A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro
Abstract
:1. Introduction
2. Results
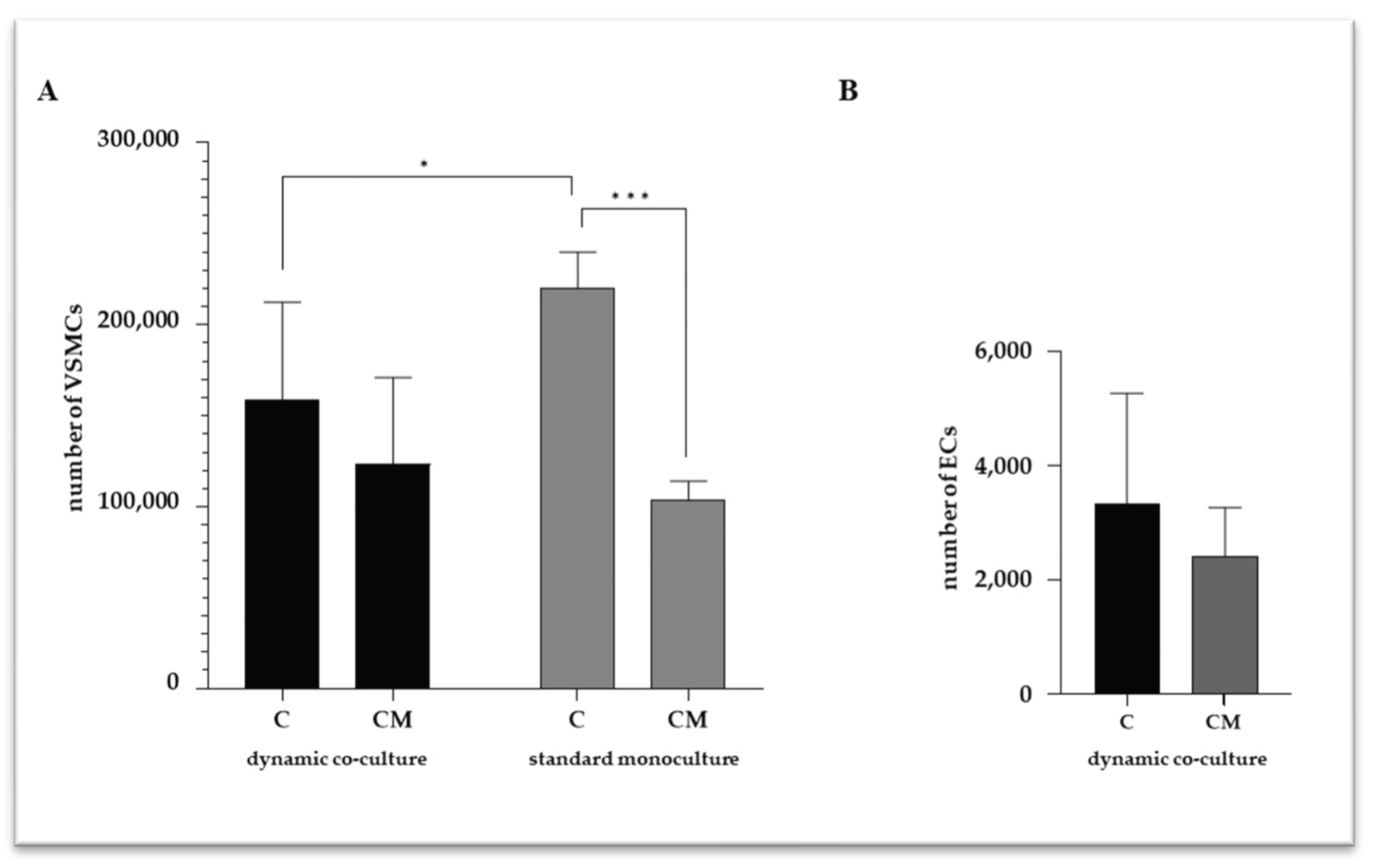
2.1. VSMC and EC Viability
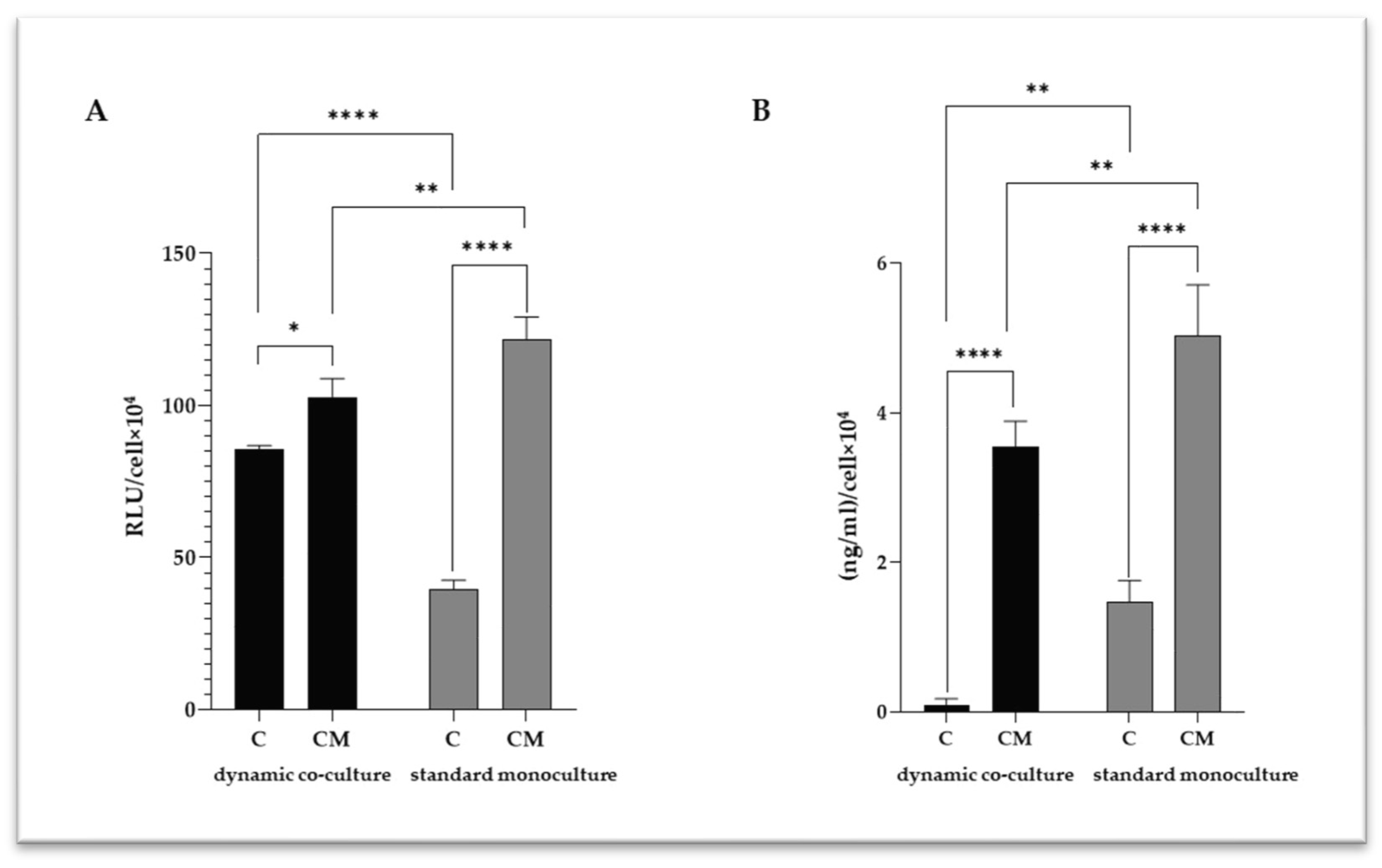
2.2. VSMC Calcification
2.3. VSMC-Released Inflammatory Mediators
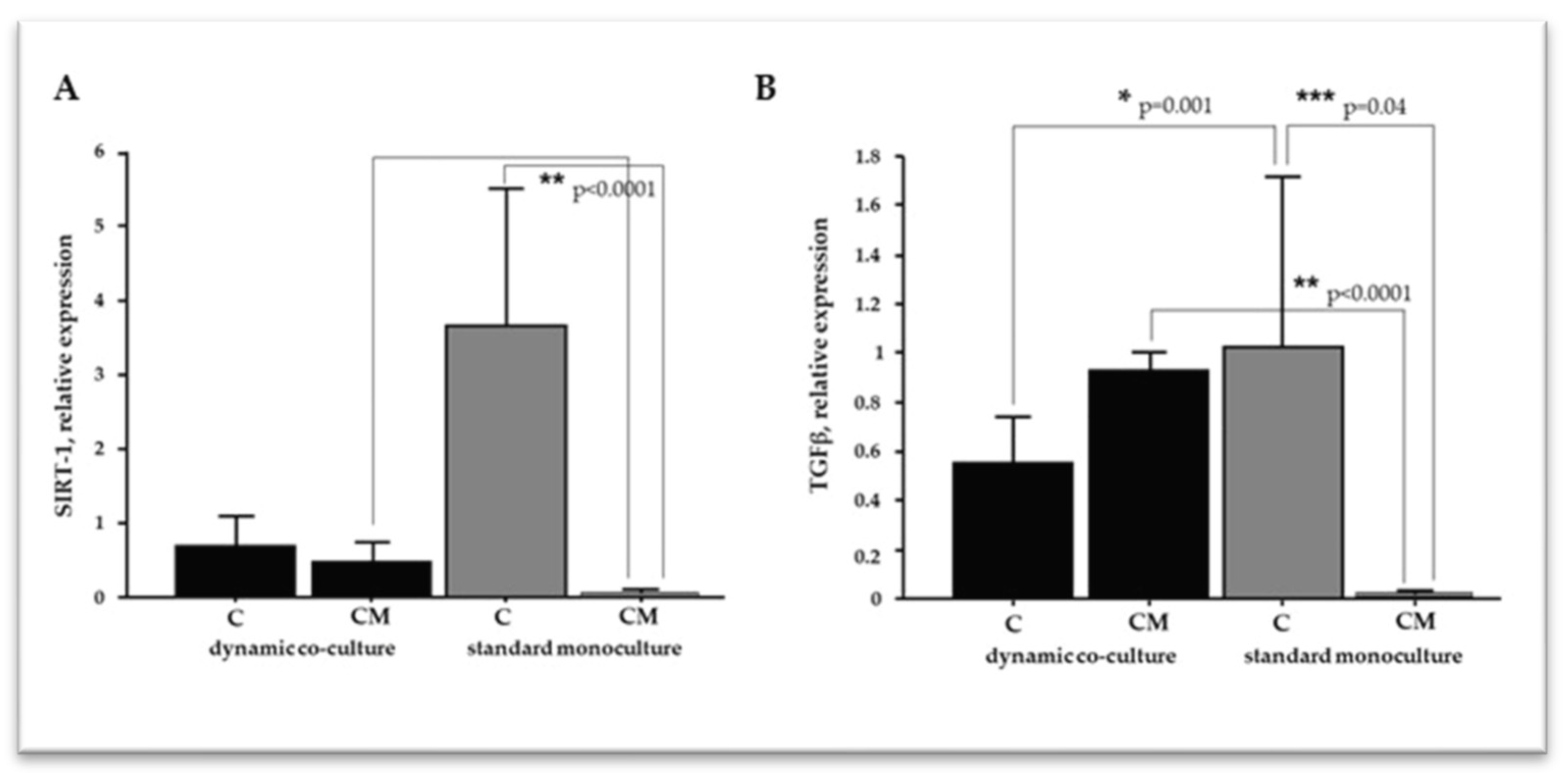
2.4. The Microenvironment Conditions Influence VSMC Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Dynamic Co-Culture Experimental Setting
4.3. VSMC Standard Monoculture
4.4. Cell Viability
4.5. Intracellular Calcium Quantification
4.6. IL-6 Determination
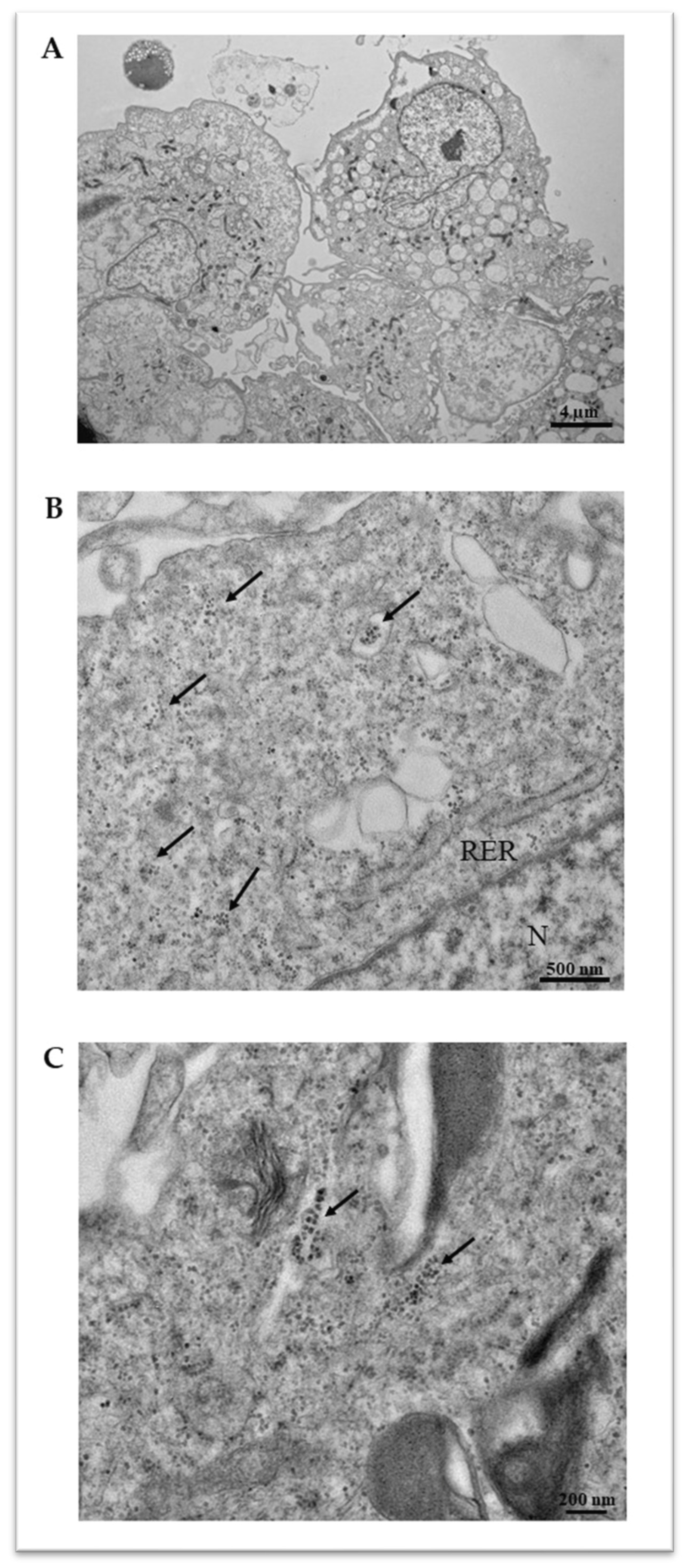
4.7. Transmission Electron Microscopy (TEM)
4.8. RNA Extraction and Real-Time PCR Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. IJMS 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An Update on the Phenotypic Switching of Vascular Smooth Muscle Cells in the Pathogenesis of Atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 6. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Urs, S.; Boucher, J.; Bernaiche, T.; Venkatesh, D.; Spicer, D.B.; Vary, C.P.H.; Liaw, L. Notch and Transforming Growth Factor-β (TGFβ) Signaling Pathways Cooperatively Regulate Vascular Smooth Muscle Cell Differentiation. J. Biol. Chem. 2010, 285, 17556–17563. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, S. The Role of Sirtuins in Osteogenic Differentiation of Vascular Smooth Muscle Cells and Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 894692. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.-Z. Histone Deacetylase SIRT1, Smooth Muscle Cell Function, and Vascular Diseases. Front. Pharmacol. 2020, 11, 537519. [Google Scholar] [CrossRef] [PubMed]
