salts 1′–3′
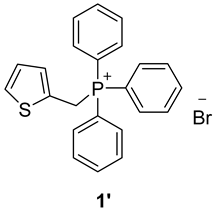
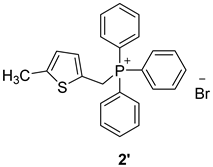

3.3. Synthesis of New Thienostilbenes 1–8
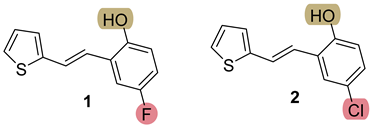
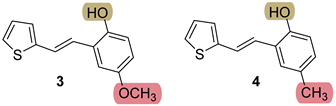
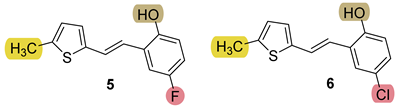

3.4. Synthesis of New Thiazolostilbenes 9–14

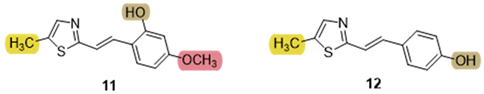
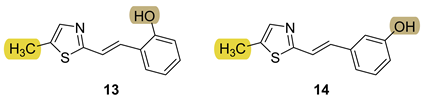
3.5. Cholinesterase Inhibition and Antioxidative Potential
3.5.1. In Vitro ChE Activity Assay
3.5.2. Antioxidative Potential
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasa, M.; Saeeda, F.; Anjuma, F.M.; Afzaala, M.; Tufaila, T.; Bashirb, M.S.; Ishtiaqb, A.; Hussainc, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 72747. [Google Scholar] [CrossRef]
- Aluko, R.E. Functional Foods and Nutraceuticals; Food Science Text Series; Springer Publishers: New York, NY, USA, 2012. [Google Scholar]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The use of stilbene scaffold in medicinal chemistry and multi-target drug design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Belwal, T.; Nabavi, S.M.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S. Naturally Occurring Chemicals against Alzheimer’s Disease; Academic: London, UK, 2020. [Google Scholar]
- Malinowska, M.A.; Sharafan, M.; Lanoue, A.; Ferrier, M.; Hano, C.; Giglioli-Guivarch, N.; Dziki, A.; Sikora, E.; Szopa, A. Trans-Resveratrol as a Health Beneficial Molecule: Activity, Sources, and Methods of Analysis. Sci. Rad. 2023, 2, 268–294. [Google Scholar] [CrossRef]
- Fornara, V.; Onelli, E.; Sparvoli, F.; Rossoni, M.; Aina, R.; Marino, G.; Citterio, S. Localization of stilbene synthase in Vitis vinifera L. during berry development. Protoplasma 2008, 233, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.E.; Santana, A.C.F.; MagalhÃes, G.J.P. Resveratrol in Alzheimer’s disease: A review of pathophysiology and therapeutic potential. Arq. Neuropsiquiatr. 2020, 78, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gomez Silva, C.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martínez, C.; Canle, L.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef]
- Richardson, F.S.; Riehl, J.P. Circularly polarized luminescence spectroscopy. Chem. Rev. 1977, 77, 773–792. [Google Scholar] [CrossRef]
- Jang, J.H.; Surh, Y.J. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 2003, 34, 1100–1110. [Google Scholar] [CrossRef]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005. [Google Scholar]
- Galano, A.; Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Alarcón-Ángeles, G.; Rojas-Hernández, A. Role of the reacting free radicals on the antioxidant mechanism of curcumin. Chem. Phys. 2009, 363, 13–23. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Thimmappa, S.A. Resveratrol—A boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Freskgård, P.O.; Urich, E. Antibody therapies in CNS diseases. Neuropharmacology 2017, 120, 38–55. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008, 9, 6. [Google Scholar] [CrossRef]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. BioFactors 2018, 44, 83–90. [Google Scholar] [CrossRef]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Yuan, W.; Shang, Z.; Qiang, X.; Tan, Z.; Deng, Y. Synthesis of pterostilbene and resveratrol carbamate derivatives as potential dual cholinesterase inhibitors and neuroprotective agents. Res. Chem. Intermed. 2014, 40, 787–800. [Google Scholar] [CrossRef]
