The Bursaphelenchus xylophilus Effector BxNMP1 Targets PtTLP-L2 to Mediate PtGLU Promoting Parasitism and Virulence in Pinus thunbergii
Abstract
1. Introduction
2. Results
2.1. Identification of the B. xylophilus Candidate Effector BxNMP1
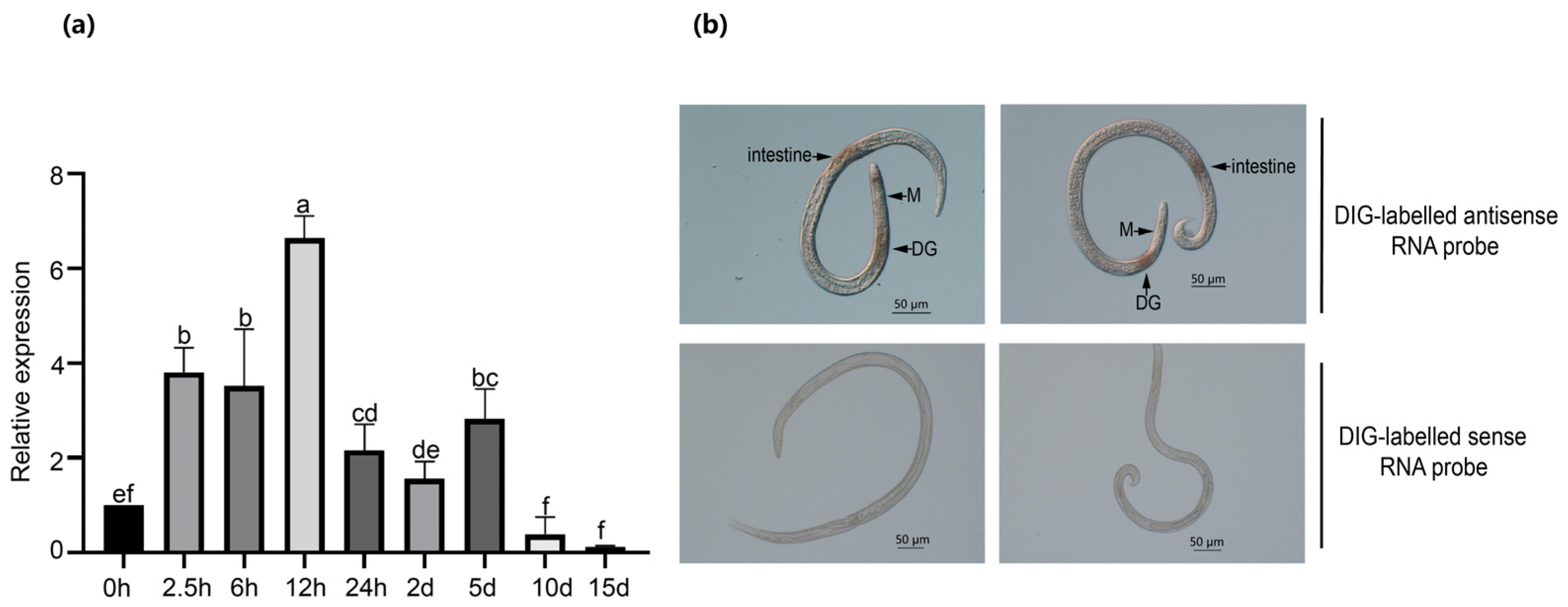
2.2. BxNMP1 Is Upregulated during Infection and Is Localized to Dorsal Gland Cells and the Intestine
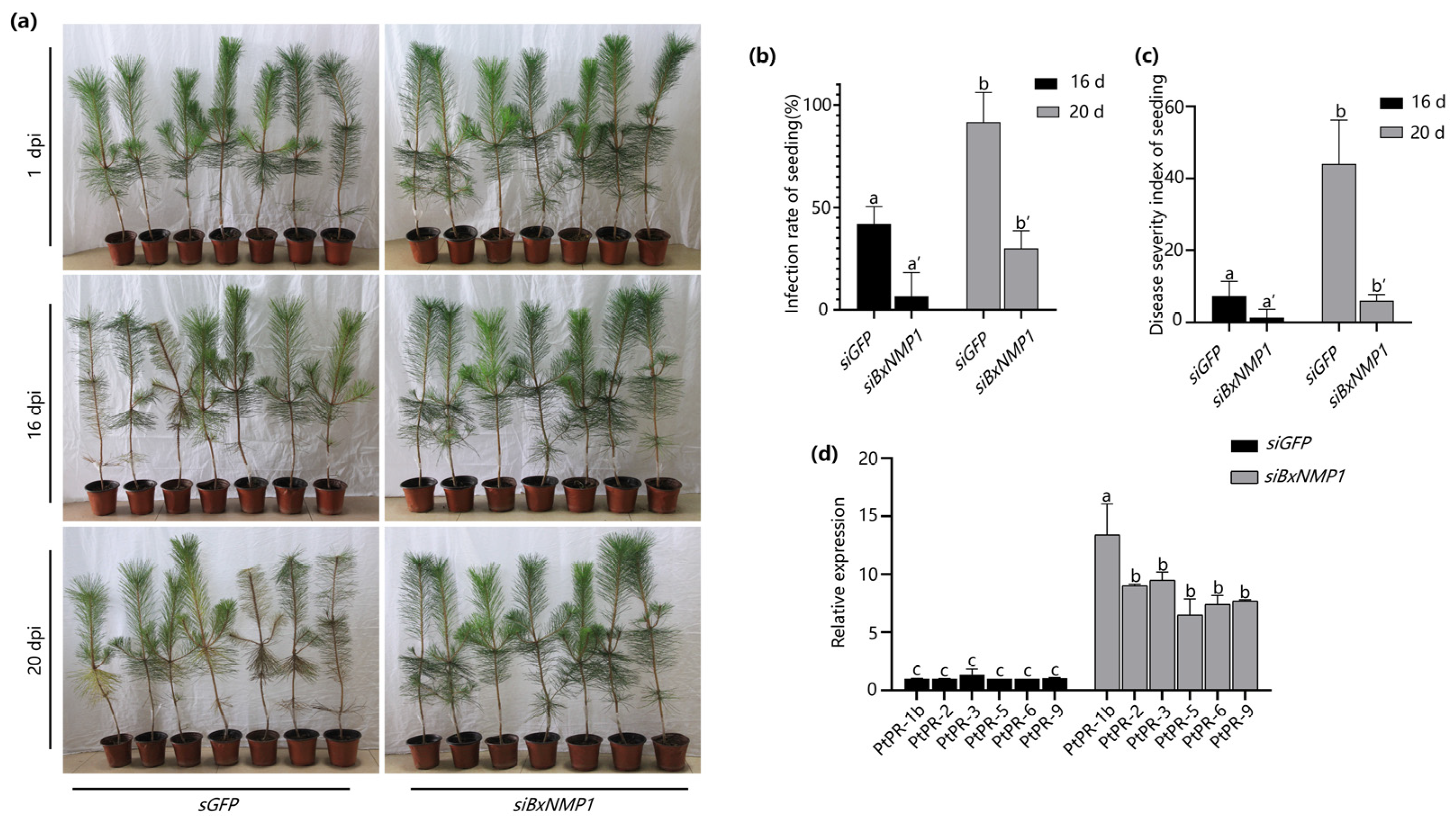
2.3. BxNMP1 Contributes to B. xylophilus Pathogenicity
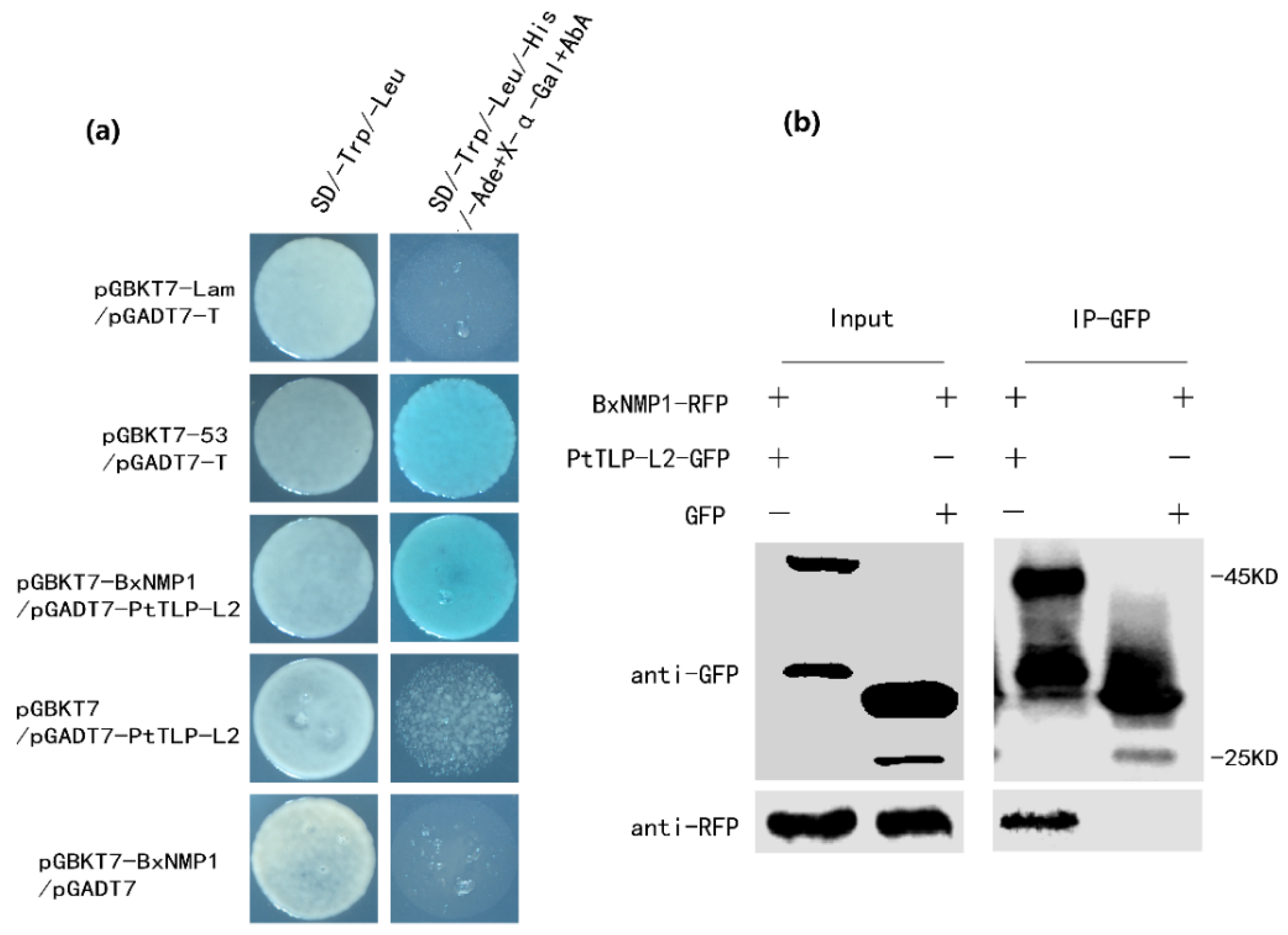
2.4. BxNMP1 Interacts with the Host Plant Thaumatin-like Protein L2
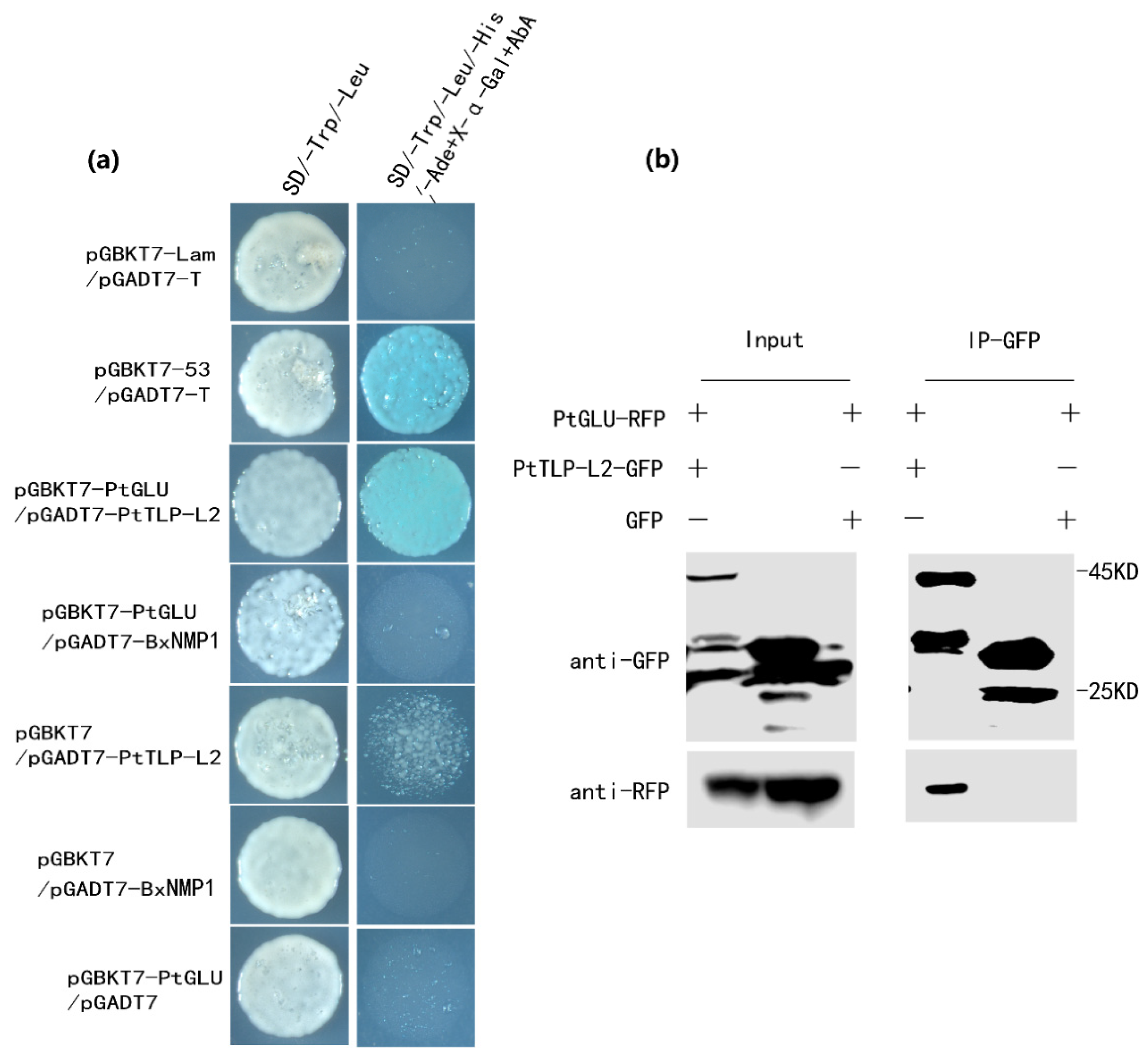
2.5. PtTLP-L2 Interacts with β-1,3-Glucanase in P. thunbergii
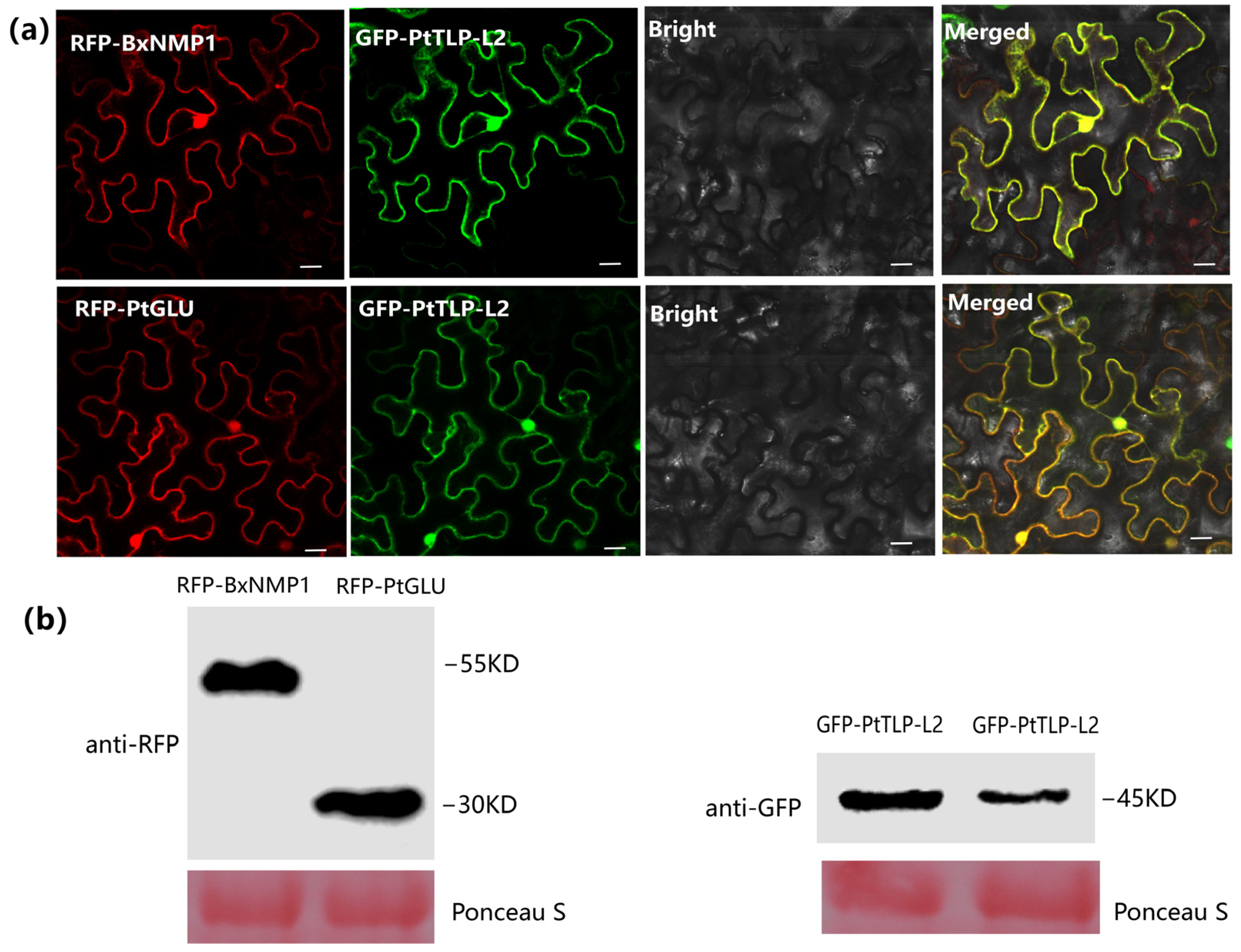
2.6. BxNMP1, PtTLP-L2, and PtGLU Colocalize in the Nucleus and Cytoplasm in N. benthamiana
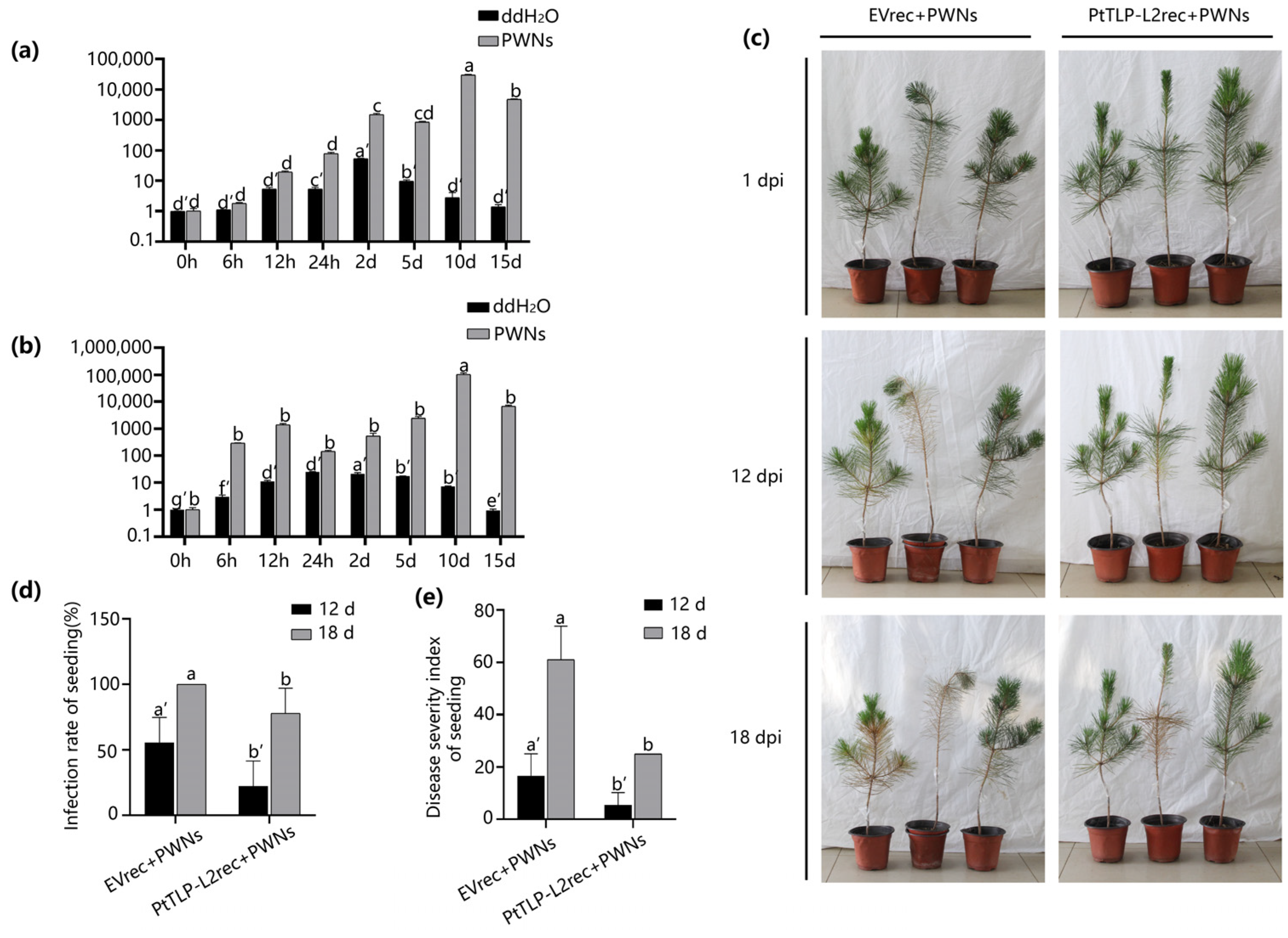
2.7. PtTLP-L2 Enhances the Resistance of P. thunbergii
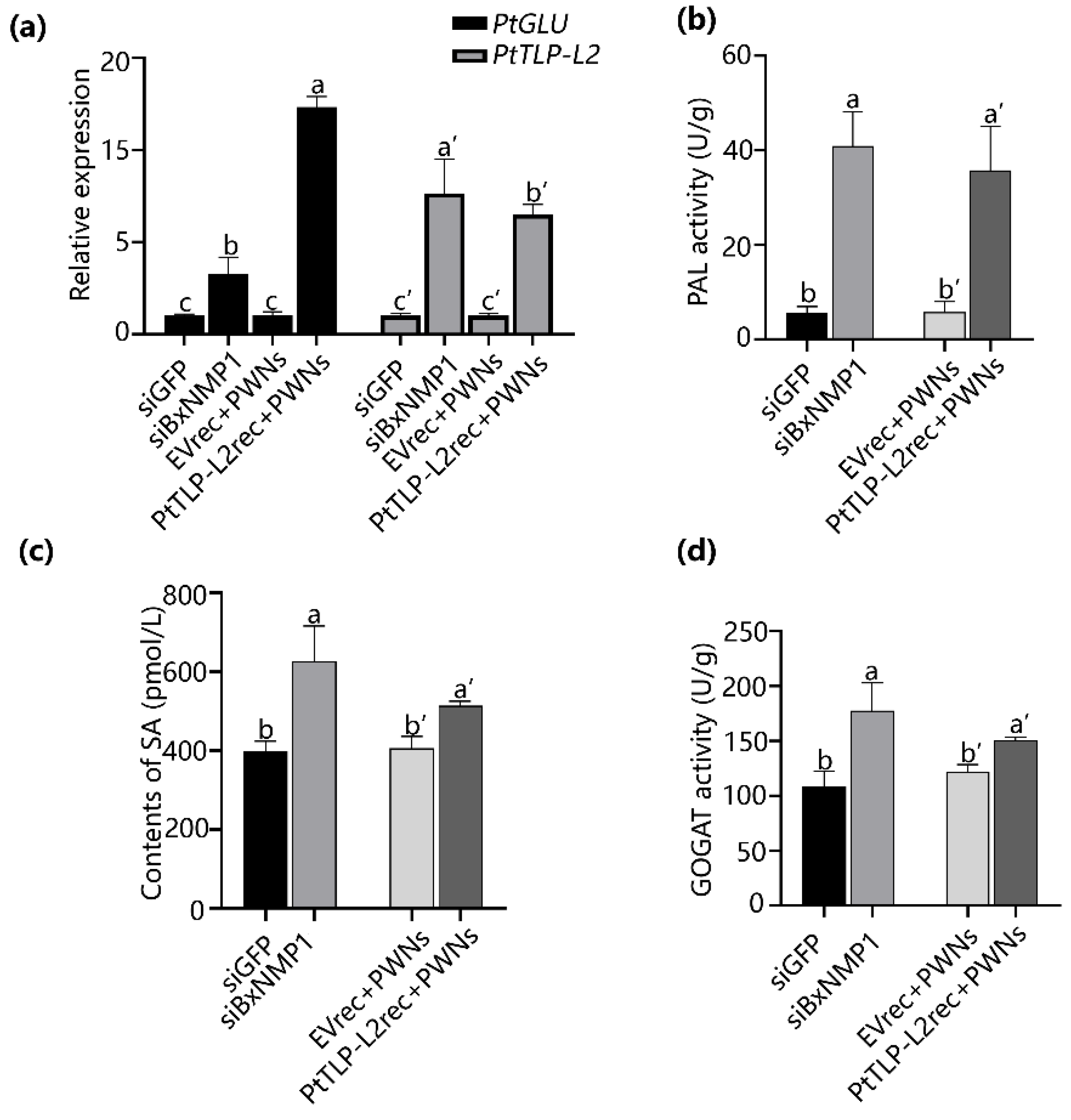
2.8. BxNMP1 Inhibits the Expression of Targets and Suppresses the Salicylic Acid Pathway in P. thunbergii
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Total RNA Extraction and cDNA Synthesis
4.3. BxNMP1 Gene Cloning, Plasmid Construction, and Real-Time Quantitative PCR
4.4. Sequence Analysis and 3D Structure Modelling
4.5. In Situ Hybridization
4.6. Transient Expression of BxNMP1 in N. benthamiana
4.7. Electrolyte Leakage Assay
4.8. Total Protein Extraction and Western Blot Analysis
4.9. Detection of the Effect of BXNMP1 on B. xylophilus Pathogenicity
4.10. Y2H and Co-IP Assays
4.11. Confocal Microscopy
4.12. PtTLP-L2 and PtGLU Gene Cloning, Plasmid Construction, and Real-Time RT–qPCR Analyses
4.13. Expression and Purification of the Recombinant PtTLP-L2 Protein
4.14. Detection of Salicylic Acid and Related Enzyme Activities
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J. Interspecific Communication between Pinewood Nematode, Its Insect Vector, and Associated Microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Vernaldi, S.; Maekawa, T. Evolution and Conservation of Plant NLR Functions. Front. Immunol. 2013, 4, 297. [Google Scholar] [CrossRef]