- Hénaut, L.; Massy, Z.A. New Insights into the Key Role of Interleukin 6 in Vascular Calcification of Chronic Kidney Disease. Nephrol. Dial. Transplant. 2018, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.-C.; Chen, Y.-H.; Wu, T.-Y.; Kao, L.-Z.; Hung, S.-Y.; Liou, H.-H.; Chen, P.-C.; Tsai, P.-J.; Lin, H.-K.; Lee, Y.-C.; et al. Phosphate Burden Induces Vascular Calcification through a NLRP3-Caspase-1-Mediated Pyroptotic Pathway. Life Sci. 2023, 332, 122123. [Google Scholar] [CrossRef]
- Kelynack, K.J.; Holt, S.G. An In Vitro Murine Model of Vascular Smooth Muscle Cell Mineralization. In Kidney Research; Hewitson, T.D., Smith, E.R., Holt, S.G., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1397, pp. 209–220. ISBN 978-1-4939-3351-8. [Google Scholar]
- Patel, J.J.; Bourne, L.E.; Davies, B.K.; Arnett, T.R.; MacRae, V.E.; Wheeler-Jones, C.P.; Orriss, I.R. Differing Calcification Processes in Cultured Vascular Smooth Muscle Cells and Osteoblasts. Exp. Cell Res. 2019, 380, 100–113. [Google Scholar] [CrossRef]
- Pustlauk, W.; Westhoff, T.H.; Claeys, L.; Roch, T.; Geißler, S.; Babel, N. Induced Osteogenic Differentiation of Human Smooth Muscle Cells as a Model of Vascular Calcification. Sci. Rep. 2020, 10, 5951. [Google Scholar] [CrossRef]
- Ceccherini, E.; Cecchettini, A.; Gisone, I.; Persiani, E.; Morales, M.A.; Vozzi, F. Vascular Calcification: In Vitro Models under the Magnifying Glass. Biomedicines 2022, 10, 2491. [Google Scholar] [CrossRef]
