Association of Perinatal Cardiovascular Features with Angiotensin System Expressions in Maternal Preeclampsia
Abstract
1. Introduction
2. Results
2.1. Cardiovascular Features in the Studied Groups
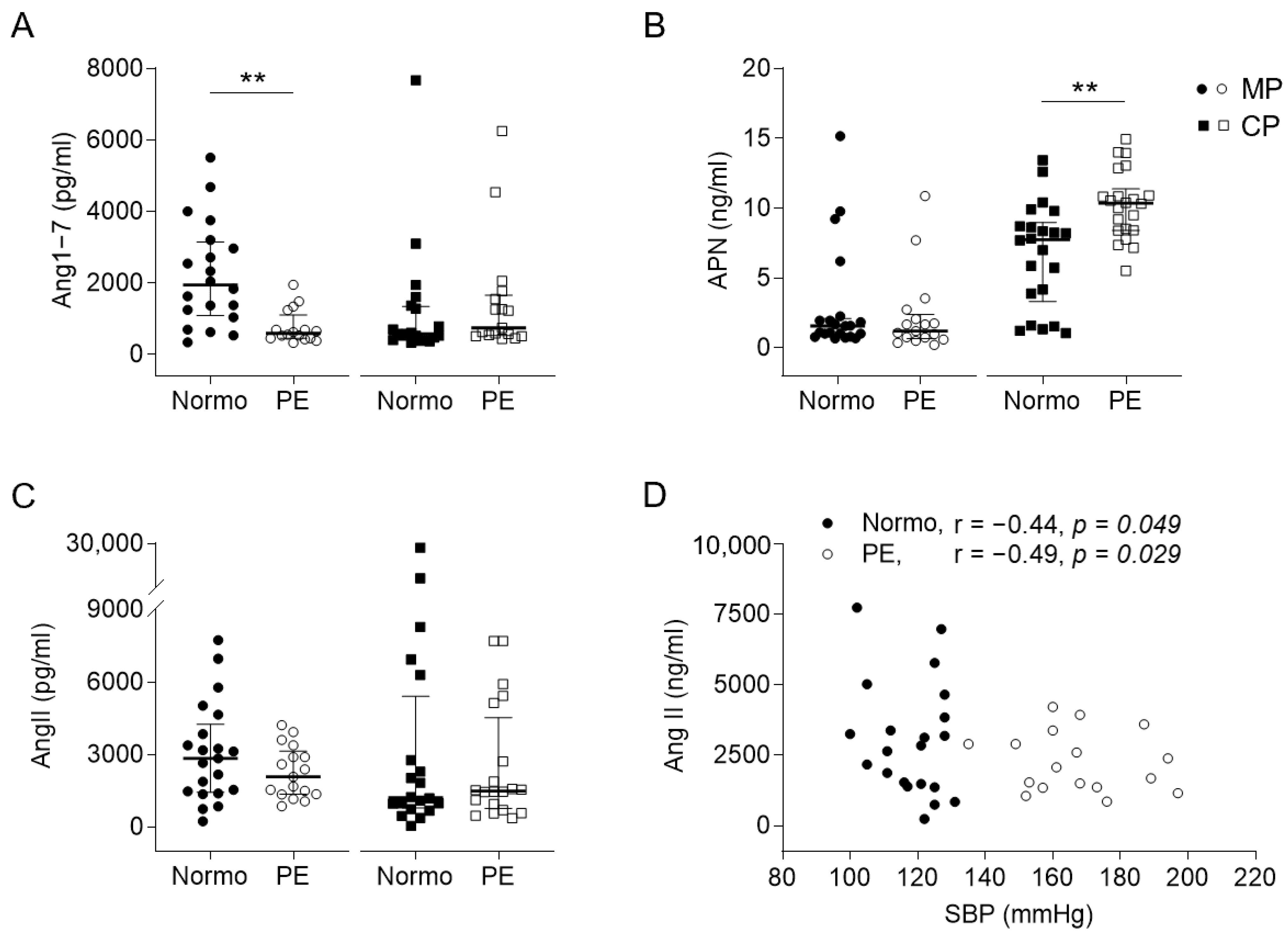
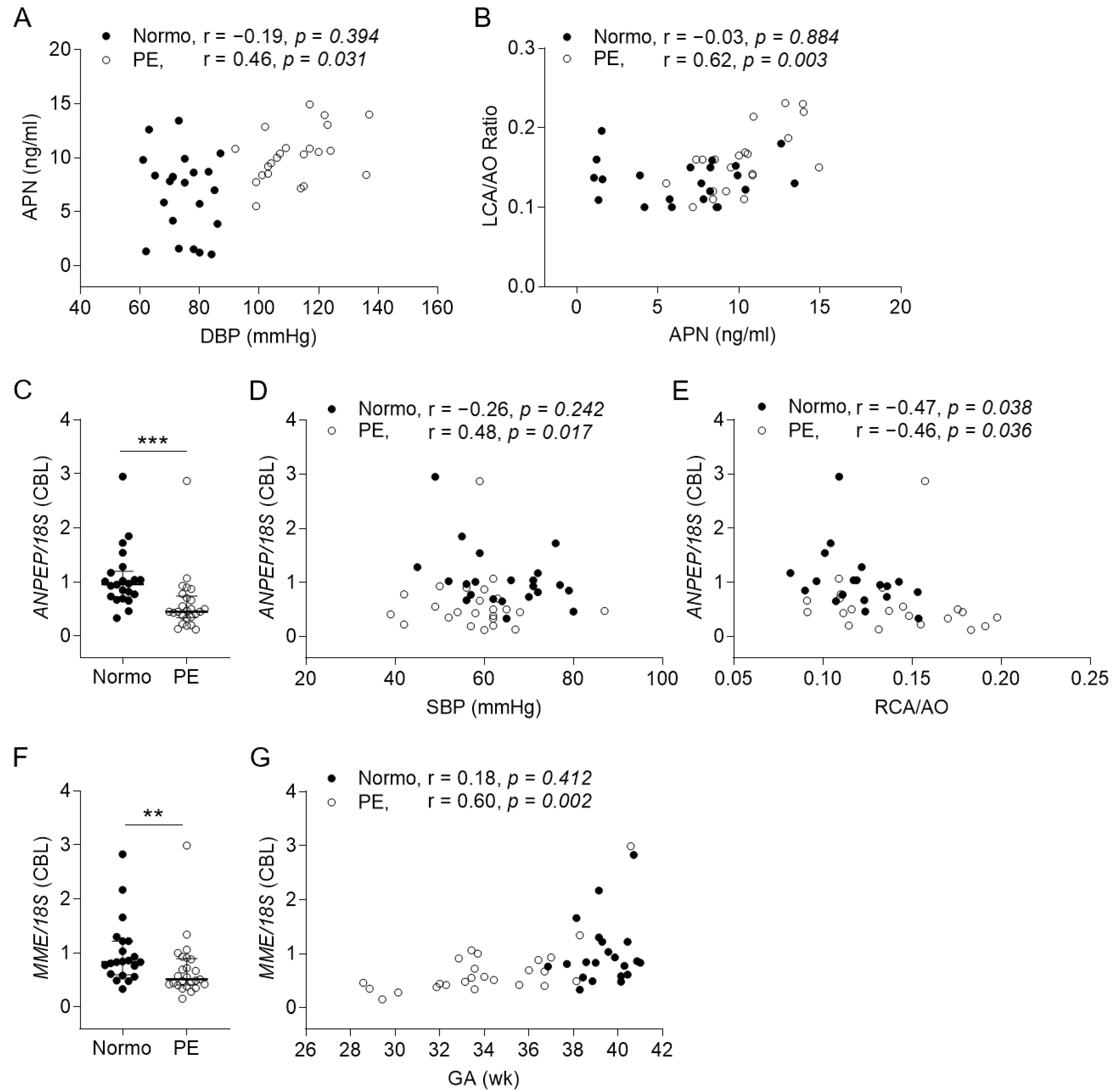
2.2. Expressions and Correlations of Angiotensin-Related Molecules to Perinatal Cardiovascular Features
2.3. In Vitro Effect of MP and CP on Endothelial Cells
2.4. Local Expression of Angiotensin Receptors and Function of Umbilical Artery
2.5. Independent Factor(s) of Perinatal Cardiovascular Features under Multivariable Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects, Sampling, and Cardiovascular Measurements
4.2. Measurements of Angiotensin-Related Molecules
4.3. Cells, Tissues, and Real-Time RT-PCR
4.4. Protein Extraction and Western Blot
4.5. Immunohistochemistry
4.6. Isometric Tension Measurement
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgins, S. Pre-eclampsia as Underlying Cause for Perinatal Deaths: Time for Action. Glob. Health Sci. Pract. 2015, 3, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, V.; Scherjon, S.A.; Plosch, T. Preeclampsia As Modulator of Offspring Health. Biol. Reprod. 2016, 94, 53. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W. Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation 2014, 129, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Baker, P.N.; Granger, J.P.; Davidge, S.T.; Tong, C. Long-Term Impacts of Preeclampsia on the Cardiovascular System of Mother and Offspring. Hypertension 2023, 80, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.J.; Fraser, A.; Tilling, K.; Benfield, L.; Davey Smith, G.; Sattar, N.; Nelson, S.M.; Lawlor, D.A. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: The Avon Longitudinal Study of Parents and Children. Circulation 2010, 122, 1192–1199. [Google Scholar] [CrossRef]