- Kohandel, Z.; Darrudi, M.; Naseri, K.; Samini, F.; Aschner, M.; Pourbagher-Shahri, A.-M.; Samarghandian, S. The Role of Resveratrol in aging and aenescence: A focus on molecular mechanisms. Curr. Mol. Med. 2024, 24, 867–875. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Brain targeting of resveratrol through intranasal lipid vesicles labelled with gold nanoparticles: In vivo evaluation and bioaccumulation investigation using computed tomography and histopathological examination. J. Drug Target 2019, 27, 1127–1134. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Landgraf Guiguer, E.; Cressoni Araújo, A.; Buchaim, R.L.; de Alvares Goulart, R.; Rubira, C.J.; Barbalho, S.M. The role of resveratrol in mild cognitive impairment and Alzheimer’s disease: A systematic review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Khan, M.M.U.; Singh, H.; Verma, A. Resveratrol: A potential therapeutic natural polyphenol for neurodegenerative diseases associated with mitochondrial dysfunction. Front. Pharmacol. 2022, 13, 922232. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Fairuz Yahaya, M.; Tooyama, I.; Damanhuri, H.A. A mechanistic evaluation of antioxidant nutraceuticals on their potential against age-associated neurodegenerative diseases. Antioxidants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. The effect of resveratrol on neurodegenerative disorders: Possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr. Pharm. Des. 2019, 25, 2178–2191. [Google Scholar] [CrossRef]
- Vasanthi, C.; Sureshkumar, R. Neuroprotection by resveratrol: A review on brain delivery strategies for Alzheimer’s and Parkinson’s disease. J. Appl. Pharm. Sci. 2022, 12, 1–17. [Google Scholar]
- Socała, K.; Żmudzka, E.; Lustyk, K.; Zagaja, M.; Brighenti, V.; Costa, A.M.; Andres-Mach, M.; Pytka, K.; Martinelli, I.; Mandrioli, J.; et al. Therapeutic potential of stilbenes in neuropsychiatric and neurological disorders: A comprehensive review of preclinical and clinical evidence. Phytother. Res. 2024, 38, 1400–1461. [Google Scholar] [CrossRef]
- Mlakić, M.; Đurčević, E.; Odak, I.; Barić, D.; Juričević, I.; Šagud, I.; Burčul, F.; Lasić, Z.; Marinić, Ž.; Škorić, I. Thieno-thiazolostilbenes, thienobenzo-thiazoles, and naphtho-oxazoles: Computational study and cholinesterase inhibitory activity. Molecules 2023, 28, 3781. [Google Scholar] [CrossRef]
- Mlakić, M.; Fodor, L.; Odak, I.; Horváth, O.; Lovrić, M.J.; Barić, D.; Milašinović, V.; Molčanov, K.; Marinić, Ž.; Lasić, Z.; et al. Resveratrol-maltol and resveratrol-thiophene hybrids as cholinesterase inhibitors and antioxidants: Synthesis, biometal chelating capability and crystal structure. Molecules 2022, 27, 6379. [Google Scholar] [CrossRef]
- Mlakić, M.; Rajić, L.; Ljubić, A.; Vušak, V.; Zelić, B.; Gojun, M.; Odak, I.; Čule, I.; Šagud, I.; Šalić, A.; et al. Synthesis of new heterocyclic resveratrol analogues in milli- and microreactors: Intensification of the Wittig reaction. J. Flow Chem. 2022, 12, 429–440. [Google Scholar] [CrossRef]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur. J. Med. Chem. 2022, 241, 114616. [Google Scholar] [CrossRef]
