Benzoxazinoids Biosynthetic Gene Cluster Identification and Expression Analysis in Maize under Biotic and Abiotic Stresses
Abstract
:1. Introduction
2. Results
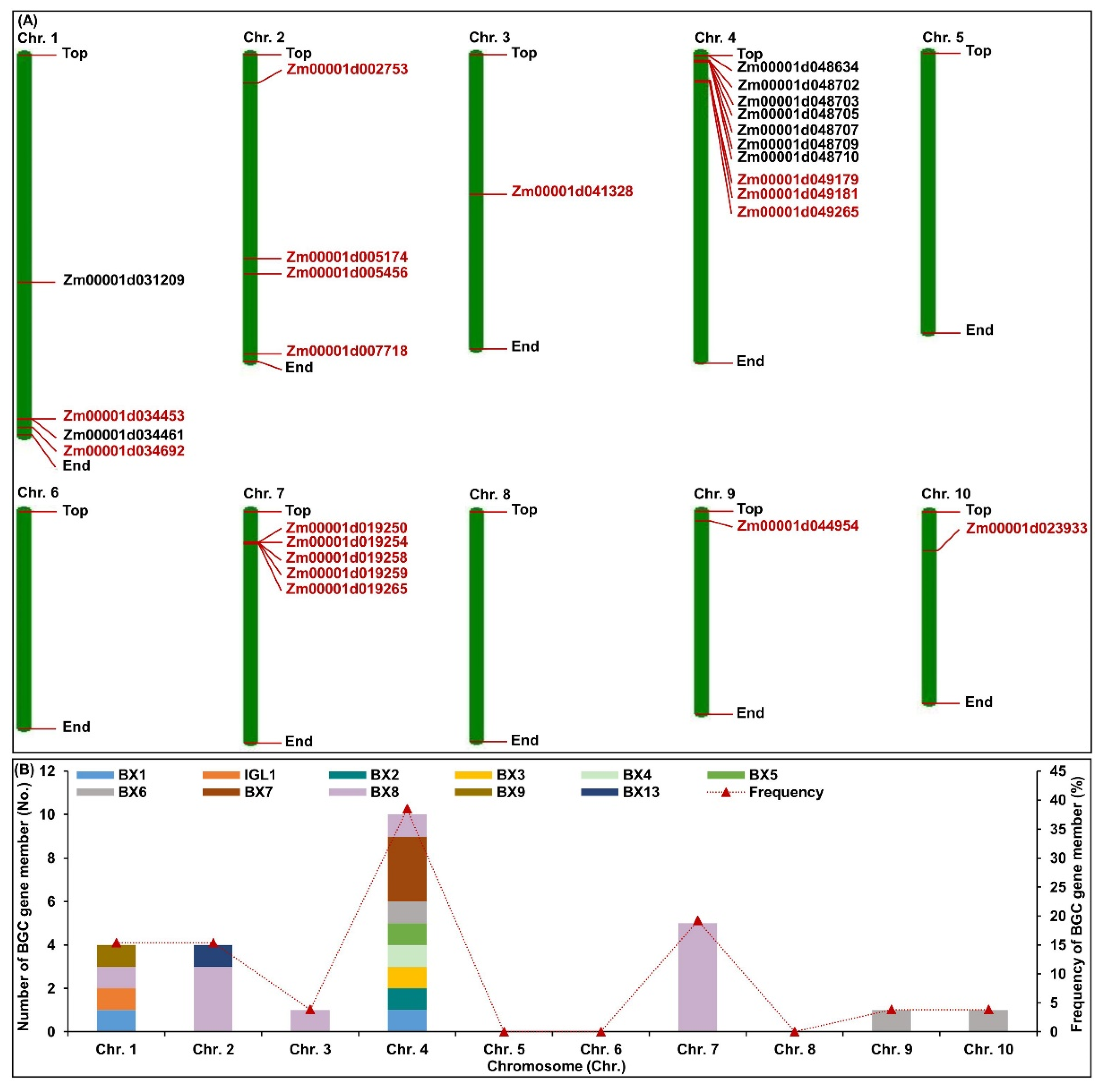