- Mitchum, M.G.; Hussey, R.S.; Baum, T.J.; Wang, X.; Elling, A.A.; Wubben, M.; Davis, E.L. Nematode Effector Proteins: An Emerging Paradigm of Parasitism. New Phytol. 2013, 199, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Goverse, A.; Smant, G. The Activation and Suppression of Plant Innate Immunity by Parasitic Nematodes. Annu. Rev. Phytopathol. 2014, 52, 243–265. [Google Scholar] [CrossRef]
- Mei, Y.; Wright, K.M.; Haegeman, A.; Bauters, L.; Diaz-Granados, A.; Goverse, A.; Gheysen, G.; Jones, J.T.; Mantelin, S. The Globodera Pallida SPRYSEC Effector GpSPRY-414-2 That Suppresses Plant Defenses Targets a Regulatory Component of the Dynamic Microtubule Network. Front. Plant Sci. 2018, 9, 1019. [Google Scholar] [CrossRef]
- Espada, M.; Silva, A.C.; Eves van den Akker, S.; Cock, P.J.A.; Mota, M.; Jones, J.T. Identification and Characterization of Parasitism Genes from the Pinewood Nematode Bursaphelenchus Xylophilus Reveals a Multilayered Detoxification Strategy. Mol. Plant Pathol. 2016, 17, 286–295. [Google Scholar] [CrossRef]
- Tsai, I.J.; Tanaka, R.; Kanzaki, N.; Akiba, M.; Yokoi, T.; Espada, M.; Jones, J.T.; Kikuchi, T. Transcriptional and Morphological Changes in the Transition from Mycetophagous to Phytophagous Phase in the Plant-Parasitic Nematode Bursaphelenchus xylophilus. Mol. Plant Pathol. 2016, 17, 77–83. [Google Scholar] [CrossRef]
- Hu, L.-J.; Wu, X.-Q.; Ding, X.-L.; Ye, J.-R. Comparative Transcriptomic Analysis of Candidate Effectors to Explore the Infection and Survival Strategy of Bursaphelenchus Xylophilus during Different Interaction Stages with Pine Trees. BMC Plant Biol. 2021, 21, 224. [Google Scholar] [CrossRef]
- Hu, L.-J.; Wu, X.-Q.; Li, H.-Y.; Zhao, Q.; Wang, Y.-C.; Ye, J.-R. An Effector, BxSapB1, Induces Cell Death and Contributes to Virulence in the Pine Wood Nematode Bursaphelenchus xylophilus. Mol. Plant Microbe Interact. 2019, 32, 452–463. [Google Scholar] [CrossRef]