- Ceccherini, E.; Gisone, I.; Persiani, E.; Ippolito, C.; Falleni, A.; Cecchettini, A.; Vozzi, F. Novel in Vitro Evidence on the Beneficial Effect of Quercetin Treatment in Vascular Calcification. Front. Pharmacol. 2024, 15, 1330374. [Google Scholar] [CrossRef]
- Persiani, E.; Ceccherini, E.; Gisone, I.; Cecchettini, A.; Vozzi, F. Protocol to Generate an in Vitro Model to Study Vascular Calcification Using Human Endothelial and Smooth Muscle Cells. STAR Protoc. 2023, 4, 102328. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Lin, X.; Shan, S.-K.; Xu, F.; Zhong, J.-Y.; Wu, F.; Duan, J.-Y.; Guo, B.; Li, F.-X.-Z.; Wang, Y.; Zheng, M.-H.; et al. The Crosstalk between Endothelial Cells and Vascular Smooth Muscle Cells Aggravates High Phosphorus-Induced Arterial Calcification. Cell Death Dis. 2022, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Barbero, N.; Gutiérrez-Muñoz, C.; Blanco-Colio, L.M. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int. J. Mol. Sci. 2021, 22, 7284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.J.; Haga, J.H.; Chien, S. Molecular Basis of the Effects of Shear Stress on Vascular Endothelial Cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Endothelial-Vascular Smooth Muscle Cells Interactions in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B. We Have Contact: Endothelial Cell-Smooth Muscle Cell Interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying Active Vascular Microcalcification by 18F-Sodium Fluoride Positron Emission Tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.M.; Proudfoot, D. Calcium Phosphate Crystals Induce Cell Death in Human Vascular Smooth Muscle Cells: A Potential Mechanism in Atherosclerotic Plaque Destabilization. Circ. Res. 2008, 103, 181305. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small Entities with Large Impact: Microcalcifications and Atherosclerotic Plaque Vulnerability. Curr. Opin. Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Miano, J.M. Transforming Growth Factor-Β1 (TGF-Β1) Utilizes Distinct Pathways for the Transcriptional Activation of MicroRNA 143/145 in Human Coronary Artery Smooth Muscle Cells. J. Biol. Chem. 2011, 286, 30119–30129. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.N.; Hilyard, A.C.; Lagna, G.; Hata, A. SMAD Proteins Control DROSHA-Mediated microRNA Maturation. Nature 2008, 454, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; Van Eys, G.J.J.M. Regulation and Characteristics of Vascular Smooth Muscle Cell Phenotypic Diversity. NHJL 2007, 15, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamaté, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of Transforming Growth Factor-β Signaling Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef]
- Takemura, A.; Iijima, K.; Ota, H.; Son, B.-K.; Ito, Y.; Ogawa, S.; Eto, M.; Akishita, M.; Ouchi, Y. Sirtuin 1 Retards Hyperphosphatemia-Induced Calcification of Vascular Smooth Muscle Cells. ATVB 2011, 31, 2054–2062. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Serracino Inglott, F.; Alexander, M.Y.; Weston, R. Loss of SIRT1 in Diabetes Accelerates DNA Damage-Induced Vascular Calcification. Cardiovasc. Res. 2021, 117, 836–849. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Inglott, F.S.; Alexander, M.Y.; Weston, R. Suppression of SIRT1 in Diabetic Conditions Induces Osteogenic Differentiation of Human Vascular Smooth Muscle Cells via RUNX2 Signalling. Sci. Rep. 2019, 9, 878. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Helenius, G.; Johansson, B.R.; Li, J.Y.; Mattsson, E.; Risberg, B. Co-Culture of Endothelial Cells and Smooth Muscle Cells Affects Gene Expression of Angiogenic Factors. J. Cell Biochem. 2003, 89, 1250–1259. [Google Scholar] [CrossRef]
- Qi, Y.-X.; Jiang, J.; Jiang, X.-H.; Wang, X.-D.; Ji, S.-Y.; Han, Y.; Long, D.-K.; Shen, B.-R.; Yan, Z.-Q.; Chien, S.; et al. PDGF-BB and TGF-Β1 on Cross-Talk between Endothelial and Smooth Muscle Cells in Vascular Remodeling Induced by Low Shear Stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.S.B.; Simes, D.C. Inflammation and Calcification in the Vascular Tree; Insights Into Atherosclerosis. In Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 189–201. ISBN 978-0-12-805417-8. [Google Scholar]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galán, J.E.; Askenase, P.W.; et al. Critical Role for NALP3/CIAS1/Cryopyrin in Innate and Adaptive Immunity through Its Regulation of Caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Holmar, J.; Noels, H.; Böhm, M.; Bhargava, S.; Jankowski, J.; Orth-Alampour, S. Development, Establishment and Validation of in Vitro and Ex Vivo Assays of Vascular Calcification. Biochem. Biophys. Res. Commun. 2020, 530, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]


| Caspase-1 | IL-6 | ||
|---|---|---|---|
| dynamic co-culture | Intracellular calcium content | R = 0.99 p = 0.0155 | R = 1.00 p = 0.0002 |