2.1. Genome-Wide Identification and Chromosome Distribution of BGC Gene Members
2.2. Characterization and Subcellular Location of BGC Gene Members
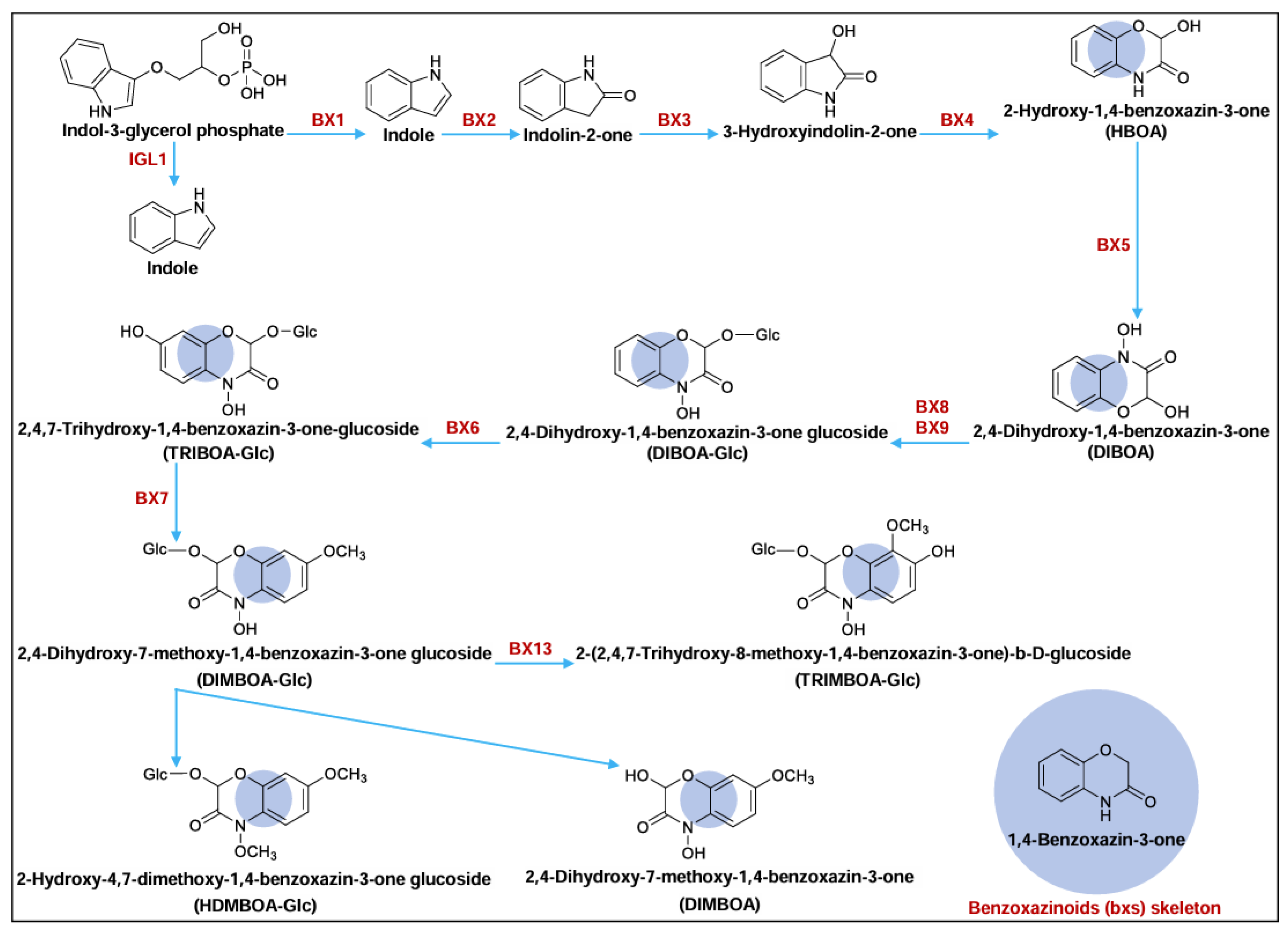
2.3. Sequence Analysis and Classification of BGC Gene Members