- Huang, X.; Hu, L.; Wu, X. Identification of a Novel Effector BxSapB3 That Enhances the Virulence of Pine Wood Nematode Bursaphelenchus xylophilus. Acta Biochim. Biophys. Sin. 2019, 51, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hu, L.-J.; Wu, X.-Q.; Wang, Y.-C. A Key Effector, BxSapB2, Plays a Role in the Pathogenicity of the Pine Wood Nematode Bursaphelenchus xylophilus. For. Pathol. 2020, 50, e12600. [Google Scholar] [CrossRef]
- Wen, T.-Y.; Wu, X.-Q.; Hu, L.-J.; Qiu, Y.-J.; Rui, L.; Zhang, Y.; Ding, X.-L.; Ye, J.-R. A Novel Pine Wood Nematode Effector, BxSCD1, Suppresses Plant Immunity and Interacts with an Ethylene-Forming Enzyme in Pine. Mol. Plant Pathol. 2021, 22, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, L.-J.; Wu, X.-Q.; Ye, J.-R. A Bursaphelenchus Xylophilus Effector, Bx-FAR-1, Suppresses Plant Defense and Affects Nematode Infection of Pine Trees. Eur. J. Plant Pathol. 2020, 157, 637–650. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, T.-Y.; Wu, X.-Q.; Hu, L.-J.; Qiu, Y.-J.; Rui, L. The Bursaphelenchus Xylophilus Effector BxML1 Targets the Cyclophilin Protein (CyP) to Promote Parasitism and Virulence in Pine. BMC Plant Biol. 2022, 22, 216. [Google Scholar] [CrossRef]
- Qiu, Y.-J.; Wu, X.-Q.; Wen, T.-Y.; Hu, L.-J.; Rui, L.; Zhang, Y.; Ye, J.-R. The Bursaphelenchus Xylophilus Candidate Effector BxLip-3 Targets the Class I Chitinases to Suppress Immunity in Pine. Mol. Plant Pathol. 2023, 24, 1033–1046. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The Families of Pathogenesis-Related Proteins, Their Activities, and Comparative Analysis of PR-1 Type Proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant Thaumatin-like Proteins: Function, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumar, K.R.R.; Kumar, D.; Shukla, P.; Kirti, P.B. Characterization of a Pathogen Induced Thaumatin-Like Protein Gene AdTLP from Arachis Diogoi, a Wild Peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Movahedi, A.; Wei, H.; Zhuge, Q. Thaumatin-like Protein(Pe-TLP)Acts as a Positive Factor in Transgenic Poplars Enhanced Resistance to Spots Disease. Physiol. Mol. Plant Pathol. 2020, 112, 101512. [Google Scholar] [CrossRef]