- Visentin, S.; Londero, A.P.; Bellamio, B.; Giunta, G.; Cosma, C.; Faggian, D.; Plebani, M.; Cosmi, E. Fetal Endothelial Remodeling in Late-Onset Gestational Hypertension. Am. J. Hypertens. 2016, 29, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Z.; Aye, C.Y.; Lewandowski, A.J.; Davis, E.F.; Khoo, C.P.; Newton, L.; Yang, C.T.; Al Haj Zen, A.; Simpson, L.J.; O’Brien, K.; et al. Association of Maternal Antiangiogenic Profile at Birth with Early Postnatal Loss of Microvascular Density in Offspring of Hypertensive Pregnancies. Hypertension 2016, 68, 749–759. [Google Scholar] [CrossRef]
- Lin, I.C.; Hsu, T.Y.; Tain, Y.L.; Tsai, C.C.; Huang, H.C.; Lai, Y.J.; Chou, M.H.; Huang, C.F.; Yu, H.R.; Huang, L.T. Coronary Dilatation and Endothelial Inflammation in Neonates Born to Mothers with Preeclampsia. J. Pediatr. 2021, 228, 58–65.e53. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Chang, H.; Zhang, S.; Yang, L.; Wang, X.; Cheng, X.; Zhang, M.; Ma, X.L.; Liu, H. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J. Hypertens. 2008, 26, 1629–1635. [Google Scholar] [CrossRef]
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Matsubara, Y.; Hyodo, S.; Katayama, T.; Ito, M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J. Obstet. Gynaecol. Res. 2010, 36, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.H.; Fathalla, M.M.; Mekki, A.R.; Elsadek Bel, K. Implication of umbilical cord in preeclampsia. Med. Princ. Pract. 2011, 20, 124–128. [Google Scholar] [CrossRef]
- Weber, M.A. Interrupting the renin-angiotensin system: The role of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of hypertension. Am. J. Hypertens. 1999, 12, 189S–194S. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Burke, S.D.; Zsengeller, Z.K.; Khankin, E.V.; Lo, A.S.; Rajakumar, A.; DuPont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Investig. 2016, 126, 2561–2574. [Google Scholar] [CrossRef]
- Singh, S.; Moodley, J.; Naicker, T. Differential expression of the angiotensin receptors (AT1, AT2, and AT4) in the placental bed of HIV-infected preeclamptic women of African ancestry. Hypertens. Res. 2023, 46, 1970–1982. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Ebrahimian, T.; He, Y.; Gratton, J.P.; Schiffrin, E.L.; Touyz, R.M. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J. Hypertens. 2006, 24, 2417–2422. [Google Scholar] [CrossRef]
