Fetal Cannabinoid Syndrome: Behavioral and Brain Alterations of the Offspring Exposed to Dronabinol during Gestation and Lactation
Abstract
1. Introduction
2. Results
2.1. Behavioral Evaluation
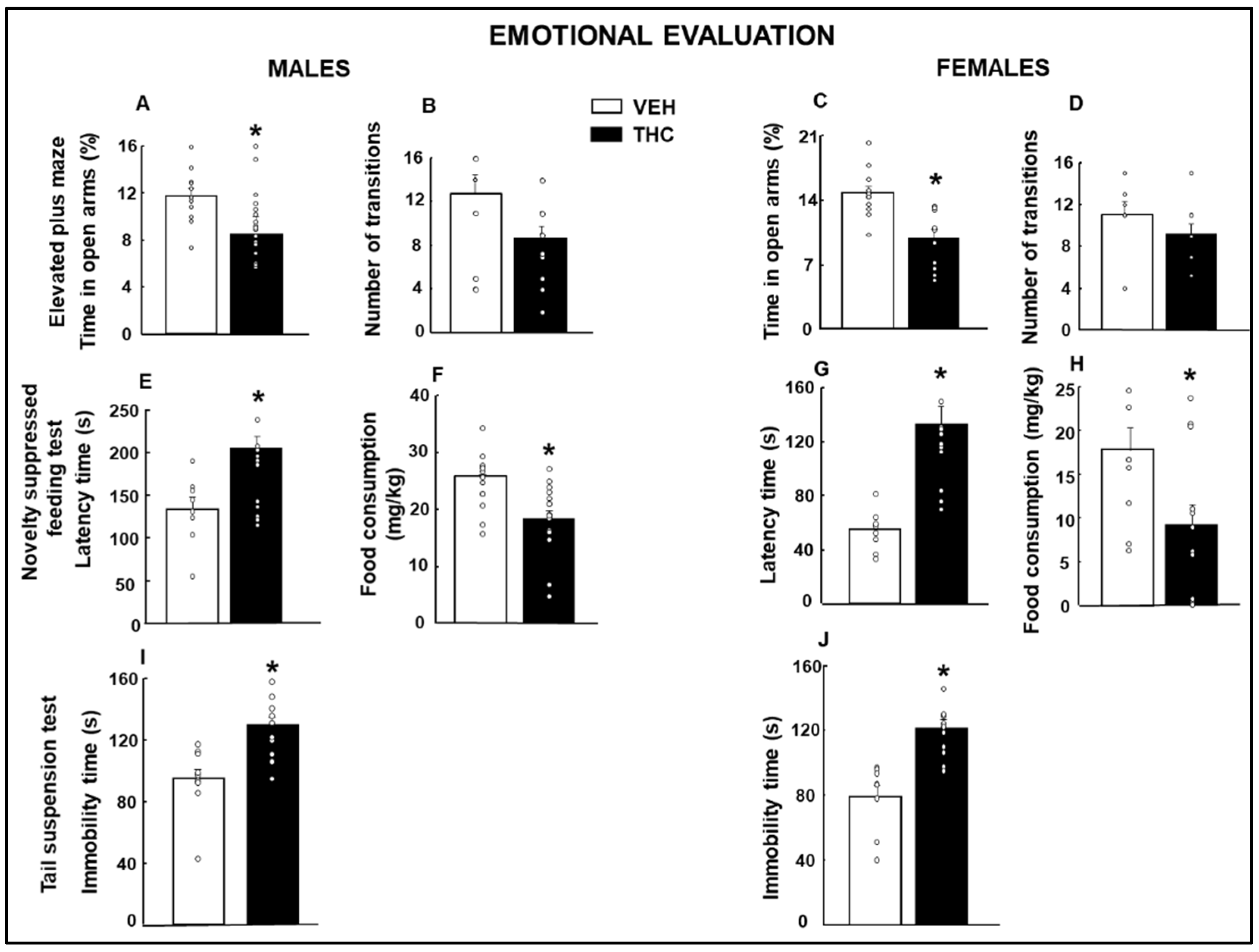
2.1.1. Emotional Evaluation
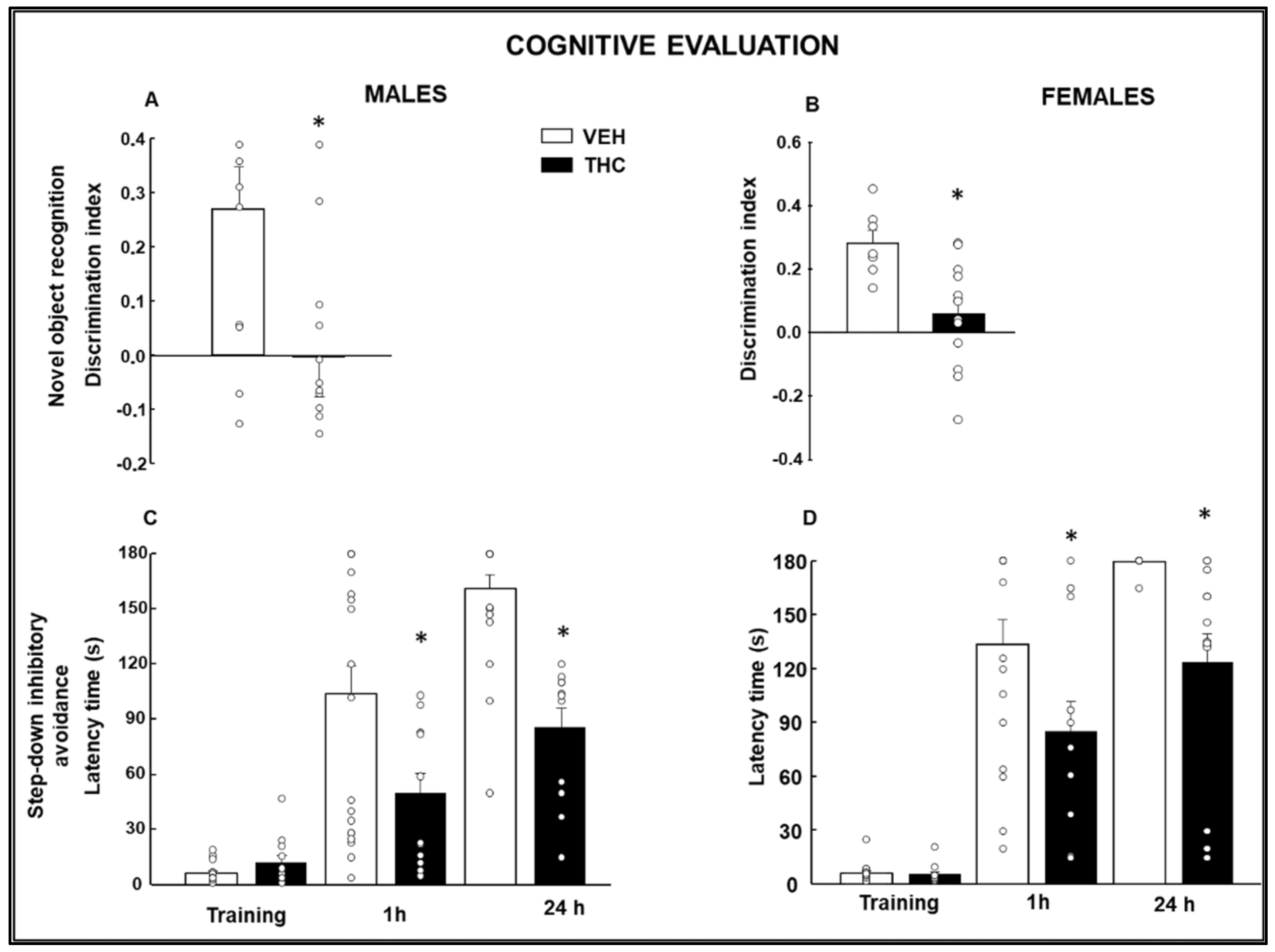
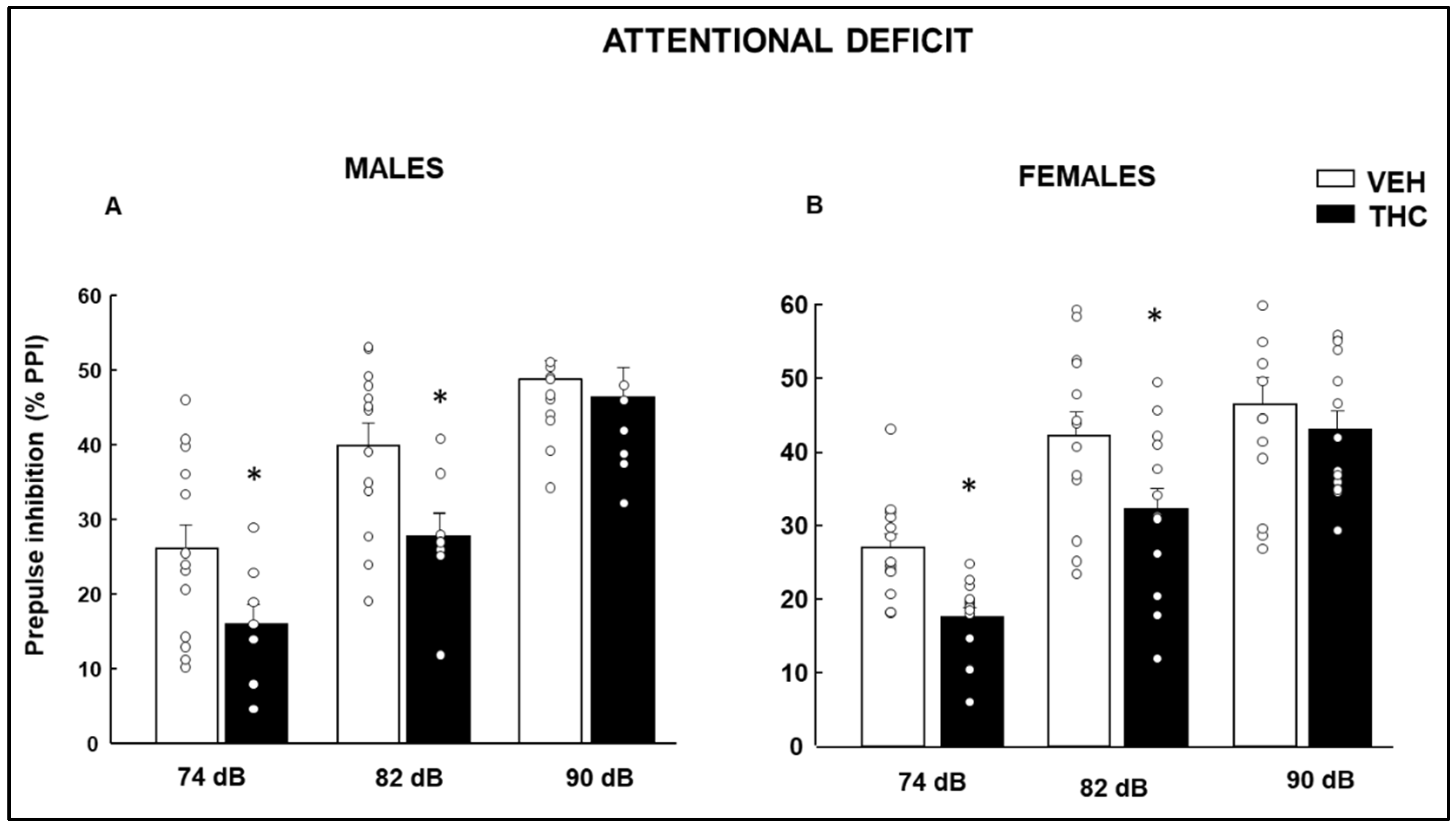
2.1.2. Cognitive Evaluation
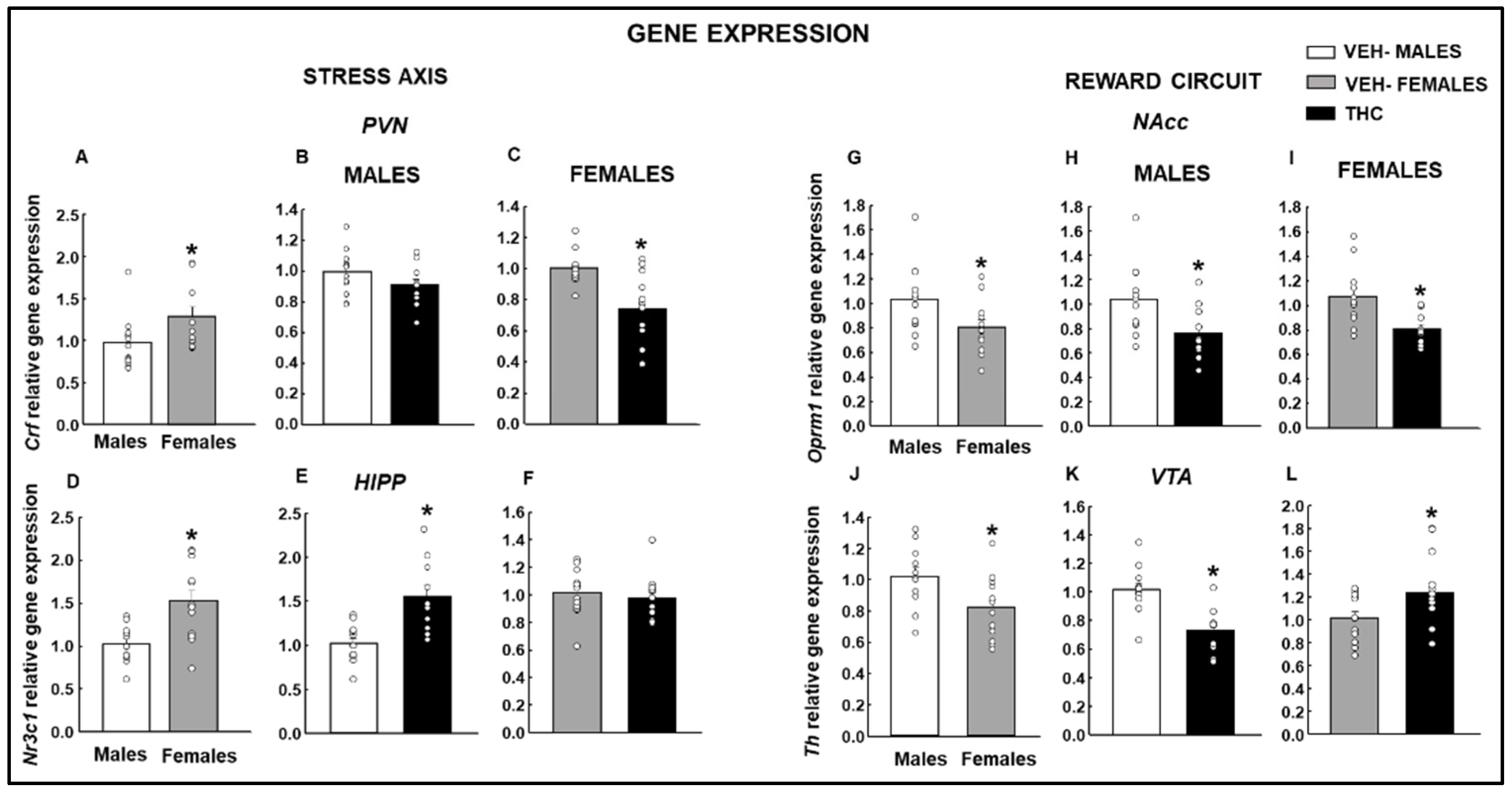