- Kumar, R.; Mukherjee, P.K. Trichoderma Virens Alt a 1 Protein May Target Maize PR5/Thaumatin-like Protein to Suppress Plant Defence: An in Silico Analysis. Physiol. Mol. Plant Pathol. 2020, 112, 101551. [Google Scholar] [CrossRef]
- Wang, F.; Shen, S.; Cui, Z.; Yuan, S.; Qu, P.; Jia, H.; Meng, L.; Hao, X.; Liu, D.; Ma, L.; et al. Puccinia Triticina Effector Protein Pt_21 Interacts with Wheat Thaumatin-like Protein TaTLP1 to Inhibit Its Antifungal Activity and Suppress Wheat Apoplast Immunity. Crop J. 2023, 11, 1431–1440. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-Glucanases: Their Biological Functions and Transgenic Expression against Phytopathogenic Fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Karr, A.L. β-1,3-Glucanase and Chitinase Activities in Soybean Root Nodules. J. Plant Physiol. 2002, 159, 245–256. [Google Scholar] [CrossRef]
- Somssich, I.E.; Hahlbrock, K. Pathogen Defence in Plants—A Paradigm of Biological Complexity. Trends Plant Sci. 1998, 3, 86–90. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of Plant Hormones in Plant Defence Responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic Acid: Biosynthesis, Metabolism and Physiological Role in Plants. In Salicylic Acid: A Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–14. ISBN 978-1-4020-5184-5. [Google Scholar]
- Zhang, Y.; Li, X. Salicylic Acid: Biosynthesis, Perception, and Contributions to Plant Immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Gawroński, P.; Górecka, M.; Bederska, M.; Rusaczonek, A.; Ślesak, I.; Kruk, J.; Karpiński, S. Isochorismate Synthase 1 Is Required for Thylakoid Organization, Optimal Plastoquinone Redox Status, and State Transitions in Arabidopsis Thaliana. J. Exp. Bot. 2013, 64, 3669–3679. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic Priming by a Secreted Fungal Effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Howe, P.J.; Maier, T.R.; Hussey, R.S.; Mitchum, M.G.; Davis, E.L.; Baum, T.J. Arabidopsis Spermidine Synthase Is Targeted by an Effector Protein of the Cyst Nematode Heterodera Schachtii. Plant Physiol. 