- Kaschina, E.; Namsolleck, P.; Unger, T. AT2 receptors in cardiovascular and renal diseases. Pharmacol. Res. 2017, 125, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Nonn, O.; Fischer, C.; Geisberger, S.; El-Heliebi, A.; Kroneis, T.; Forstner, D.; Desoye, G.; Staff, A.C.; Sugulle, M.; Dechend, R.; et al. Maternal Angiotensin Increases Placental Leptin in Early Gestation via an Alternative Renin-Angiotensin System Pathway: Suggesting a Link to Preeclampsia. Hypertension 2021, 77, 1723–1736. [Google Scholar] [CrossRef]
- Williams, P.J.; Mistry, H.D.; Innes, B.A.; Bulmer, J.N.; Broughton Pipkin, F. Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta 2010, 31, 448–455. [Google Scholar] [CrossRef]
- Yoshihara, M.; Mizutani, S.; Matsumoto, K.; Kato, Y.; Masuo, Y.; Tano, S.; Mizutani, H.; Kotani, T.; Mizutani, E.; Shibata, K.; et al. Crosstalk between foetal vasoactive peptide hormones and placental aminopeptidases regulates placental blood flow: Its significance in preeclampsia. Placenta 2022, 121, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Wangesteen, R.; Rodriguez-Gomez, I.; Garcia-Estan, J. Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers. Int. J. Mol. Sci. 2020, 21, 5615. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier/AP: Amsterdam, The Netherlands, 2013; p. 3. 3932p. [Google Scholar]
- Sartori, C.; Rimoldi, S.F.; Duplain, H.; Stuber, T.; Garcin, S.; Rexhaj, E.; Allemann, Y.; Scherrer, U. Developmental Origins of Hypoxic Pulmonary Hypertension and Systemic Vascular Dysfunction: Evidence from Humans. Adv. Exp. Med. Biol. 2016, 903, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.Q.; Li, S.J.; Zhang, Q.; Cai, H.B.; Chen, H.P. Interactions between inflammatory and oxidative stress in preeclampsia. Hypertens. Pregnancy 2009, 28, 56–62. [Google Scholar] [CrossRef]
- Nascimento, M.I.D.; Cunha, A.A.; Netto, N.F.R.; Santos, R.A.D.; Barroso, R.R.; Alves, T.R.C.; Soares, W.E. COVID-19 and Preeclampsia: A Systematic Review of Pathophysiological Interactions. Rev. Bras. Ginecol. Obstet. 2023, 45, 347–355. [Google Scholar] [CrossRef]