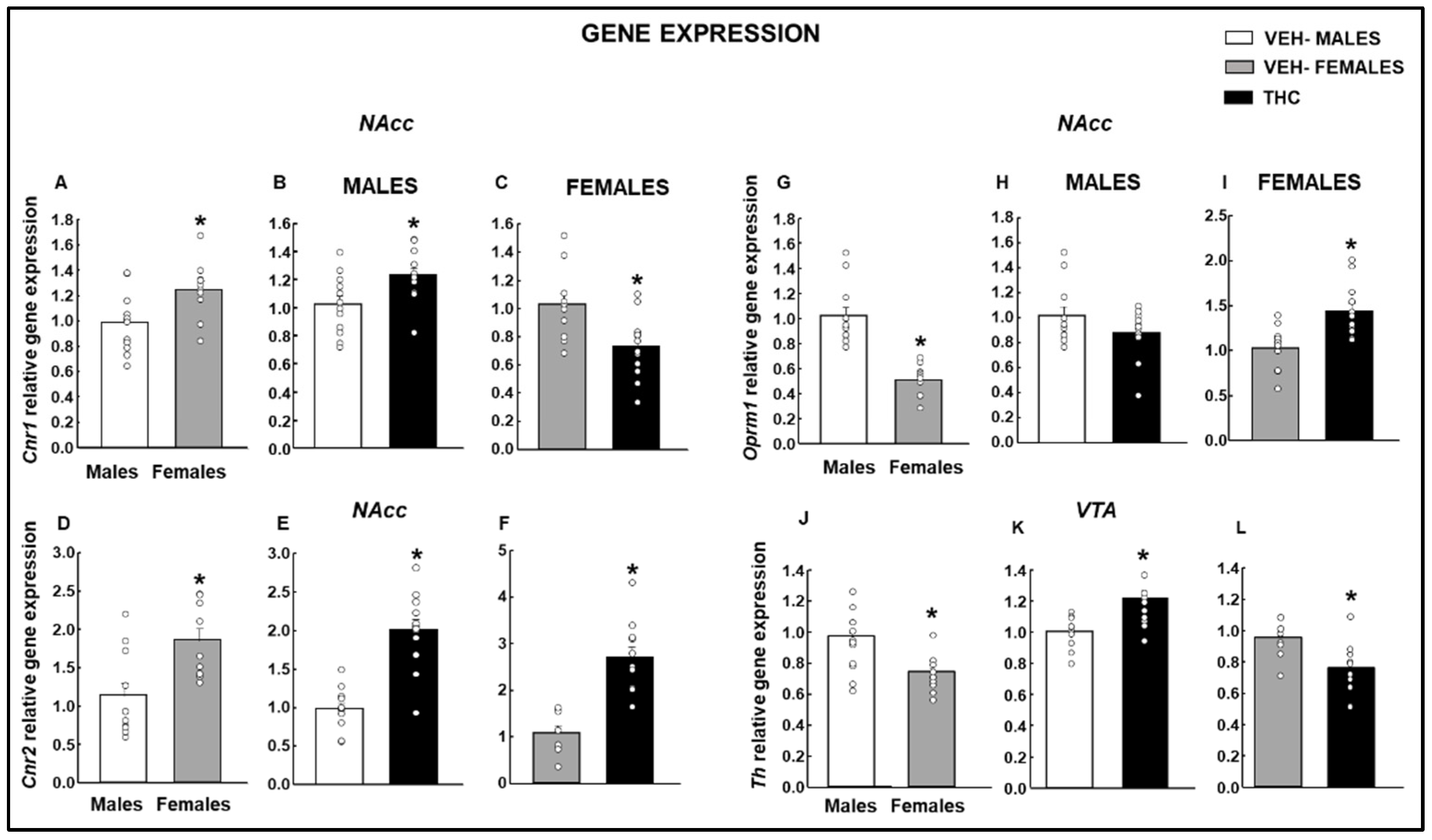
2.2. Gene Expression Studies under Basal Conditions
2.2.1. Stress Axis
2.2.2. Reward Circuit
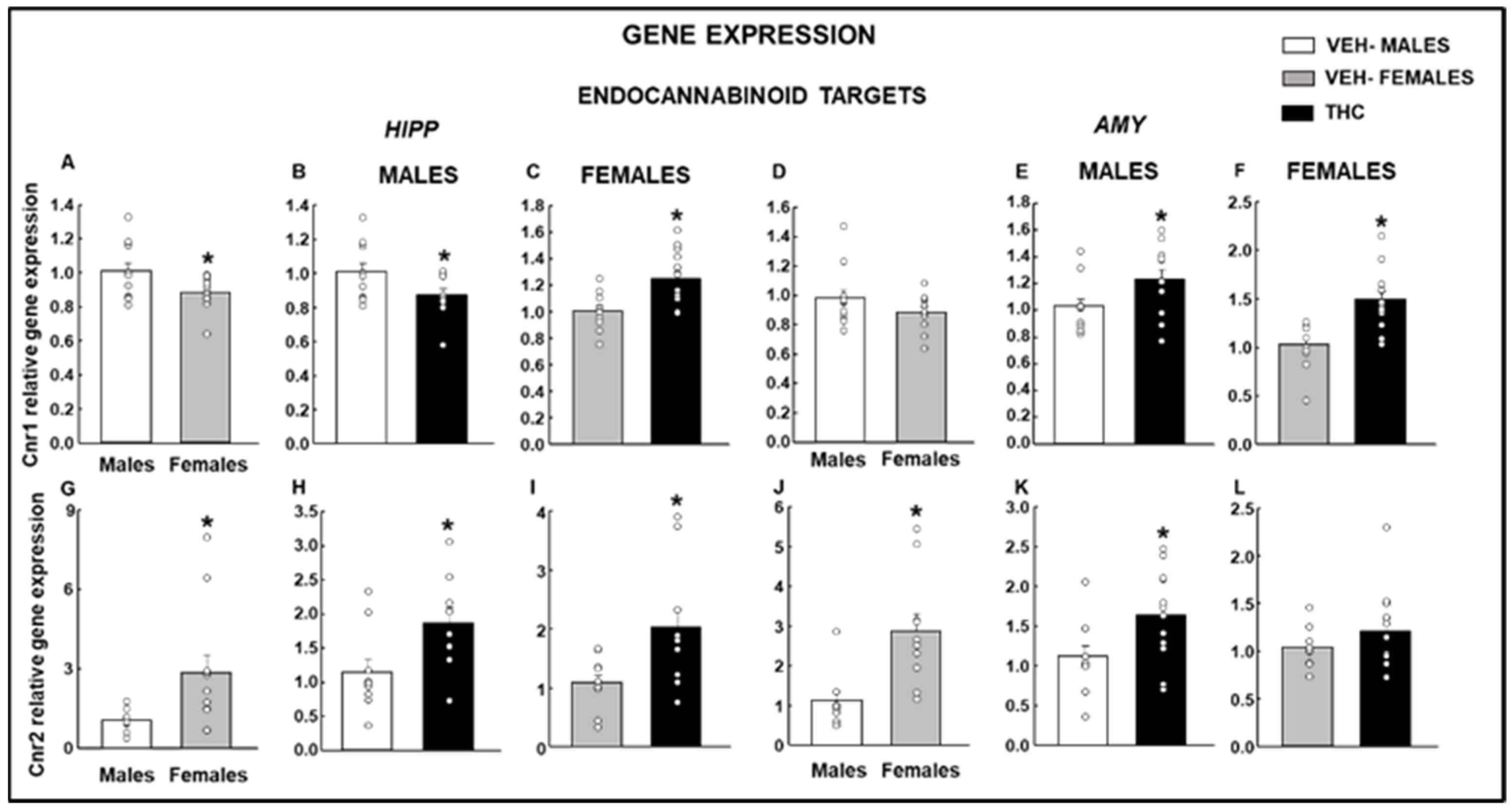
2.2.3. Endocannabinoid Targets
2.3. Immunohistochemistry
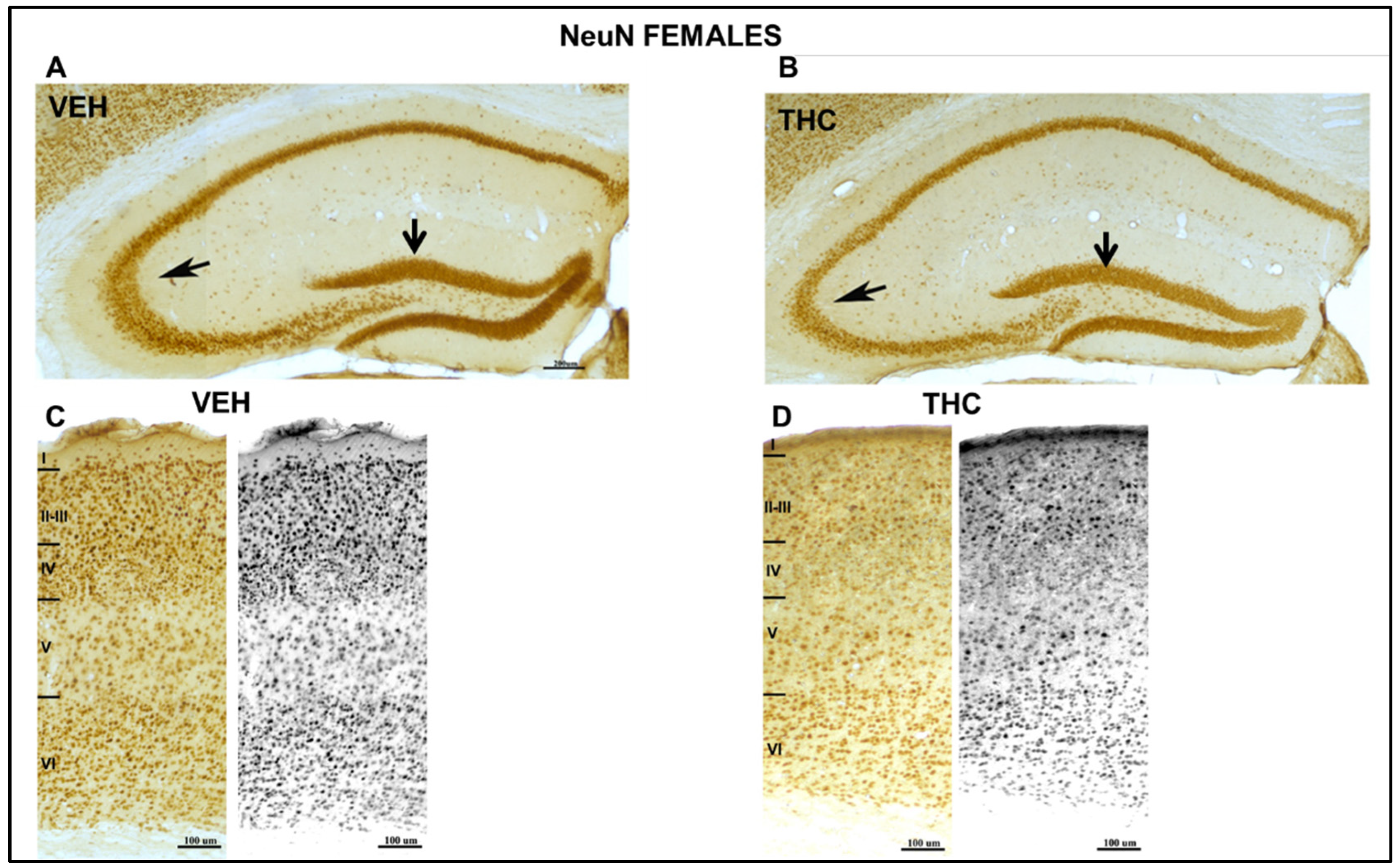
2.3.1. NeuN Immunolabeling in the HIPP’s DG and CA in the Somatosensorial Cortex
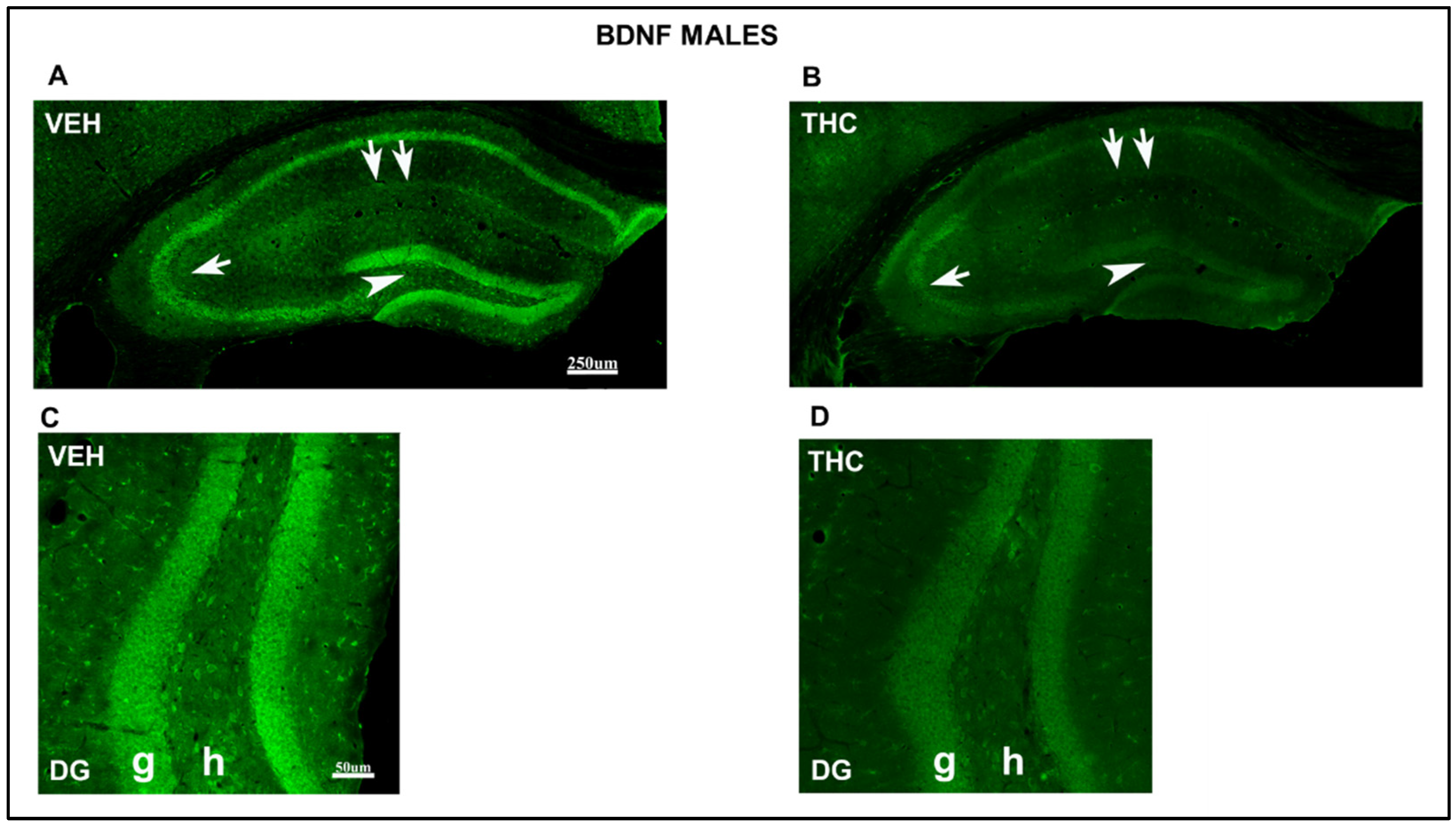