- Mlakić, M.; Faraho, I.; Odak, I.; Talić, S.; Vukovinski, A.; Raspudić, A.; Bosnar, M.; Zadravec, R.; Ratković, A.; Lasić, K.; et al. Synthesis, photochemistry and computational study of novel 1,2,3-triazole heterostilbenes: Expressed biological activity of their electrocyclization photoproducts. Bioorg. Chem. 2022, 121, 105701. [Google Scholar] [CrossRef]
- Modrić, M.; Božičević, M.; Faraho, I.; Bosnar, M.; Škorić, I. Design, synthesis and biological evaluation of new 1,3-thiazole derivatives as potential anti-inflammatory agents. J. Mol. Struct. 2021, 1239, 130526. [Google Scholar] [CrossRef]
- Šagud, I.; Maček Hrvat, N.; Grgičević, A.; Čadež, T.; Hodak, J.; Dragojević, M.; Lasić, K.; Kovarik, Z.; Škorić, I. Design, synthesis and cholinesterase inhibitory properties of new oxazole benzylamine derivatives. J. Enzyme Inhib. Med. Chem. 2020, 35, 460–467. [Google Scholar] [CrossRef]
- Mlakić, M.; Faraho, I.; Odak, I.; Kovačević, B.; Raspudić, A.; Šagud, I.; Bosnar, M.; Škorić, I.; Barić, D. Cholinesterase inhibitory and anti-inflammatory activity of the naphtho- and thienobenzo-triazole photoproducts: Experimental and computational study. Int. J. Mol. Sci. 2023, 24, 14676. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Selec, I.; Ćaleta, I.; Odak, I.; Barić, D.; Ratković, A.; Molčanov, K.; Škorić, I. New thienobenzo/naphtho-triazoles as butyrylcholinesterase inhibitors. Design, synthesis and computational study. Int. J. Mol. Sci. 2023, 24, 5879. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Odak, I.; Faraho, I.; Bosnar, M.; Banjanac, M.; Lasić, Z.; Marinić, Ž.; Barić, D.; Škorić, I. Synthesis, photochemistry, computational study and potential application of new styryl-thiophene and naphtho-thiophene benzylamines. Int. J. Mol. Sci. 2023, 24, 610. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Odak, I.; Barić, D.; Talić, S.; Šagud, I.; Štefanić, Z.; Molčanov, K.; Lasić, Z.; Kovačević, B.; Škorić, I. New resveratrol analogs as improved biologically active structures: Design, synthesis and computational modeling. Bioorg. Chem. 2024, 143, 106965. [Google Scholar] [CrossRef] [PubMed]
- Kikaš, I.; Horváth, O.; Škorić, I. Functionalization of the benzobicyclo [3.2.1]octadiene skeleton via photocatalytic and thermal oxygenation of a furan derivative. Tetrahedron Lett. 2011, 52, 6255–6259. [Google Scholar] [CrossRef]
- Fauconneau, B.; Waffo-Teguo, P.; Huguet, F.; Barrier, L.; Decendit, A.; Merillon, J.M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997, 61, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Gulcin, I.; Saleh, H.A. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Vrbanac, J.; Slauter, R. ADME in Drug Discovery. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development, 3rd ed.; Faqi, A.S., Ed.; Academic Press: Detroit, MI, USA, 2017; pp. 39–67. [Google Scholar]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Cl–π interactions in protein–ligand complexes. Protein Sci. 2008, 17, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Ishola, A.A.; Oyinloye, B.E.; Ajiboye, B.O.; Kappo, A.P. Molecular Docking Studies of Flavonoids from Andrographis paniculata as Potential Acetylcholinesterase, Butyrylcholinesterase and Monoamine Oxidase Inhibitors towards the Treatment of Neuro-degenerative Diseases. Biointerface Res. Appl. Chem. 2021, 11, 9871–9879. [Google Scholar]