- Tamanna, S.; Clifton, V.L.; Rae, K.; van Helden, D.F.; Lumbers, E.R.; Pringle, K.G. Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front. Physiol. 2020, 11, 590787. [Google Scholar] [CrossRef]
- Tamanna, S.; Lumbers, E.R.; Morosin, S.K.; Delforce, S.J.; Pringle, K.G. ACE2: A key modulator of the renin-angiotensin system and pregnancy. Am. J. Physiology.Regul. Integr. Comp. Physiol. 2021, 321, R833–R843. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Lin, I.C.; Sheen, J.M.; Tain, Y.L.; Chou, M.H.; Huang, L.T.; Yang, K.D. Vascular endothelial growth factor-a in lactobacillus casei cell wall extract-induced coronary arteritis of a murine model. Circ.J. Off. J. Jpn. Circ. Soc. 2014, 78, 752–762. [Google Scholar] [CrossRef]



| Normotension Group (n = 48) | Preeclampsia Group (n = 49) | p Value | |
|---|---|---|---|
| Maternal or gestational characteristics | |||
| Age (y) | 34.13 (30.69–36.23) | 34.83 (31.67–38.25) | 0.159 |
| BW (kg) | 70.00 (63.00–75.00) | 76.70 (67.50–86.65) | <0.001 ‡ |
| BH (cm) | 160.00 (155.00–163.00) | 158.00 (154.50–165.00) | 0.763 |
| BMI (kg/m2) | 26.72 (24.46–29.34) | 31.17 (27.02–33.87) | <0.001 ‡ |
| Gravidity | 1.50 (1.00–2.00) | 2.00 (1.00–3.00) | 0.052 |
| Parity | 1.00 (1.00–2.00) | 2.00 (1.00–2.00) | 0.062 |
| SBP (mmHg) | 120.50 (111.00–125.00) | 167.00 (154.00–185.50) | <0.001 ‡ |
| DBP (mmHg) | 73.00 (65.00–79.00) | 111.00 (103.00–119.00) | <0.001 ‡ |
| MBP (mmHg) | 88.50 (80.00–93.75) | 129.00 (120.50–139.50) | <0.001 ‡ |
| Severe PE, n (%) | 0 (0.00) | 42 (85.71) | N.A. |
| Essential HTN, n (%) | 0 (0.00) | 4 (8.16) | N.A. |
| Proteinuria, n (%) | 0 (0.00) | 47 (95.92) | N.A. |
| GA of PE (wk) | N.A. | 27.00 (21.50–31.25) | N.A. |
| Neonatal characteristics | |||
| Male, n (%) | 23 (47.92) | 19 (38.78) | 0.364 |
| GA (wk) | 39.14 (38.43–40.14) | 33.43 (31.72–35.22) | <0.001 ‡ |
| BBW (g) | 3100.00 (2873.75–3307.50) | 1500.00 (1080.00–1805.00) | <0.001 ‡ |