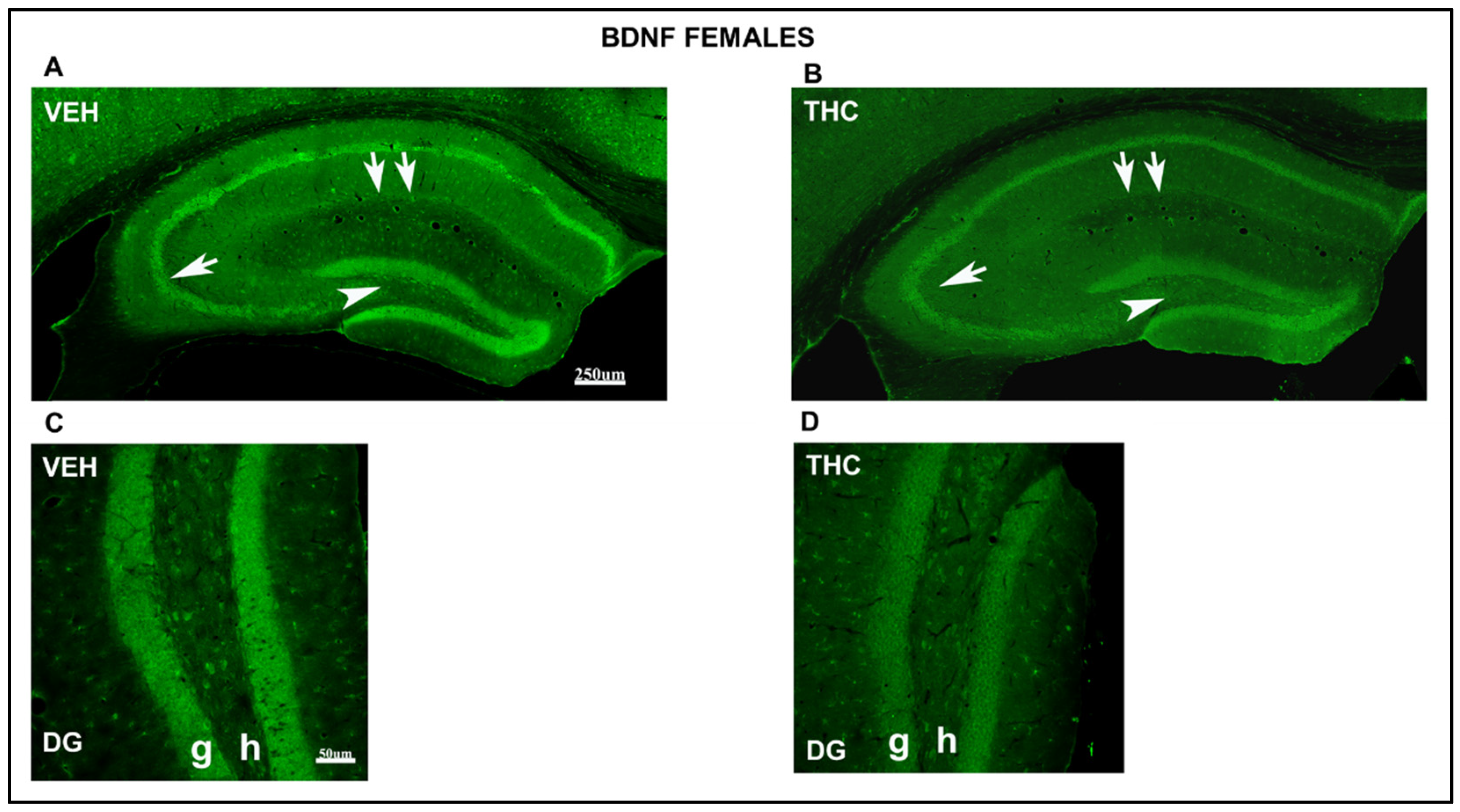
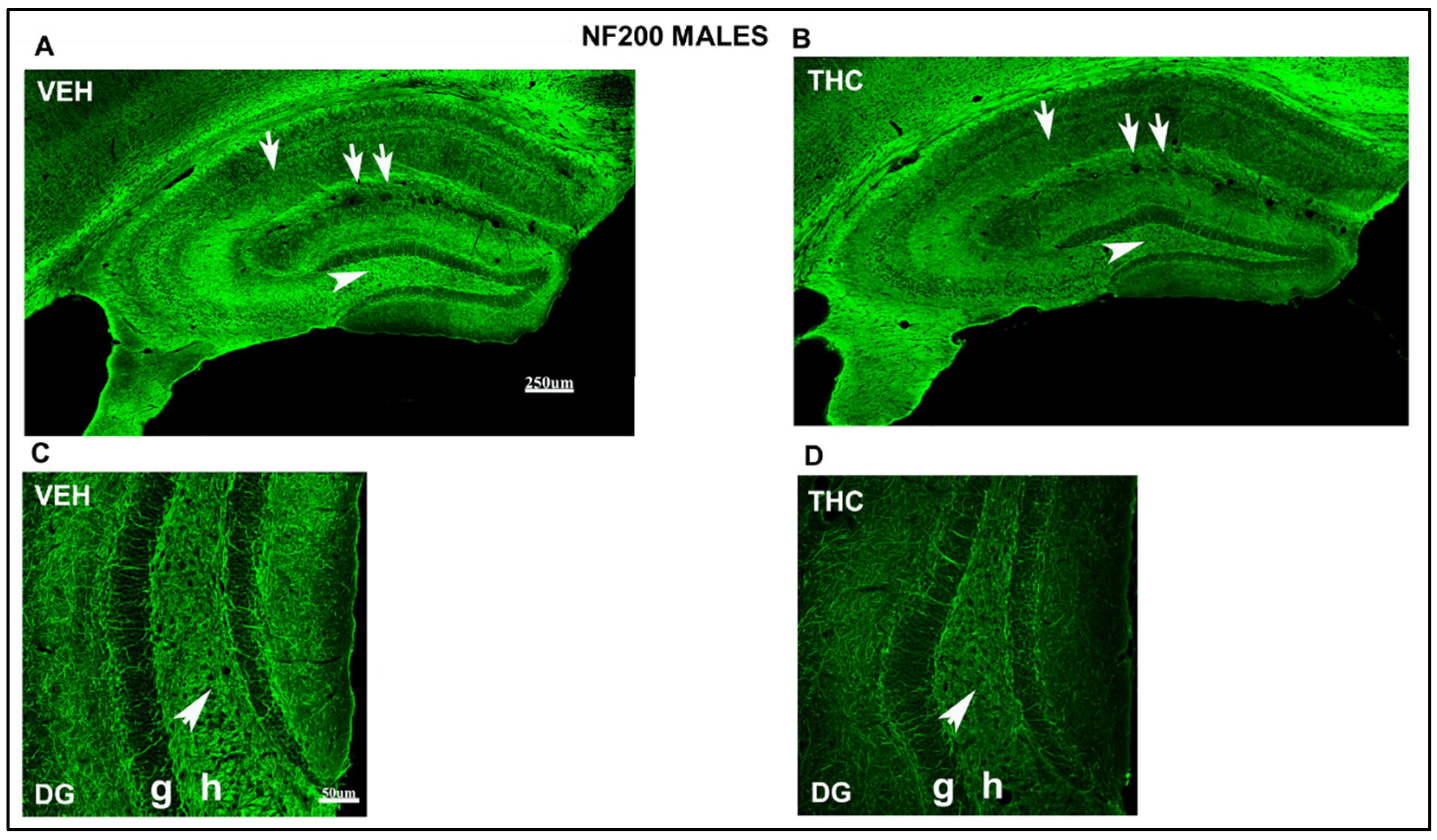
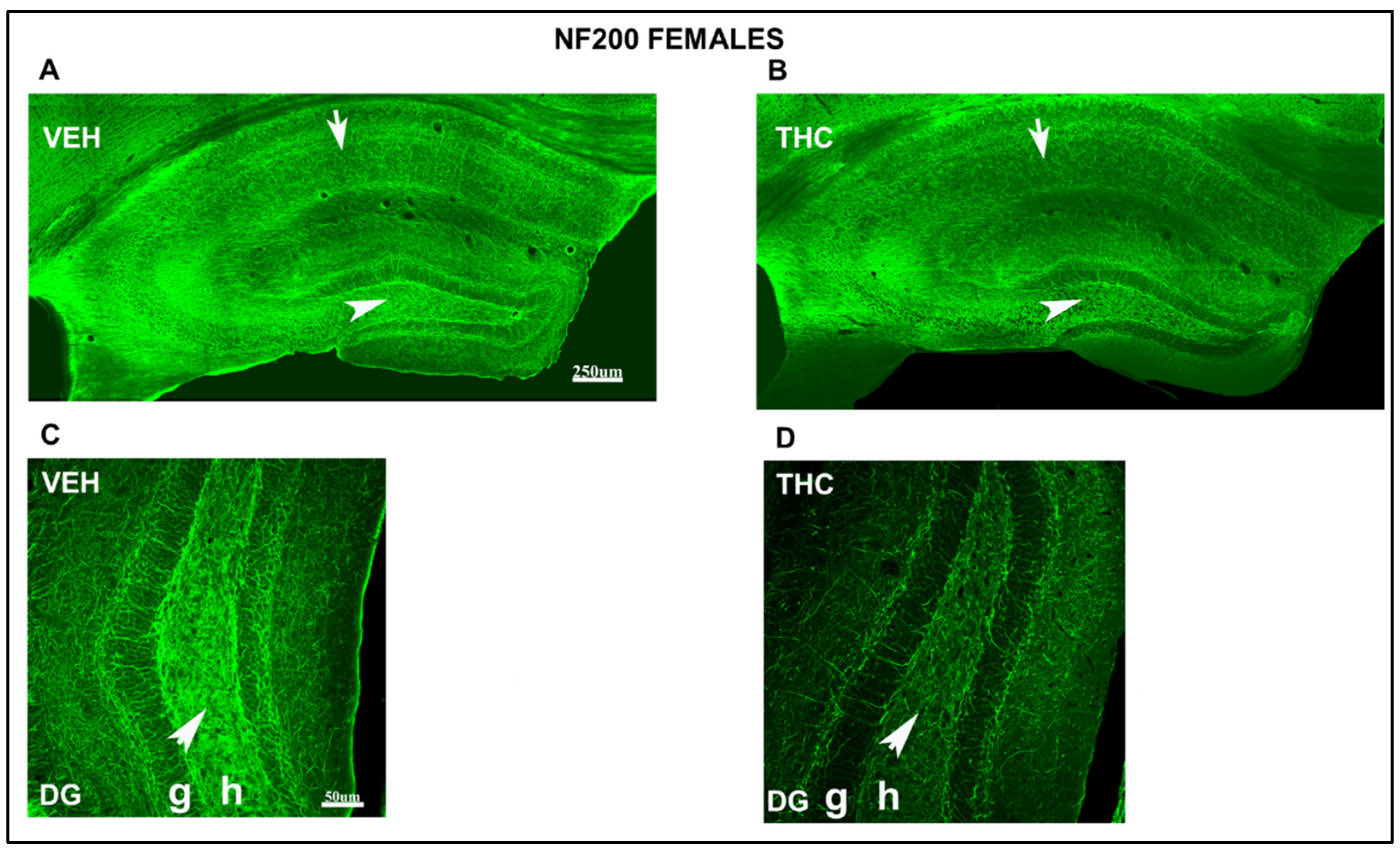
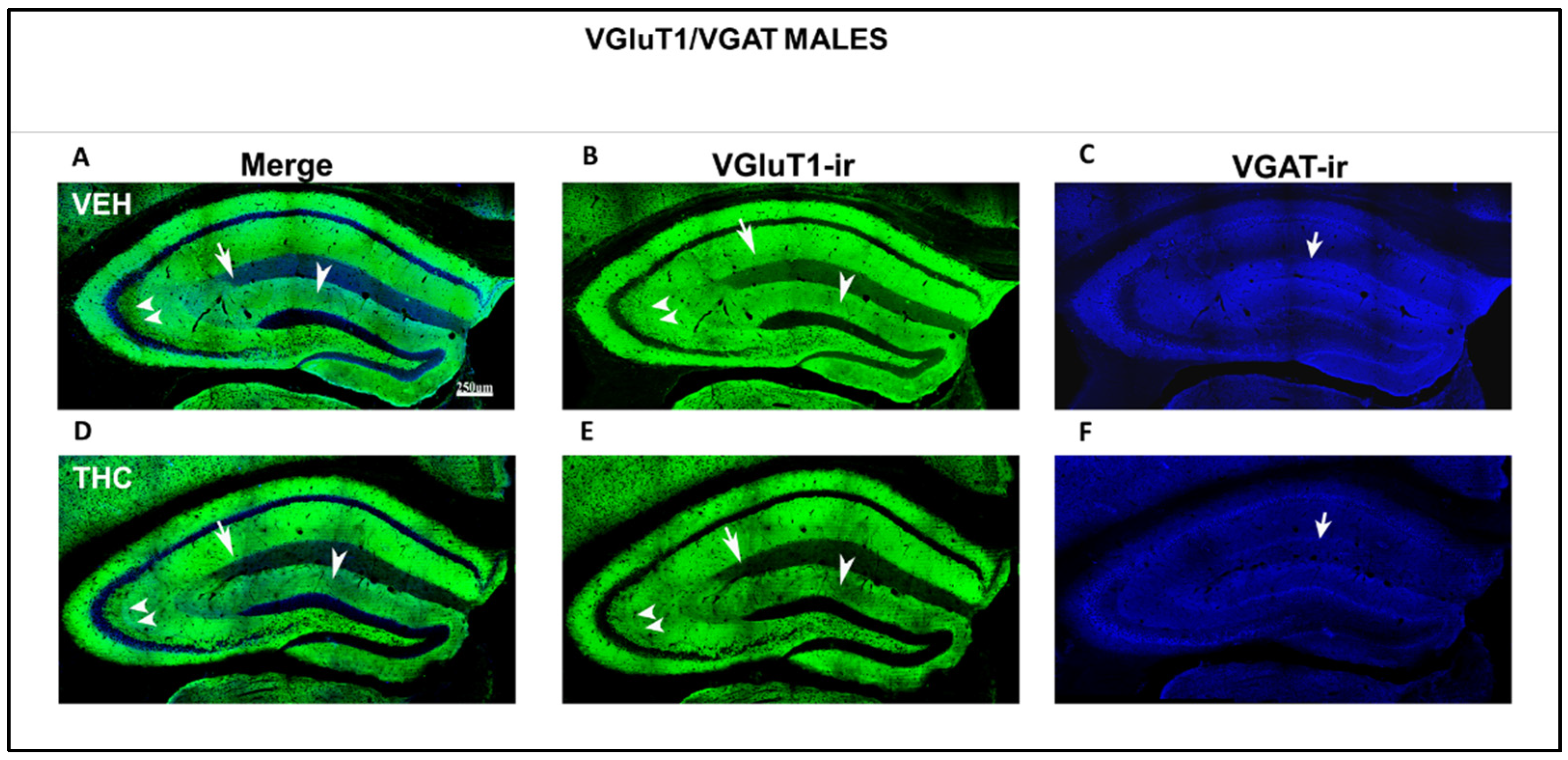
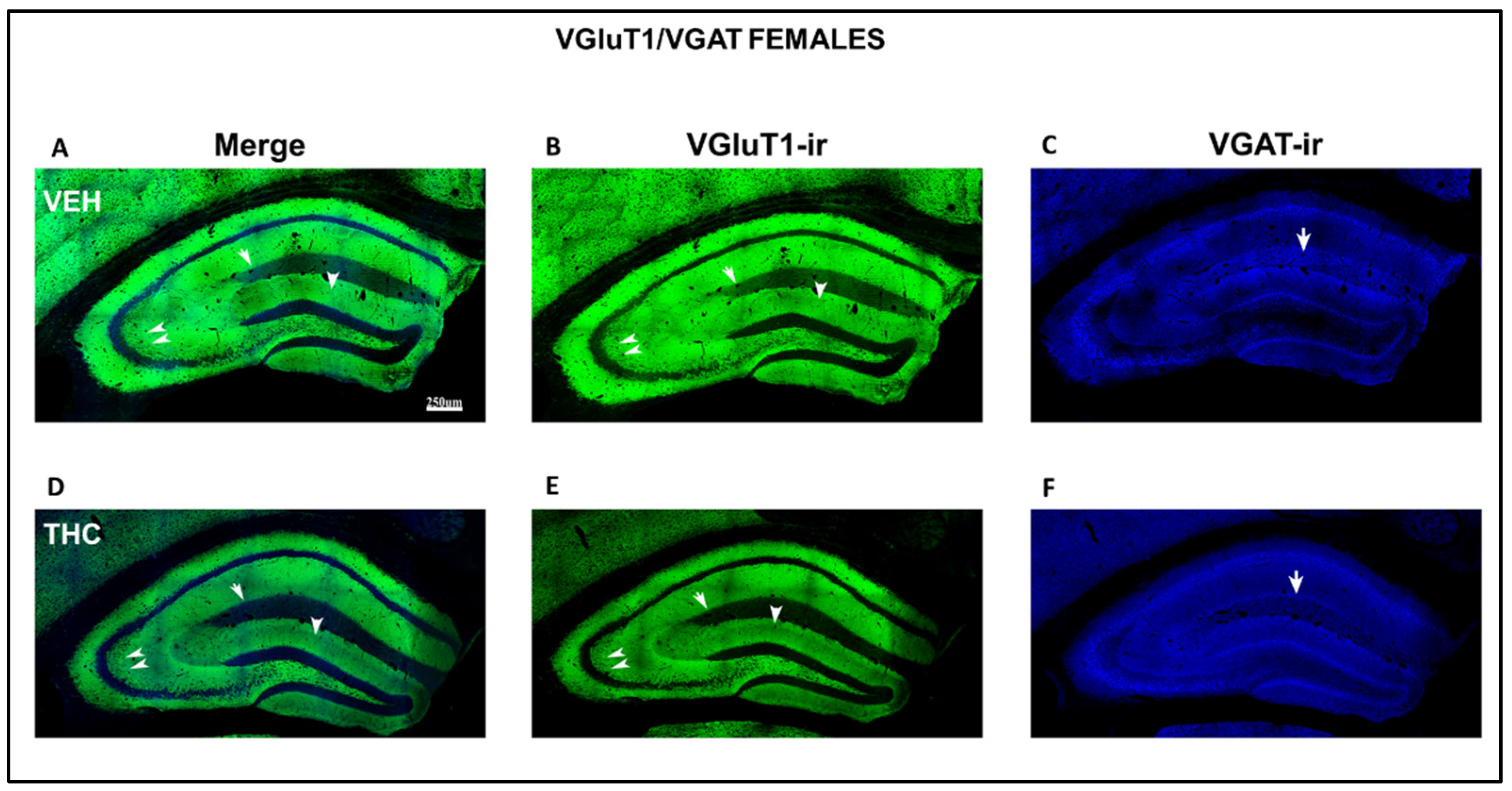
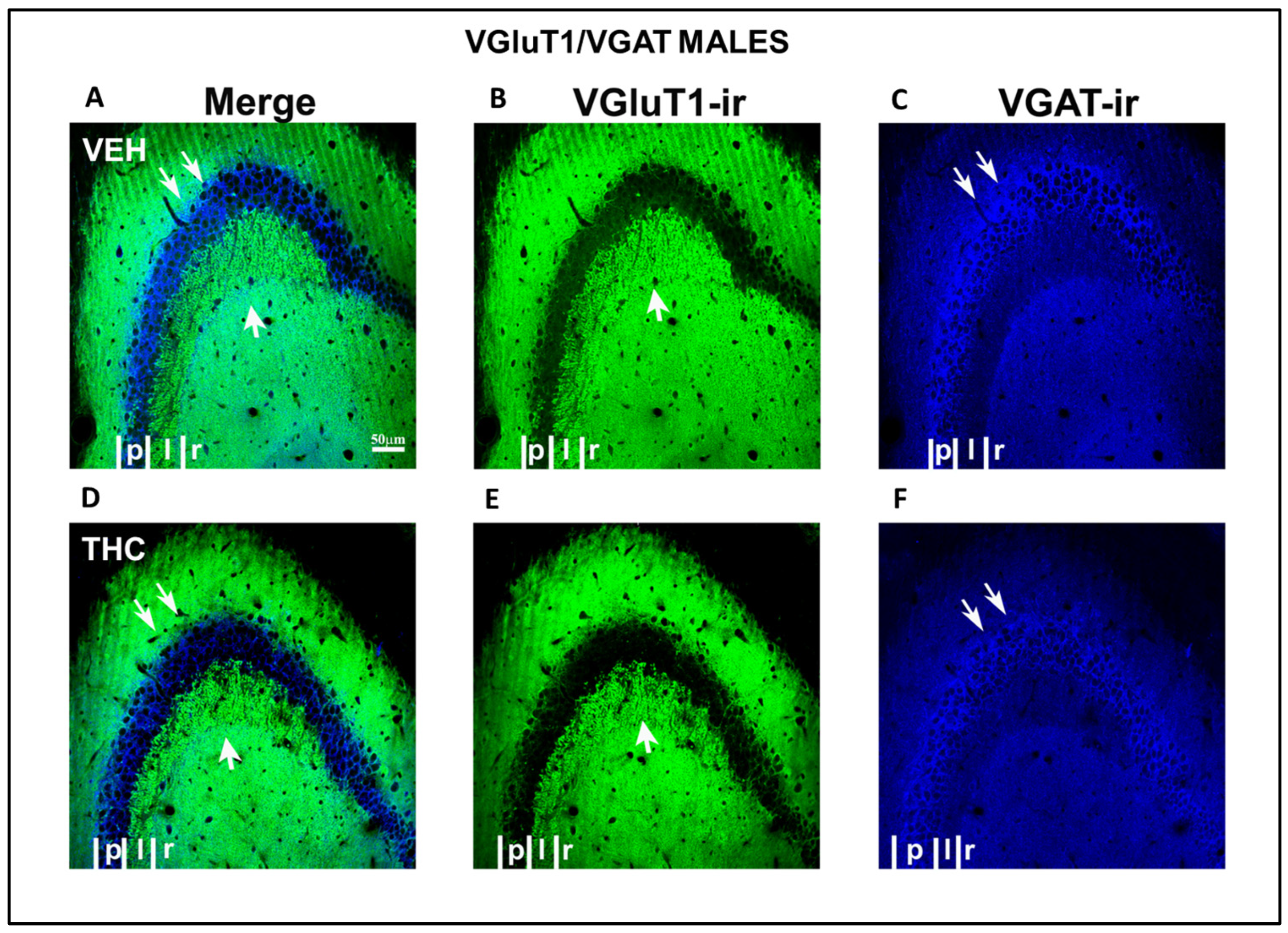
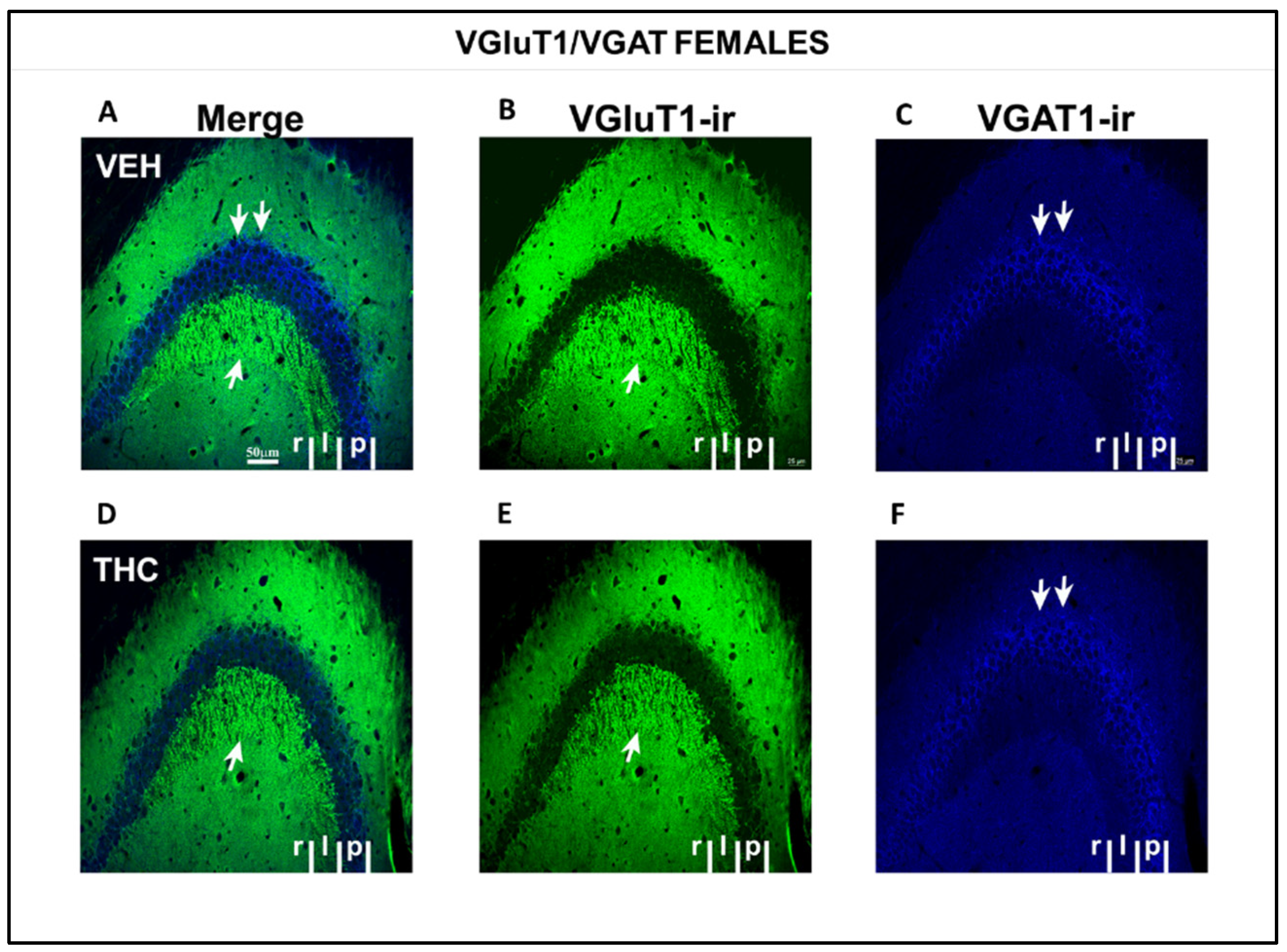
2.3.2. Immunolabeling BDNF, NF200, VGluT1, and VGAT in the DG and CA of the HIPP
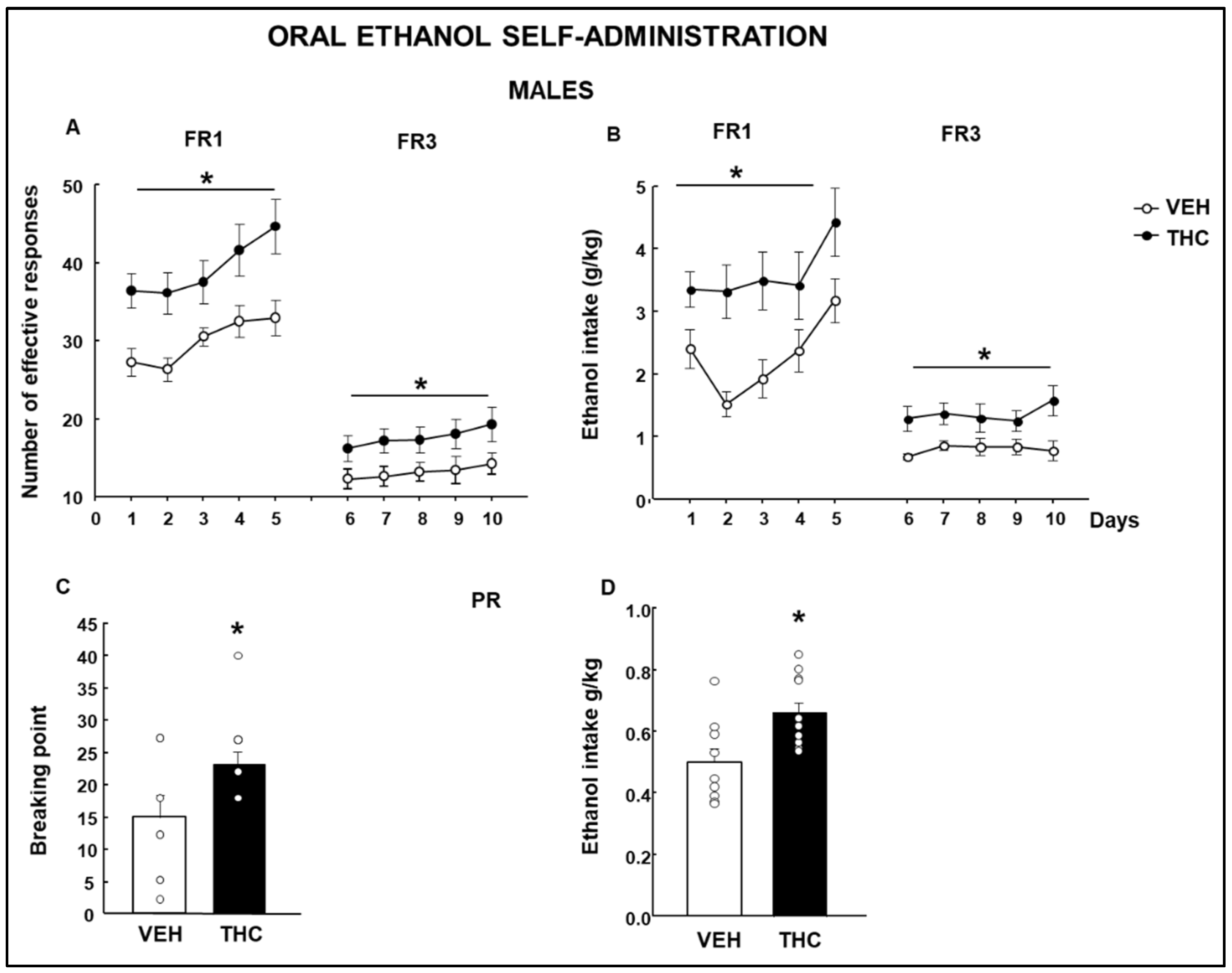