2010, 152, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chronis, D.; Kenning, C.; Peret, B.; Hewezi, T.; Davis, E.L.; Baum, T.J.; Hussey, R.; Bennett, M.; Mitchum, M.G. The Novel Cyst Nematode Effector Protein 19C07 Interacts with the Arabidopsis Auxin Influx Transporter LAX3 to Control Feeding Site Development. Plant Physiol. 2011, 155, 866–880. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S. Beet Cyst Nematode HsSNARE1 Interacts with Both AtSNAP2 and AtPR1 and Promotes Disease in Arabidopsis. J. Adv. Res. 2022, 47, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, R.; Dai, Y.; Zhang, Y.; Lin, S.; Ye, J. Comprehensive Analysis of Copy Number Variations on Glycoside Hydrolase 45 Genes among Different Bursaphelenchus Xylophilus Strains. Int. J. Mol. Sci. 2022, 23, 15323. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, K.; de Sain, M.; Weide, R.; Gouget, A.; Klamer, S.; Canut, H.; Govers, F. The Lectin Receptor Kinase LecRK-I.9 Is a Novel Phytophthora Resistance Component and a Potential Host Target for a RXLR Effector. PLoS Pathog. 2011, 7, e1001327. [Google Scholar] [CrossRef]
- Trudel, J.; Grenier, J.; Potvin, C.; Asselin, A. Several Thaumatin-Like Proteins Bind to β-1,3-Glucans. Plant Physiol. 1998, 118, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Menu-Bouaouiche, L.; Vriet, C.; Peumans, W.J.; Barre, A.; Van Damme, E.J.M.; Rougé, P. A Molecular Basis for the Endo-Β1,3-Glucanase Activity of the Thaumatin-like Proteins from Edible Fruits. Biochimie 2003, 85, 123–131. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yang, S.; Liu, G.; Zeng, Q.; Liu, Y. Molecular Characterization and Functional Analysis of a Pathogenesis-Related β-1,3-Glucanase Gene in Spruce (Picea asperata). Eur. J. Plant Pathol. 2022, 164, 177–192. [Google Scholar] [CrossRef]
- Tanz, S.K.; Castleden, I.; Small, I.D.; Millar, A.H. Fluorescent Protein Tagging as a Tool to Define the Subcellular Distribution of Proteins in Plants. Front. Plant Sci. 2013, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of Resistance to Pine Wood Nematode Infection in Pinus Thunbergiiusing Suppression Subtractive Hybridization. BMC Plant Biol. 2012, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.F. Pathogenesis in Pine Wilt Caused by Pinewood Nematode, Bursaphelenchus xylophilus. J. Nematol. 1988, 20, 236–244. [Google Scholar]