| standard monoculture | R = 0.79 p = 0.0061 | R = 0.81 p = 0.0159 | |
| GENE | PRIMER, 5’→3’ | Genbank | pb | LOCALIZATION | Ta |
|---|---|---|---|---|---|
| SIRT-1 | F: TCCTCTAGTTCTTGTGGCAGTA R: CATCTCCATCAGTCCCAAATCC | NM_012238 | 169 | chr 10q21.3 | 58 |
| TGFβ1 | F: TGAACCCGTGTTGCTCTC R: GCCAGGAATTGTTGCTGTATT | NM_000660.7 | 104 | chr 19q13.2 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccherini, E.; Persiani, E.; Cabiati, M.; Guiducci, L.; Del Ry, S.; Gisone, I.; Falleni, A.; Cecchettini, A.; Vozzi, F. A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro. Int. J. Mol. Sci. 2024, 25, 7427. https://doi.org/10.3390/ijms25137427
Ceccherini E, Persiani E, Cabiati M, Guiducci L, Del Ry S, Gisone I, Falleni A, Cecchettini A, Vozzi F. A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro. International Journal of Molecular Sciences. 2024; 25(13):7427. https://doi.org/10.3390/ijms25137427
Chicago/Turabian StyleCeccherini, Elisa, Elisa Persiani, Manuela Cabiati, Letizia Guiducci, Silvia Del Ry, Ilaria Gisone, Alessandra Falleni, Antonella Cecchettini, and Federico Vozzi. 2024. "A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro" International Journal of Molecular Sciences 25, no. 13: 7427. https://doi.org/10.3390/ijms25137427
APA StyleCeccherini, E., Persiani, E., Cabiati, M., Guiducci, L., Del Ry, S., Gisone, I., Falleni, A., Cecchettini, A., & Vozzi, F. (2024). A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro. International Journal of Molecular Sciences, 25(13), 7427. https://doi.org/10.3390/ijms25137427