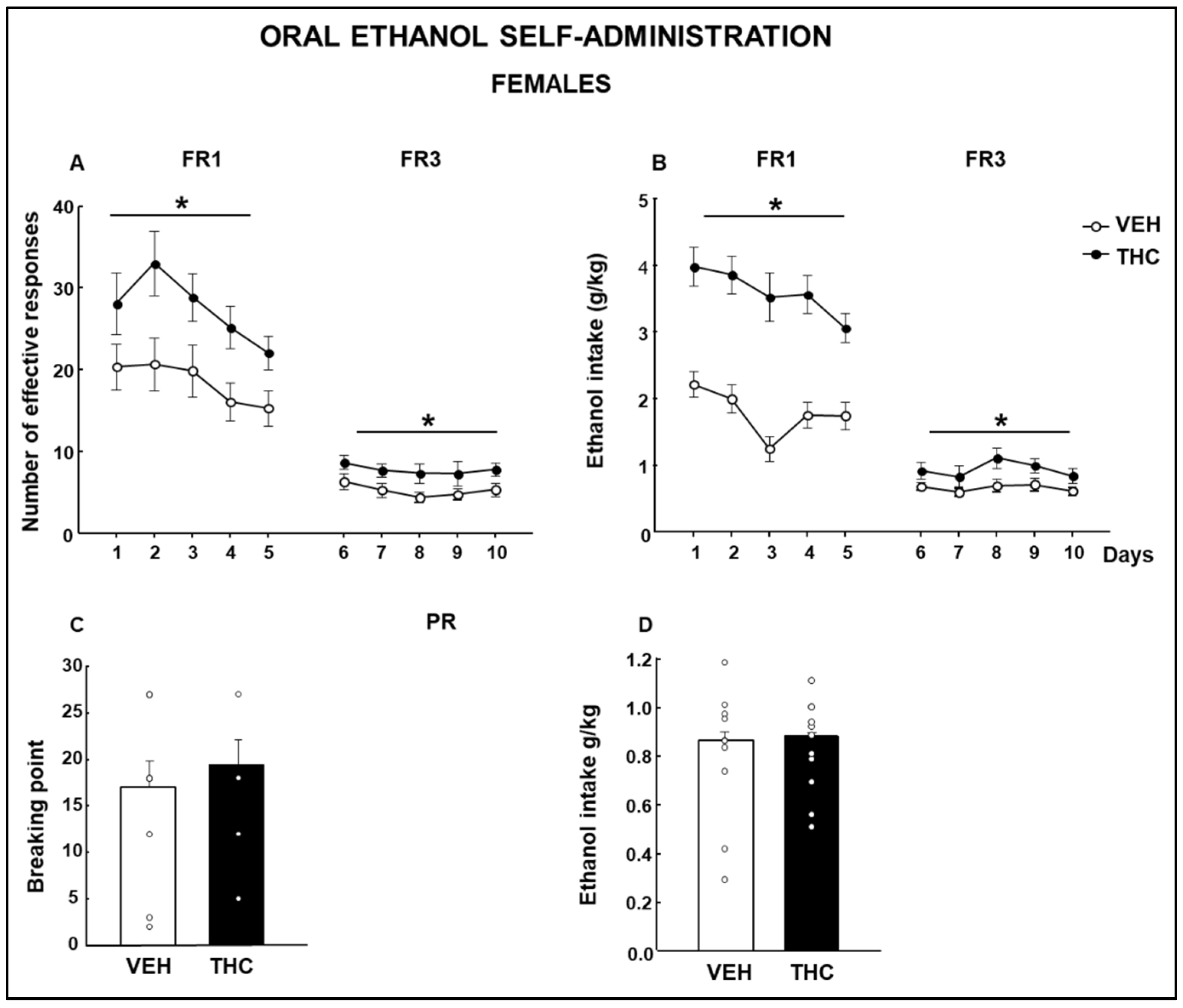
2.4. Oral Ethanol Self-Administration (OEA)
Gene Expression Studies in the OEA Paradigm-Exposed Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Perinatal Dronabinol Exposure Procedure
4.3. Experimental Design
4.3.1. Evaluation of Emotional/Cognitive Disturbances and Associated Neuromolecular Changes Induced by Perinatal Dronabinol Exposure
4.3.2. Evaluation of Oral Ethanol Self-Administration and Gene Expression in Animals Exposed to Dronabinol during the Perinatal Period
4.4. Emotional Evaluation
4.4.1. Light–Dark Box (LDB)
4.4.2. Novelty-Suppressed Feeding Test (NSFT)
4.4.3. Tail Suspension Test (TST)
4.4.4. Prepulse Inhibition (PPI)
4.5. Cognitive Evaluation
4.5.1. Novel Object Recognition (NOR)
4.5.2. Step-Down Inhibitory Avoidance (SDIA)
4.6. Oral Ethanol Self-Administration (OEA)
4.7. Conventional and Confocal Immunohistochemistry
4.8. Gene Expression Analyses by RT-PCR
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Drug Report; World Health Organisation: Geneva, Switzerland, 2023. [Google Scholar]
- NSDUH. The National Survey on Drug Use and Health: 2020; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2020.