- Yamaguchi, R.; Matsunaga, K.; Hirao, T.; Tamura, M.; Watanabe, A. Spatiotemporal Analysis of Pine Wilt Disease: Relationship between Pinewood Nematode Distribution and Defence Response in Pinus Thunbergii Seedlings. For. Pathol. 2019, 49, e12518. [Google Scholar] [CrossRef]
- Thorpe, P.; Mantelin, S.; Cock, P.J.; Blok, V.C.; Coke, M.C.; Eves-van den Akker, S.; Guzeeva, E.; Lilley, C.J.; Smant, G.; Reid, A.J.; et al. Genomic Characterisation of the Effector Complement of the Potato Cyst Nematode Globodera Pallida. BMC Genom. 2014, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Pogorelko, G.; Juvale, P.S.; Rutter, W.B.; Hewezi, T.; Hussey, R.; Davis, E.L.; Mitchum, M.G.; Baum, T.J. A Cyst Nematode Effector Binds to Diverse Plant Proteins, Increases Nematode Susceptibility and Affects Root Morphology. Mol. Plant Pathol. 2016, 17, 832–844. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Liu, R.; Ling, J.; Li, Y.; Yang, Y.; Xie, B.; Zhao, J.; Mao, Z. A Meloidogyne Incognita Effector Minc03329 Suppresses Plant Immunity and Promotes Parasitism. J. Integr. Agric. 2023, 22, 799–811. [Google Scholar] [CrossRef]
- Ramesh Sundar, A.; Velazhahan, R.; Nagarathinam, S.; Vidhyasekaran, P. Induction of Pathogenesis-Related Proteins in Sugarcane Leaves and Cell-Cultures by a Glycoprotein Elicitor Isolated from Colletotrichum Falcatum. Biol. Plant. 2008, 52, 321–328. [Google Scholar] [CrossRef]
- Cui, Z.; Liang, F.; Zhang, J.; Wang, F.; Liu, D.; Wang, H. Transgenic Expression of TaTLP1, a Thaumatin-like Protein Gene, Reduces Susceptibility to Common Root Rot and Leaf Rust in Wheat. Crop J. 2021, 9, 1214–1218. [Google Scholar] [CrossRef]