| BL (cm) | 49.75 (48.00–50.38) | 40.00 (36.50–42.50) | <0.001 ‡ |
| BHC (cm) | 33.00 (32.00–34.00) | 29.00 (26.50–31.00) | <0.001 ‡ |
| SGA, n (%) | 0 (0.00) | 17 (34.69) | <0.001 ‡ |
| Cesarean section, n (%) | 11 (22.92) | 46 (93.88) | <0.001 ‡ |
| Apgar score at 1 min | 9.00 (9.00–9.00) | 8.00 (6.00–9.00) | <0.001 ‡ |
| Apgar score at 5 min | 10.00 (10.00–10.00) | 10.00 (9.00–10.00) | <0.001 ‡ |
| Neonatal cardiovascular measurements | |||
| HR (bpm) | 139.50 (125.00–148.75) | 140.00 (123.50–155.50) | 0.751 |
| SBP (mmHg) | 62.50 (58.00–71.75) | 57.50 (50.50–63.00) | 0.002 † |
| DBP (mmHg) | 37.50 (31.25–44.75) | 29.50 (26.00–34.00) | <0.001 ‡ |
| MBP (mmHg) | 46.50 (42.00–53.00) | 38.50 (33.00–43.75) | <0.001 ‡ |
| LA (mm) | 12.00 (11.00–13.00) | 10.70 (9.15–11.90) | <0.001 ‡ |
| AO (mm) | 10.00 (9.50–10.88) | 8.10 (7.00–9.00) | <0.001 ‡ |
| LVID (mm) | 18.55 (17.00–19.60) | 15.90 (14.10–17.20) | <0.001 ‡ |
| IVS (mm) | 3.28 (3.05–3.53) | 2.79 (2.48–3.24) | <0.001 ‡ |
| LVPW (mm) | 2.60 (2.50–2.90) | 2.08 (1.89–2.39) | <0.001 ‡ |
| LCA (mm) | 1.31 (1.18–1.52) | 1.31 (1.05–1.55) | 0.721 |
| LCA/AO ratio | 0.13 (0.11–0.15) | 0.16 (0.13–0.19) | <0.001 ‡ |
| RCA (mm) | 1.19 (1.01–1.36) | 1.10 (0.94–1.33) | 0.464 |
| RCA/AO ratio | 0.11 (0.10–0.14) | 0.15 (0.11–0.18) | <0.001 ‡ |
| FS (%) | 30.70 (28.00–34.65) | 29.40 (24.70–33.30) | 0.285 |
| EF (%) | 61.87 (57.17–66.70) | 59.61 (52.75–66.20) | 0.427 |
| EDV (mL) | 10.51 (8.39–12.09) | 7.00 (4.87–8.65) | <0.001 ‡ |
| ESV (mL) | 3.91 (2.79–5.28) | 2.70 (1.84–3.75) | <0.001 ‡ |
| SV (mL) | 6.38 (5.55–7.12) | 4.04 (2.82–5.34) | <0.001 ‡ |
| CO (L/min) | 0.84 (0.72–0.98) | 0.55 (0.43–0.70) | <0.001 ‡ |
| CI (L/min/mm2) | 4.14 (3.39–4.73) | 4.14 (3.62–5.76) | 0.290 |
| PE | Normotension | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | S.E. | p | Adjusted R2 | Coefficient | S.E. | p | Adjusted R2 | ||
| SBP | SBP | ||||||||
| Constant | 65.17 | 3.11 | <0.001 ‡ | 21.97% | Constant | −89.35 | 65.60 | 0.187 | 16.00% |
| GA | 0.73 | GA | 3.88 | 1.67 | 0.030 * | ||||
| Lg ANPEP/S18 (CBL) | 19.02 | 6.96 | 0.012 * | SQRT cGMP (UA) | 0.071 | ||||