- Grant, K.S.; Conover, E.; Chambers, C.D. Update on the developmental consequences of cannabis use during pregnancy and lactation. Birth Defects Res. 2020, 112, 1126–1138. [Google Scholar] [CrossRef]
- Badowski, S.; Smith, G. Cannabis use during pregnancy and postpartum. Can. Fam. Physician 2020, 66, 98–103. [Google Scholar] [PubMed]
- Metz, T.D.; Borgelt, L.M. Marijuana Use in Pregnancy and While Breastfeeding. Obs. Gynecol. 2018, 132, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.G.; Turner, J.D.; Parrott, A.C.; Goodwin, J.E.; Fulton, S.E.; Min, M.O.; Fox, H.C.; Braddick, F.M.; Axelsson, E.L.; Lynch, S.; et al. During pregnancy, recreational drug-using women stop taking ecstasy (3,4-methylenedioxy-N-methylamphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: Initial findings from the Development and Infancy Study. J. Psychopharmacol. 2010, 24, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Bara, A.; Ferland, J.N.; Rompala, G.; Szutorisz, H.; Hurd, Y.L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 2021, 22, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rosado, A.; Manzanares, J.; Fernandez-Ruiz, J.; Ramos, J.A. Prenatal Δ9-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: Sex-dependent differences. Brain Res. Dev. Brain Res. 2000, 120, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Corchero, J.; Garcia-Gil, L.; Manzanares, J.; Fernandez-Ruiz, J.J.; Fuentes, J.A.; Ramos, J.A. Perinatal Δ9-tetrahydrocannabinol exposure reduces proenkephalin gene expression in the caudate-putamen of adult female rats. Life Sci. 1998, 63, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Borelli, A.C.; Tomasini, M.C.; Morgano, L.; Antonelli, T.; Tanganelli, S.; Cuomo, V.; Ferraro, L. Long-lasting alterations of hippocampal GABAergic neurotransmission in adult rats following perinatal Δ9-THC exposure. Neurobiol. Learn. Mem. 2017, 139, 135–143. [Google Scholar] [CrossRef]
- Moreno, M.; Trigo, J.M.; Escuredo, L.; Rodriguez de Fonseca, F.; Navarro, M. Perinatal exposure to Δ9-tetrahydrocannabinol increases presynaptic dopamine D2 receptor sensitivity: A behavioral study in rats. Pharmacol. Biochem. Behav. 2003, 75, 565–575. [Google Scholar] [CrossRef]
- Frau, R.; Miczan, V.; Traccis, F.; Aroni, S.; Pongor, C.I.; Saba, P.; Serra, V.; Sagheddu, C.; Fanni, S.; Congiu, M.; et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat. Neurosci. 2019, 22, 1975–1985. [Google Scholar] [CrossRef]
- Beiersdorf, J.; Hevesi, Z.; Calvigioni, D.; Pyszkowski, J.; Romanov, R.; Szodorai, E.; Lubec, G.; Shirran, S.; Botting, C.H.; Kasper, S.; et al. Adverse effects of Δ9-tetrahydrocannabinol on neuronal bioenergetics during postnatal development. JCI Insight 2020, 5, e135418. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, D.E.; Martin, B.R.; Gamagaris, Z.; Miller, N.; Fico, T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989, 44, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chiang, C.N. Maternal-fetal transfer of abused substances: Pharmacokinetic and pharmacodynamic data. NIDA Res. Monogr. 1985, 60, 110–147. [Google Scholar]
- Brancato, A.; Castelli, V.; Lavanco, G.; Marino, R.A.M.; Cannizzaro, C. In utero Δ9-tetrahydrocannabinol exposure confers vulnerability towards cognitive impairments and alcohol drinking in the adolescent offspring: Is there a role for neuropeptide Y? J. Psychopharmacol. 2020, 34, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Vela, G.; Martin, S.; Garcia-Gil, L.; Crespo, J.A.; Ruiz-Gayo, M.; Fernandez-Ruiz, J.J.; Garcia-Lecumberri, C.; Pelaprat, D.; Fuentes, J.A.; Ramos, J.A.; et al. Maternal exposure to Δ9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998, 807, 101–109. [Google Scholar] [CrossRef]
- Spano, M.S.; Ellgren, M.; Wang, X.; Hurd, Y.L. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry 2007, 61, 554–563. [Google Scholar] [CrossRef]
- Szutorisz, H.; DiNieri, J.A.; Sweet, E.; Egervari, G.; Michaelides, M.; Carter, J.M.; Ren, Y.; Miller, M.L.; Blitzer, R.D.; Hurd, Y.L. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 2014, 39, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Borsoi, M.; Pelissier-Alicot, A.L.; Manzoni, O.J.J. Perinatal THC exposure via lactation induces lasting alterations to social behavior and prefrontal cortex function in rats at adulthood. Neuropsychopharmacology 2020, 45, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.; Knight, E.J.; Leemaqz, S.; Poston, L.; McCowan, L.; Kenny, L.; Myers, J.; Walker, J.J.; et al. The deleterious effects of cannabis during pregnancy on neonatal outcomes. Med. J. Aust. 2020, 212, 519–524. [Google Scholar] [CrossRef]
- Moore, B.F.; Sauder, K.A.; Shapiro, A.L.B.; Crume, T.; Kinney, G.L.; Dabelea, D. Fetal Exposure to Cannabis and Childhood Metabolic Outcomes: The Healthy Start Study. J. Clin. Endocrinol. Metab. 2022, 107, e2862–e2869. [Google Scholar] [CrossRef]
- Corsi, D.J.; Walsh, L.; Weiss, D.; Hsu, H.; El-Chaar, D.; Hawken, S.; Fell, D.B.; Walker, M. Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA 2019, 322, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S.; Richardson, G.A.; Coble, P.A.; Day, N.L.; Stoffer, D.S. The effects of prenatal alcohol and marijuana exposure: Disturbances in neonatal sleep cycling and arousal. Pediatr. Res. 1988, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Watkinson, B.; Gray, R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998, 20, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Jutras-Aswad, D.; DiNieri, J.A.; Harkany, T.; Hurd, Y.L. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Jakubovic, A.; Hattori, T.; McGeer, P.L. Radioactivity in suckled rats after giving 14C-tetrahydrocannabinol to the mother. Eur. J. Pharmacol. 1973, 22, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, M.; Wall, M.E. Presence of Δ9-tetrahydrocannabinol in human milk. N. Engl. J. Med. 1982, 307, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Monfort, A.; Ferreira, E.; Leclair, G.; Lodygensky, G.A. Pharmacokinetics of Cannabis and Its Derivatives in Animals and Humans During Pregnancy and Breastfeeding. Front. Pharmacol. 2022, 13, 919630. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.J.; Little, R.E. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol. 1990, 12, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; de Fonseca, F.R. Early Cannabinoid Exposure as a Source of Vulnerability to Opiate Addiction: A Model in Laboratory Rodents. Span. J. Ol. Psychol. 1998, 1, 39–58. [Google Scholar] [CrossRef]
- Rubio, P.; Rodriguez de Fonseca, F.; Munoz, R.M.; Ariznavarreta, C.; Martin-Calderon, J.L.; Navarro, M. Long-term behavioral effects of perinatal exposure to delta 9-tetrahydrocannabinol in rats: Possible role of pituitary-adrenal axis. Life Sci. 1995, 56, 2169–2176. [Google Scholar] [CrossRef]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Sandini, T.M.; Onofrychuk, T.J.; Roebuck, A.J.; Hammond, S.A.; Udenze, D.; Hayat, S.; Herdzik, M.A.; McElroy, D.L.; Orvold, S.N.; Greba, Q.; et al. Repeated Exposure to High-THC Cannabis Smoke during Gestation Alters Sex Ratio, Behavior, and Amygdala Gene Expression of Sprague Dawley Rat Offspring. eNeuro 2023, 10, 10–16. [Google Scholar] [CrossRef]
- Sarikahya, M.H.; Cousineau, S.; De Felice, M.; Lee, K.; Wong, K.K.; DeVuono, M.V.; Jung, T.; Rodriguez-Ruiz, M.; Ng, T.H.J.; Gummerson, D.; et al. Prenatal THC Exposure Induces Sex-Dependent Neuropsychiatric Endophenotypes in Offspring and Long-Term Disruptions in Fatty-Acid Signaling Pathways Directly in the Mesolimbic Circuitry. eNeuro 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Sagheddu, C.; Traccis, F.; Serra, V.; Congiu, M.; Frau, R.; Cheer, J.F.; Melis, M. Mesolimbic dopamine dysregulation as a signature of information processing deficits imposed by prenatal THC exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110128. [Google Scholar] [CrossRef]
- Navarrete, F.; Garcia-Gutierrez, M.S.; Gasparyan, A.; Austrich-Olivares, A.; Femenia, T.; Manzanares, J. Cannabis Use in Pregnant and Breastfeeding Women: Behavioral and Neurobiological Consequences. Front. Psychiatry 2020, 11, 586447. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A. The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Cannabinoids—A Systematic Review. Brain Sci. 2018, 8, 40. [Google Scholar] [CrossRef]
- Antonelli, T.; Tomasini, M.C.; Tattoli, M.; Cassano, T.; Tanganelli, S.; Finetti, S.; Mazzoni, E.; Trabace, L.; Steardo, L.; Cuomo, V.; et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 2005, 15, 2013–2020. [Google Scholar] [CrossRef]
- Borgen, L.A.; Davis, W.M.; Pace, H.B. Effects of prenatal Δ9-tetrahydrocannabinol on the development of rat offspring. Pharmacol. Biochem. Behav. 1973, 1, 203–206. [Google Scholar] [CrossRef]
- Campolongo, P.; Trezza, V.; Ratano, P.; Palmery, M.; Cuomo, V. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 2011, 214, 5–15. [Google Scholar] [CrossRef]
- Newsom, R.J.; Kelly, S.J. Perinatal delta-9-tetrahydrocannabinol exposure disrupts social and open field behavior in adult male rats. Neurotoxicol Teratol. 2008, 30, 213–219. [Google Scholar] [CrossRef]
- Silva, L.; Zhao, N.; Popp, S.; Dow-Edwards, D. Prenatal tetrahydrocannabinol (THC) alters cognitive function and amphetamine response from weaning to adulthood in the rat. Neurotoxicol Teratol. 2012, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Campolongo, P.; Cassano, T.; Macheda, T.; Dipasquale, P.; Carratu, M.R.; Gaetani, S.; Cuomo, V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology 2008, 198, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Berrendero, F.; Manzanares, J.; Perez, A.; Corchero, J.; Fuentes, J.A.; Fernandez-Ruiz, J.J.; Ramos, J.A. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse 1998, 30, 298–308. [Google Scholar] [CrossRef]
- Manzanares, J.; Corchero, J.; Romero, J.; Fernandez-Ruiz, J.J.; Ramos, J.A.; Fuentes, J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999, 20, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ruat, J.; Hartmann, A.; Heinz, D.E.; Nemcova, P.; Stoffel, R.; Deussing, J.M.; Chen, A.; Wotjak, C.T. CB1 receptors in corticotropin-releasing factor neurons selectively control the acoustic startle response in male mice. Genes. Brain Behav. 2021, 20, e12775. [Google Scholar] [CrossRef]
- Weimar, H.V.; Wright, H.R.; Warrick, C.R.; Brown, A.M.; Lugo, J.M.; Freels, T.G.; McLaughlin, R.J. Long-term effects of maternal cannabis vapor exposure on emotional reactivity, social behavior, and behavioral flexibility in offspring. Neuropharmacology 2020, 179, 108288. [Google Scholar] [CrossRef]
- Locci, A.; Yan, Y.; Rodriguez, G.; Dong, H. Sex differences in CRF1, CRF, and CRFBP expression in C57BL/6J mouse brain across the lifespan and in response to acute stress. J. Neurochem. 2021, 158, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.P.; Marco, E.M.; Llorente, R.; Lopez-Gallardo, M. Endocannabinoid system and synaptic plasticity: Implications for emotional responses. Neural Plast. 2007, 2007, 52908. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). Cannabis (Marijuana) DrugFacts. Available online: https://nida.nih.gov/publications/drugfacts/cannabis-marijuana (accessed on 14 May 2024).