- Junaid, M.; Islam, N.; Hossain, M.K.; Ullah, M.O.; Halim, M.A. Metal based donepezil analogues designed to inhibit human acetylcholinesterase for Alzheimer’s disease. PLoS ONE 2019, 14, e0211935. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Kiametis., A.S.; Treptow, W. Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature. J. Chem. Inf. Model. 2020, 60, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, C.; Bercu, J.; Cayley, A.; Cross, K.; Glowienke, S.; Kruhlak, N.; Muster, W.; Nicolette, J.; Vijayaraj Reddy, M.; Saiakhov, R.; et al. Management of pharmaceutical ICH M7 (Q)SAR predictions—The impact of model updates. Regul. Toxicol. Pharmacol. 2020, 118, 104807. [Google Scholar] [CrossRef]
- European Medicines Agency Science Medicines Health. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-s2-r1-genotoxicity-testing-and-data-interpretation-pharmaceuticals-intended-human-use-step-5_en.pdf (accessed on 19 June 2024).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, Using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock-823 Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.; Burshteyn, F.; Cassidy, M.; Gary, E.; Love, J.; Height, J.; Franklin, M. Crystal Structure of Recombinant Human Acetylcholinesterase in Complex with Donepezil. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
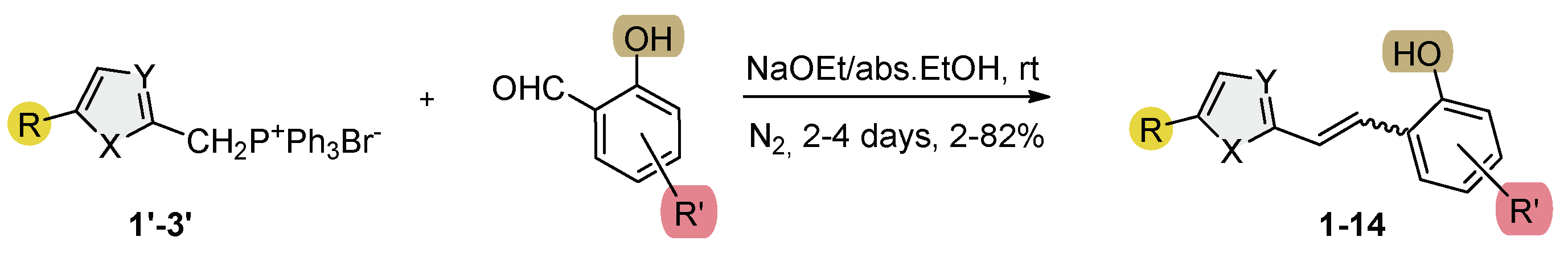




| Compound | eeAChE | eqBChE | DPPH | CUPRAC | |||
|---|---|---|---|---|---|---|---|
| IC50 (μM) | Inhibition ** (%) | IC50 (μM) | Inhibition ** (%) | IC50 (μM) | Inhibition ** (%) | mol TE/mol of Compound | |
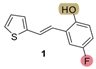 | 177.7 | 80.9 ± 1.9 (500) | 66.0 | 83.5 ± 0.8 (400) | 159.2 | 77.2 ± 0.5 (500) | 0.925 |
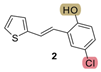 | 82.3 | 83.0 ± 2.5 (250) | 53.0 | 80.4 ± 6.4 (200) | 265.1 | 65.0 ± 2.4 (500) | 0.713 |
 | 404.6 | 77.8 ± 5.2 (500) | 206.4 | 68.5 ± 6.2 (500) | 36.2 | 87.7 ± 0.8 (500) | 1.111 |
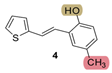 | 225.0 | 82.1 ± 1.1 (250) | 36.5 | 81.1 ± 2.2 (250) | 51.4 | 90.3 ± 0.6 (400) | 0.852 |
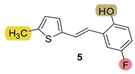 | 154.9 | 84.6 ± 2.7 (250) | 22.9 | 75.3 ± 2.3 (100) | 130.3 | 83.0 ± 1.9 (400) | 0.204 |
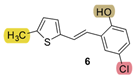 | 27.1 | 82.4 ± 0.8 (80) | 39.7 | 84.0 ± 1.8 (100) | - * | 42.9 ± 2.7 (400) | 1.407 |
 | 62.9 | 81.1 ± 1.1 (200) | 42.5 | 83.1 ± 0.8 (200) | 23.8 | 86.2 ± 2.3 (50) | 2.350 |
 | 92.4 | 78.2 ± 7.7 (150) | 24.8 | 88.5 ± 5.0 (250) | 26.3 | 89.3 ± 1.2 (250) | 1.148 |
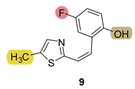 | - * | 17.6 ± 3.1 (100) | - * | 10.5 ± 4.1 (250) | 428.4 | 55.4 ± 0.7 (500) | 1.000 |
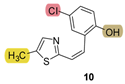 | - * | 34.2 ± 2.1 (500) | - * | 22.4 ± 9.0 (500) | - * | 24.0 ± 0.7 (500) | 0.630 |
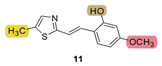 | - * | 29.3 ± 1.8 (500) | - * | 9.8 ± 2.7 (500) | 30.1 | 89.8 ± 0.0 (524) | n.d. *** |
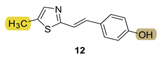 | - * | 9.1 ± 1.4 (500) | 189.5 | 68.4 ± 1.3 (500) | 139.8 | 74.1 ± 0.4 (524) | n.d. *** |
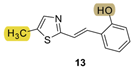 | - * | 17.1 ± 3.9 (500) | 30.7 | 78.2 ± 3.8 (500) | 699.2 | 59.8 ± 2.3 (1047) | n.d. *** |
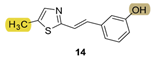 | - * | 10.7 ± 2.4 (250) | - * | 15.6 ± 3.6 (250) | - * | 11.2 ± 2.7 (250) | n.d. *** |
| Resveratrol | n.d. *** | n.d. *** | 74.0 [48] | n.d. *** | |||
| Galantamine | 0.15 [48] | 7.9 [46] | n.d. *** | n.d. *** | |||