| Constant | 41.76 | 4.49 | <0.001 ‡ | 30.05% | |||||
| GA | 0.268 | ||||||||
| SQRT cAMP (UA) | 12.20 | 3.85 | 0.005 † | ||||||
| Lg DBP | Lg DBP | ||||||||
| Constant | 1.58 | 0.04 | <0.001 ‡ | 43.51% | Constant | 2.09 | 0.20 | <0.001 ‡ | 35.65% |
| GA | 0.264 | GA | 0.944 | ||||||
| Lg AT2R/GAPDH (UA) | −0.33 | 0.11 | 0.012 * | AT2R (UA, WB) | −0.33 | 0.13 | 0.024 * | ||
| Lg ACE2/GAPDH (UA) | 0.751 | Constant | 1.80 | 0.07 | <0.001 ‡ | 29.81% | |||
| GA | 0.415 | ||||||||
| SQRT cAMP (UA) | 0.732 | ||||||||
| SQRT cGMP (UA) | −0.17 | 0.05 | 0.003 † | ||||||
| LVID | LVID | ||||||||
| Constant | −1.48 | 0.69 | 0.053 | 55.67% | Constant | 0.78 | 0.47 | 0.132 | 31.64% |
| GA | 0.07 | 0.02 | 0.003 † | GA | 0.222 | ||||
| AT4R/GAPDH (CP-EC) | 0.62 | 0.26 | 0.033 * | AT2R (UA, WB) | 0.72 | 0.29 | 0.033 * | ||
| IVS | IVS | ||||||||
| N.A. | Constant | 0.20 | 0.05 | 0.001 † | 26.55% | ||||
| GA | 0.602 | ||||||||
| AT4R/GAPDH (CP-EC) | 0.12 | 0.05 | 0.024 * | ||||||
| LVPW | LVPW | ||||||||
| Constant | 0.36 | 0.05 | <0.001 ‡ | 27.89% | Constant | 0.21 | 0.03 | <0.001 ‡ | 15.24% |
| GA | 0.749 | GA | 0.208 | ||||||
| AT4R/GAPDH (CP-EC) | −0.12 | 0.05 | 0.025 * | SQRT cAMP (UA) | 0.462 | ||||
| SQRT cGMP (UA) | 0.04 | 0.02 | 0.034 * | ||||||
| AO | AO | ||||||||
| Constant | −7.81 | 2.29 | 0.003 † | 69.30% | Constant | 9.81 | 0.20 | <0.001 ‡ | 34.8% |
| GA | 0.47 | 0.07 | <0.001 ‡ | GA | 0.117 | ||||
| SQRT cAMP (UA) | 0.120 | Lg ACE/GAPDH (UA) | −2.60 | 0.99 | 0.026 * | ||||
| SQRT cGMP (UA) | 0.335 | ||||||||
| LCA/AO | LCA/AO | ||||||||
| Constant | 0.06 | 0.03 | 0.072 | 38.24% | N.A. | ||||
| GA | 0.709 | ||||||||
| APN (CP) | 0.01 | 0.003 | 0.002 † | ||||||
| Lg RCA/AO | Lg RCA/AO | ||||||||
| Constant | −1.10 | 0.10 | <0.001 ‡ | 18.51% | N.A. | ||||
| GA | 0.828 | ||||||||
| APN (CP) | 0.02 | 0.01 | 0.029 * | ||||||
| Lg EDV | Lg EDV | ||||||||
| N.A. | Constant | 0.43 | 0.28 | 0.148 | 28.14% | ||||
| GA | 0.255 | ||||||||
| AT2R (UA, WB) | 0.39 | 0.17 | 0.044 * | ||||||
| Constant | 1.25 | 0.11 | <0.001 ‡ | 20.09% | |||||