- Wen, T.-Y.; Wu, X.-Q.; Ye, J.-R.; Qiu, Y.-J.; Rui, L.; Zhang, Y. Two Novel Bursaphelenchus Xylophilus Kunitz Effector Proteins Using Different Infection and Survival Strategies to Suppress Immunity in Pine. Phytopathology 2023, 113, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Vigers, A.J.; Wiedemann, S.; Roberts, W.K.; Legrand, M.; Selitrennikoff, C.P.; Fritig, B. Thaumatin-like Pathogenesis-Related Proteins Are Antifungal. Plant Sci. 1992, 83, 155–161. [Google Scholar] [CrossRef]
- Liu, D.; He, X.; Li, W.; Chen, C.; Ge, F. Molecular Cloning of a Thaumatin-like Protein Gene from Pyrus Pyrifolia and Overexpression of This Gene in Tobacco Increased Resistance to Pathogenic Fungi. Plant Cell Tiss. Organ. Cult. 2012, 111, 29–39. [Google Scholar] [CrossRef]
- Shah, J.M.; Singh, R.; Veluthambi, K. Transgenic Rice Lines Constitutively Co-Expressing Tlp-D34 and Chi11 Display Enhancement of Sheath Blight Resistance. Biol. Plant. 2013, 57, 351–358. [Google Scholar] [CrossRef]
- Wang, X.; Xue, B.; Dai, J.; Qin, X.; Liu, L.; Chi, Y.; Jones, J.T.; Li, H. A Novel Meloidogyne Incognita Chorismate Mutase Effector Suppresses Plant Immunity by Manipulating the Salicylic Acid Pathway and Functions Mainly during the Early Stages of Nematode Parasitism. Plant Pathol. 2018, 67, 1436–1448. [Google Scholar] [CrossRef]
- Sung, Y.-C.; Outram, M.A.; Breen, S.; Wang, C.; Dagvadorj, B.; Winterberg, B.; Kobe, B.; Williams, S.J.; Solomon, P.S. PR1-Mediated Defence via C-Terminal Peptide Release Is Targeted by a Fungal Pathogen Effector. New Phytol. 2021, 229, 3467–3480. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, W.; Zhao, Q. Salicylic Acid Biosynthesis Is Not from Phenylalanine in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Rekhter, D.; Ding, Y.; Lüdke, D.; Feussner, K.; Wiermer, M.; Zhang, Y.; Feussner, I. From Isochorismate to Salicylate: A New Reaction Mechanism for Salicylic Acid Biosynthesis. bioRXiv 2019. [Google Scholar] [CrossRef]
- Bagal, U.R.; Leebens-Mack, J.H.; Lorenz, W.W.; Dean, J.F. The Phenylalanine Ammonia Lyase (PAL) Gene Family Shows a Gymnosperm-Specific Lineage. BMC Genom. 2012, 13, S1. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- de Boer, J.M.; Yan, Y.; Smant, G.; Davis, E.L.; Baum, T.J. In-Situ Hybridization to Messenger RNA in Heterodera Glycines. J. Nematol. 1998, 30, 309–312. [Google Scholar] [PubMed]