| Compound 5 | Compound 6 | Compound 7 | Compound 8 | |||
|---|---|---|---|---|---|---|
| Property | Property Measure | Predicted Value | Unit | |||
| Absorption | Water solubility | −4.558 | −4.938 | −4.511 | −4.592 | log mol/L |
| Caco2 permeability | 1.338 | 1.497 | 1.348 | 1.489 | log Papp in 10−6 cm/s | |
| Intestinal absorption (human) | 91.111 | 90.018 | 92.389 | 91.477 | % Absorbed | |
| Skin Permeability | −2.046 | −1.9 | −2.109 | −1.87 | log Kp | |
| P-glycoprotein substrate | Yes | No | No | No | ||
| P-glycoprotein I inhibitor | No | No | No | No | ||
| P-glycoprotein II inhibitor | No | No | No | No | ||
| Distribution | VDss (human) | 0.387 | 0.686 | 0.453 | 0.726 | log L/kg |
| Fraction unbound (human) | 0.035 | 0.013 | 0.024 | 0.026 | Fu | |
| BBB permeability | 0.345 | 0.294 | 0.315 | 0.344 | log BB | |
| CNS permeability | −1.626 | −1.565 | −1.69 | −1.565 | log PS | |
| Metabolism | CYP2D6 substrate | No | No | No | No | |
| CYP3A4 substrate | Yes | Yes | Yes | Yes | ||
| CYP1A2 inhibitior | Yes | Yes | Yes | Yes | ||
| CYP2C19 inhibitior | Yes | Yes | Yes | Yes | ||
| CYP2C9 inhibitior | No | Yes | No | Yes | ||
| CYP2D6 inhibitior | No | No | No | No | ||
| CYP3A4 inhibitior | No | No | No | No | ||
| Excretion | Total Clearance | −0.146 | −0.126 | 0.035 | −0.109 | log mL/min/kg |
| Renal OCT2 substrate | No | No | No | No | ||
| Toxicity | AMES toxicity | No | No | No | No | |
| Max. tolerated dose (human) | 0.574 | 0.639 | 0.613 | 0.647 | log mg/kg/day | |
| hERG I inhibitor | No | No | No | No | ||
| hERG II inhibitor | No | No | No | No | ||
| Oral Rat Acute Toxicity (LD50) | 2.376 | 2.421 | 2.407 | 2.235 | mol/kg | |
| Oral Rat Chronic Toxicity (LOAEL) | 2.167 | 1.116 | 2.204 | 1.165 | log mg/kg_bw/day | |
| Hepatotoxicity | No | Yes | No | Yes | ||
| Skin Sensitisation | No | Yes | No | Yes | ||
| T.Pyriformis toxicity | 1.738 | 2.383 | 1.868 | 2.143 | log ug/L | |
| Minnow toxicity | 0.334 | 0.126 | 0.177 | 0.344 | ||
| Structure | ICH M7 Class | Derek Prediction | Sarah Prediction | Overall In Silico |
|---|---|---|---|---|
| 1 | Class 5 |  |  | Negative |
| 2 | Class 3 |  |  | Positive |
| 3 | Class 5 |  |  | Negative |
| 4 | Class 5 |  |  | Negative |
| 5 | Class 5 |  |  | Negative |
| 6 | Class 3 |  |  | Positive |
| 7 | Class 5 |  |  | Negative |
| 8 | Class 5 |  |  | Negative |
| 9 | Class 5 |  |  | Negative |
| 10 | Class 3 |  |  | Positive |
| 11 | Class 5 |  |  | Negative |
| 12 | Class 5 |  |  | Negative |
| 13 | Class 5 |  |  | Negative |
| 14 | Class 5 |  |  | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlakić, M.; Talić, S.; Odak, I.; Barić, D.; Šagud, I.; Škorić, I. Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico—Chemical Properties. Int. J. Mol. Sci. 2024, 25, 7401. https://doi.org/10.3390/ijms25137401
Mlakić M, Talić S, Odak I, Barić D, Šagud I, Škorić I. Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico—Chemical Properties. International Journal of Molecular Sciences. 2024; 25(13):7401. https://doi.org/10.3390/ijms25137401
Chicago/Turabian StyleMlakić, Milena, Stanislava Talić, Ilijana Odak, Danijela Barić, Ivana Šagud, and Irena Škorić. 2024. "Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico—Chemical Properties" International Journal of Molecular Sciences 25, no. 13: 7401. https://doi.org/10.3390/ijms25137401