| GA | 0.601 | ||||||||
| ACE/GAPDH (CP-EC) | −0.22 | 0.10 | 0.046 * | ||||||
| Constant | 1.08 | 0.02 | <0.001 ‡ | 51.55% | |||||
| GA | 0.601 | ||||||||
| Lg ACE/GAPDH (UA) | −0.41 | 0.12 | 0.005 † | ||||||
| ESV | ESV | ||||||||
| Constant | 0.30 | 1.02 | 0.776 | 18.32% | Constant | 5.28 | 0.35 | <0.001 ‡ | 41.32% |
| GA | 0.150 | GA | 0.525 | ||||||
| APN (CP) | 0.22 | 0.10 | 0.034 * | Lg ACE/GAPDH (UA) | −5.16 | 1.74 | 0.014 * | ||
| EF | EF | ||||||||
| Constant | 50.17 | 4.78 | <0.001 ‡ | 16.34% | N.A. | ||||
| GA | 0.886 | ||||||||
| SQRT cGMP (UA) | 9.59 | 4.33 | 0.039 * | ||||||
| Lg SV | Lg SV | ||||||||
| Constant | −1.38 | 0.37 | 0.001 † | 64.06% | Constant | 0.67 | 0.06 | <0.001 ‡ | 28.02% |
| GA | 0.05 | 0.01 | <0.001 ‡ | GA | 0.972 | ||||
| SQRT cAMP (UA) | 0.18 | 0.07 | 0.023 * | SQRT cAMP (UA) | 0.873 | ||||
| SQRT cGMP (UA) | 0.066 | SQRT cGMP (UA) | 0.14 | 0.04 | 0.005 † | ||||
| Constant | 0.91 | 0.05 | <0.001 ‡ | 16.21% | |||||
| GA | 0.344 | ||||||||
| APN (CP) | −0.01 | 0.006 | 0.036 * | ||||||
| Constant | 0.17 | 0.26 | 0.534 | 34.99% | |||||
| GA | 0.104 | ||||||||
| AT2R (UA, WB) | 0.42 | 0.16 | 0.025 * | ||||||
| SQRT CO | SQRT CO | ||||||||
| Constant | −0.97 | 0.46 | 0.055 | 50.25% | Constant | −0.12 | 0.33 | 0.726 | 47.28% |
| GA | 0.03 | 0.01 | 0.016 * | GA | 0.157 | ||||
| AT4R/GAPDH (CP-EC) | 0.48 | 0.17 | 0.016 * | AT2R (UA, WB) | 0.67 | 0.20 | 0.008 † | ||
| CI | CI | ||||||||
| Constant | −4.33 | 3.00 | 0.170 | 32.80% | Constant | 23.21 | 13.16 | 0.112 | 64.46% |
| GA | 0.724 | GA | −0.72 | 0.31 | 0.047 * | ||||
| Lg Ang1–7 (MP) | 3.05 | 1.06 | 0.012 * | AT2R (UA, WB) | 6.02 | 1.73 | 0.007 † | ||
| Constant | 3.20 | 0.90 | 0.001 † | 14.79% | Constant | 2.40 | 1.10 | 0.040 * | 13.48% |
| GA | 0.763 | GA | 0.464 | ||||||
| SQRT cGMP (UA) | 1.72 | 0.81 | 0.048 * | SQRT cGMP (UA) | 1.64 | 0.78 | 0.048 * | ||
| Constant | 4.75 | 0.42 | <0.001 ‡ | 35.32% | |||||
| GA | 0.339 | ||||||||
| Lg ACE/GAPDH (UA) | −5.65 | 2.13 | 0.024 * | ||||||
| SBP (maternal) | SBP (maternal) | ||||||||