- Hu, L.-J.; Wu, X.-Q.; Wen, T.-Y.; Ye, J.-R.; Qiu, Y.-J.; Rui, L.; Zhang, Y. The Key Molecular Pattern BxCDP1 of Bursaphelenchus Xylophilus Induces Plant Immunity and Enhances Plant Defense Response via Two Small Peptide Regions. Front. Plant Sci. 2022, 13, 937473. [Google Scholar] [CrossRef] [PubMed]
- Moffett, P.; Farnham, G.; Peart, J.; Baulcombe, D.C. Interaction between Domains of a Plant NBS–LRR Protein in Disease Resistance-Related Cell Death. EMBO J. 2002, 21, 4511–4519. [Google Scholar] [CrossRef] [PubMed]
- Šņepste, I.; Šķipars, V.; Krivmane, B.; Brūna, L.; Ruņģis, D. Characterization of a Pinus Sylvestris Thaumatin-like Protein Gene and Determination of Antimicrobial Activity of the in Vitro Expressed Protein. Tree Genet. Genomes 2018, 14, 58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Rui, L.; Qiu, Y.-J.; Wen, T.-Y.; Ye, J.-R.; Wu, X.-Q. The Bursaphelenchus xylophilus Effector BxNMP1 Targets PtTLP-L2 to Mediate PtGLU Promoting Parasitism and Virulence in Pinus thunbergii. Int. J. Mol. Sci. 2024, 25, 7452. https://doi.org/10.3390/ijms25137452
Yang D, Rui L, Qiu Y-J, Wen T-Y, Ye J-R, Wu X-Q. The Bursaphelenchus xylophilus Effector BxNMP1 Targets PtTLP-L2 to Mediate PtGLU Promoting Parasitism and Virulence in Pinus thunbergii. International Journal of Molecular Sciences. 2024; 25(13):7452. https://doi.org/10.3390/ijms25137452
Chicago/Turabian StyleYang, Dan, Lin Rui, Yi-Jun Qiu, Tong-Yue Wen, Jian-Ren Ye, and Xiao-Qin Wu. 2024. "The Bursaphelenchus xylophilus Effector BxNMP1 Targets PtTLP-L2 to Mediate PtGLU Promoting Parasitism and Virulence in Pinus thunbergii" International Journal of Molecular Sciences 25, no. 13: 7452. https://doi.org/10.3390/ijms25137452
APA StyleYang, D., Rui, L., Qiu, Y.-J., Wen, T.-Y., Ye, J.-R., & Wu, X.-Q. (2024). The Bursaphelenchus xylophilus Effector BxNMP1 Targets PtTLP-L2 to Mediate PtGLU Promoting Parasitism and Virulence in Pinus thunbergii. International Journal of Molecular Sciences, 25(13), 7452. https://doi.org/10.3390/ijms25137452