- Traccis, F.; Serra, V.; Sagheddu, C.; Congiu, M.; Saba, P.; Giua, G.; Devoto, P.; Frau, R.; Cheer, J.F.; Melis, M. Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats. Int. J. Mol. Sci. 2021, 22, 1666. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch. Gen. Psychiatry 1990, 47, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Koch, M. The neurobiology of startle. Prog. Neurobiol. 1999, 59, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Bast, T.; Feldon, J. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharmacol. Biochem. Behav. 2002, 73, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Daenen, E.W.; Wolterink, G.; Van Der Heyden, J.A.; Kruse, C.G.; Van Ree, J.M. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur. Neuropsychopharmacol. 2003, 13, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.G.; MacKenzie, E.M.; Yim, T.T.; Taepavarapruk, P.; Phillips, A.G. Electrical stimulation of the hippocampus disrupts prepulse inhibition in rats: Frequency- and site-dependent effects. Behav. Brain Res. 2004, 152, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Wu, H.H.; Dong, Y.L.; Zhang, T.; Wang, J.; Zhang, Y.; Wei, Y.Y.; Lu, Y.C.; Wu, S.X.; Wang, W.; et al. Neurochemical properties of BDNF-containing neurons projecting to rostral ventromedial medulla in the ventrolateral periaqueductal gray. Front. Neural Circuits 2014, 8, 137. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Caldeira, M.V.; Santos, S.D.; Duarte, C.B. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S310–S324. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Witter, M.P. Hippocampal Formation; Paxinos, G., Ed.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Li, Y.; Li, F.; He, N.; Guo, L.; Huang, X.; Lui, S.; Gong, Q. Neural hyperactivity related to working memory in drug-naive boys with attention deficit hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lallai, V.; Manca, L.; Sherafat, Y.; Fowler, C.D. Effects of Prenatal Nicotine, THC, or Co-Exposure on Cognitive Behaviors in Adolescent Male and Female Rats. Nicotine Tob. Res. 2022, 24, 1150–1160. [Google Scholar] [CrossRef]
- Scheyer, A.F.; Melis, M.; Trezza, V.; Manzoni, O.J.J. Consequences of Perinatal Cannabis Exposure. Trends Neurosci. 2019, 42, 871–884. [Google Scholar] [CrossRef]
- Frau, R.; Melis, M. Sex-specific susceptibility to psychotic-like states provoked by prenatal THC exposure: Reversal by pregnenolone. J. Neuroendocr. 2023, 35, e13240. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, Y.; Xiong, R.; Rosales, C.I.; Coles, C.; Hamada, K.; Asad, N.; Thatcher, G.R.J.; Lasek, A.W. Effect of a brain-penetrant selective estrogen receptor degrader (SERD) on binge drinking in female mice. Alcohol. Clin. Exp. Res. 2022, 46, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Gessa, G.L.; Muntoni, F.; Collu, M.; Vargiu, L.; Mereu, G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985, 348, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.S.; Shefner, S.A.; Dunwiddie, T.V. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990, 508, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.S.; Reith, M.E.; Jobe, P.C.; Dailey, J.W. Focal ethanol elevates extracellular dopamine and serotonin concentrations in the rat ventral tegmental area. Eur. J. Pharmacol. 1996, 301, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Fitzgerald, L.W.; Charlton, M.; Lane, S.; Trevisan, L.; Guitart, X.; Shoemaker, W.; Duman, R.S.; Nestler, E.J. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 1995, 21, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.M.; Ortiz, S.; Perez-Rial, S.; Manzanares, J. Time dependent alterations on tyrosine hydroxylase, opioid and cannabinoid CB1 receptor gene expressions after acute ethanol administration in the rat brain. Eur. Neuropsychopharmacol. 2008, 18, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.M.; Martin-Garcia, E.; Berrendero, F.; Robledo, P.; Maldonado, R. The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol. Depend. 2010, 108, 183–194. [Google Scholar] [CrossRef]
- Marinelli, P.W.; Quirion, R.; Gianoulakis, C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology 2003, 169, 60–67. [Google Scholar] [CrossRef]
- Fried, P.A.; Watkinson, B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J. Dev. Behav. Pediatr. 1990, 11, 49–58. [Google Scholar] [CrossRef]
- Fried, P.A.; Watkinson, B.; Gray, R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, L.; Richardson, G.A.; Willford, J.A.; Severtson, S.G.; Day, N.L. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol. 2012, 34, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Day, N.; Cornelius, M.; Goldschmidt, L.; Richardson, G.; Robles, N.; Taylor, P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol. 1992, 14, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Fried, P.A.; Hogan, M.J.; Cameron, I. Effects of prenatal marijuana on response inhibition: An fMRI study of young adults. Neurotoxicol Teratol. 2004, 26, 533–542. [Google Scholar] [CrossRef]
- Fried, P.A.; Smith, A.M. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef]
- Gasparyan, A.; Navarrete, F.; Manzanares, J. Cannabidiol and Sertraline Regulate Behavioral and Brain Gene Expression Alterations in an Animal Model of PTSD. Front. Pharmacol. 2021, 12, 694510. [Google Scholar] [CrossRef]
- Austrich-Olivares, A.; Garcia-Gutierrez, M.S.; Illescas, L.; Gasparyan, A.; Manzanares, J. Cannabinoid CB1 Receptor Involvement in the Actions of CBD on Anxiety and Coping Behaviors in Mice. Pharmaceuticals 2022, 15, 473. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Aitken, D.H.; Quirion, R.; Meaney, M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 1988, 95, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Vaugeois, J.M.; Passera, G.; Zuccaro, F.; Costentin, J. Individual differences in response to imipramine in the mouse tail suspension test. Psychopharmacology 1997, 134, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Paylor, R.; Crawley, J.N. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 1997, 132, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Izquierdo, L.A.; Barros, D.M.; Mello e Souza, T.; de Souza, M.M.; Quevedo, J.; Rodrigues, C.; Sant’Anna, M.K.; Madruga, M.; Medina, J.H. Differential involvement of cortical receptor mechanisms in working, short-term and long-term memory. Behav. Pharmacol. 1998, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Rubio, G.; Manzanares, J. Effects of naltrexone plus topiramate on ethanol self-administration and tyrosine hydroxylase gene expression changes. Addict. Biol. 2014, 19, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004. [Google Scholar]
- Palkovits, M. Punch sampling biopsy technique. Methods Enzym. 1983, 103, 368–376. [Google Scholar] [CrossRef]
- Navarrete, F.; Perez-Ortiz, J.M.; Manzanares, J. Pregabalin- and topiramate-mediated regulation of cognitive and motor impulsivity in DBA/2 mice. Br. J. Pharmacol. 2012, 167, 183–195. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, D.; Gasparyan, A.; Navarrete, F.; Manzanares, J. Fetal Cannabinoid Syndrome: Behavioral and Brain Alterations of the Offspring Exposed to Dronabinol during Gestation and Lactation. Int. J. Mol. Sci. 2024, 25, 7453. https://doi.org/10.3390/ijms25137453
Navarro D, Gasparyan A, Navarrete F, Manzanares J. Fetal Cannabinoid Syndrome: Behavioral and Brain Alterations of the Offspring Exposed to Dronabinol during Gestation and Lactation. International Journal of Molecular Sciences. 2024; 25(13):7453. https://doi.org/10.3390/ijms25137453
Chicago/Turabian StyleNavarro, Daniela, Ani Gasparyan, Francisco Navarrete, and Jorge Manzanares. 2024. "Fetal Cannabinoid Syndrome: Behavioral and Brain Alterations of the Offspring Exposed to Dronabinol during Gestation and Lactation" International Journal of Molecular Sciences 25, no. 13: 7453. https://doi.org/10.3390/ijms25137453
APA StyleNavarro, D., Gasparyan, A., Navarrete, F., & Manzanares, J. (2024). Fetal Cannabinoid Syndrome: Behavioral and Brain Alterations of the Offspring Exposed to Dronabinol during Gestation and Lactation. International Journal of Molecular Sciences, 25(13), 7453. https://doi.org/10.3390/ijms25137453