| Constant | 161.47 | 4.38 | <0.001 ‡ | 43.99% | Constant | 115.69 | 1.39 | <0.001 ‡ | 80.63% |
| GA | 0.713 | GA | 0.741 | ||||||
| Lg AT1R/GAPDH (UA) | 48.99 | 15.78 | 0.011 * | Lg AT1R/GAPDH (UA) | −24.70 | 3.78 | <0.001 ‡ | ||
| Constant | 127.86 | 17.47 | <0.001 ‡ | 32.26% | Lg AT2R/GAPDH (UA) | 0.476 | |||
| GA | 0.343 | Lg ACE/GAPDH (UA) | 0.260 | ||||||
| AT1R/GAPDH (CP-EC) | 29.91 | 10.48 | 0.013 * | Lg ACE2/GAPDH (UA) Lg MAS1/GAPDH (UA) | 0.670 0.144 | ||||
| Lg DBP (maternal) | Lg DBP (maternal) | ||||||||
| Constant | 2.01 | 0.01 | <0.001 ‡ | 38.81% | Constant | 1.86 | 0.01 | <0.001 ‡ | 26.72% |
| GA | 0.172 | GA | 0.377 | ||||||
| Lg AT1R/GAPDH (UA) | 0.13 | 0.05 | 0.020 * | Lg MAS1/GAPDH (UA) | −0.11 | 0.05 | 0.049 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, I.-C.; Wu, K.L.H.; Cheng, H.-H.; Tsai, C.-C.; Yu, H.-R.; Hsu, T.-Y.; Tain, Y.-L.; Huang, L.-T.; Lai, Y.-J. Association of Perinatal Cardiovascular Features with Angiotensin System Expressions in Maternal Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7426. https://doi.org/10.3390/ijms25137426
Lin I-C, Wu KLH, Cheng H-H, Tsai C-C, Yu H-R, Hsu T-Y, Tain Y-L, Huang L-T, Lai Y-J. Association of Perinatal Cardiovascular Features with Angiotensin System Expressions in Maternal Preeclampsia. International Journal of Molecular Sciences. 2024; 25(13):7426. https://doi.org/10.3390/ijms25137426
Chicago/Turabian StyleLin, I-Chun, Kay L. H. Wu, Hsin-Hsin Cheng, Ching-Chang Tsai, Hong-Ren Yu, Te-Yao Hsu, You-Lin Tain, Li-Tung Huang, and Yun-Ju Lai. 2024. "Association of Perinatal Cardiovascular Features with Angiotensin System Expressions in Maternal Preeclampsia" International Journal of Molecular Sciences 25, no. 13: 7426. https://doi.org/10.3390/ijms25137426
APA StyleLin, I.-C., Wu, K. L. H., Cheng, H.-H., Tsai, C.-C., Yu, H.-R., Hsu, T.-Y., Tain, Y.-L., Huang, L.-T., & Lai, Y.-J. (2024). Association of Perinatal Cardiovascular Features with Angiotensin System Expressions in Maternal Preeclampsia. International Journal of Molecular Sciences, 25(13), 7426. https://doi.org/10.3390/ijms25137426