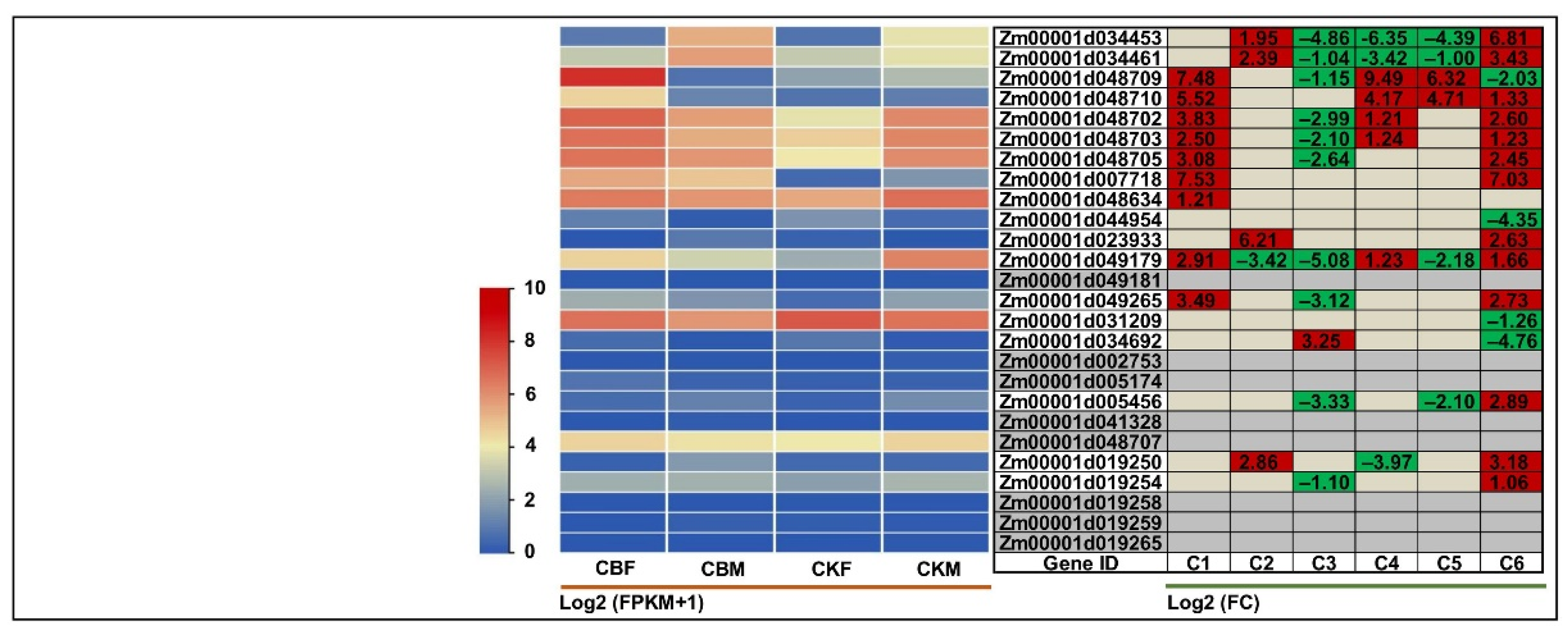
2.4. Expression Patterns of BGC Gene Members under Multiple Biotic/Abiotic Stresses
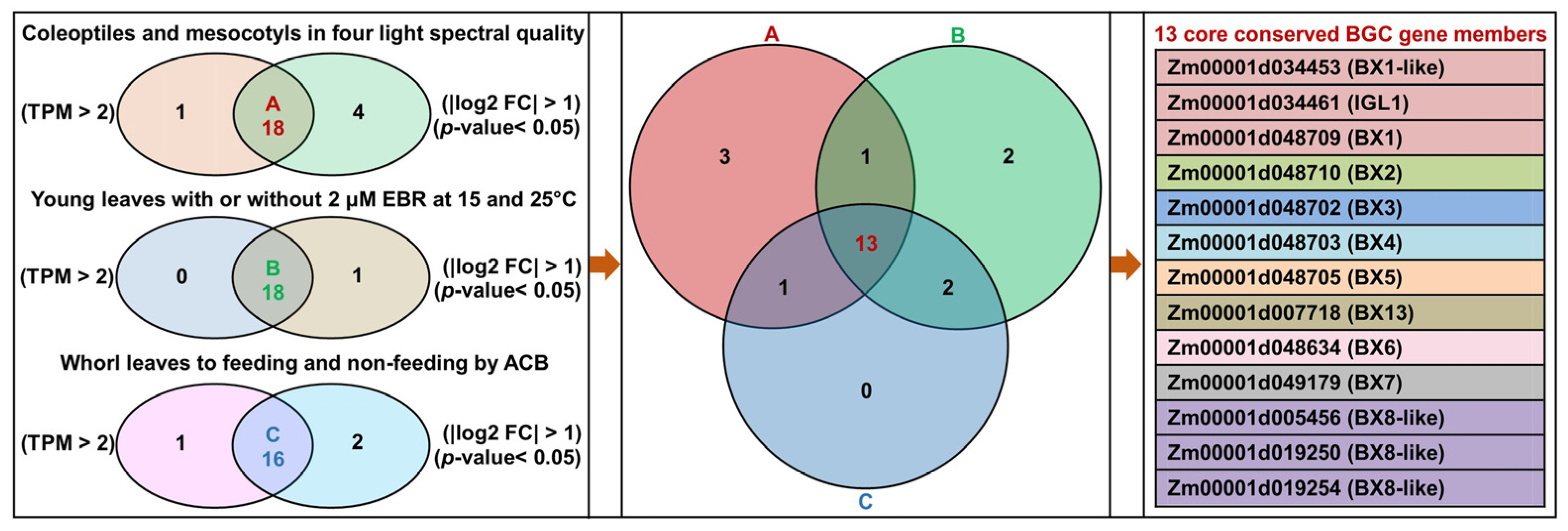
2.5. Identification of Core Conserved BGC Gene Members in Maize
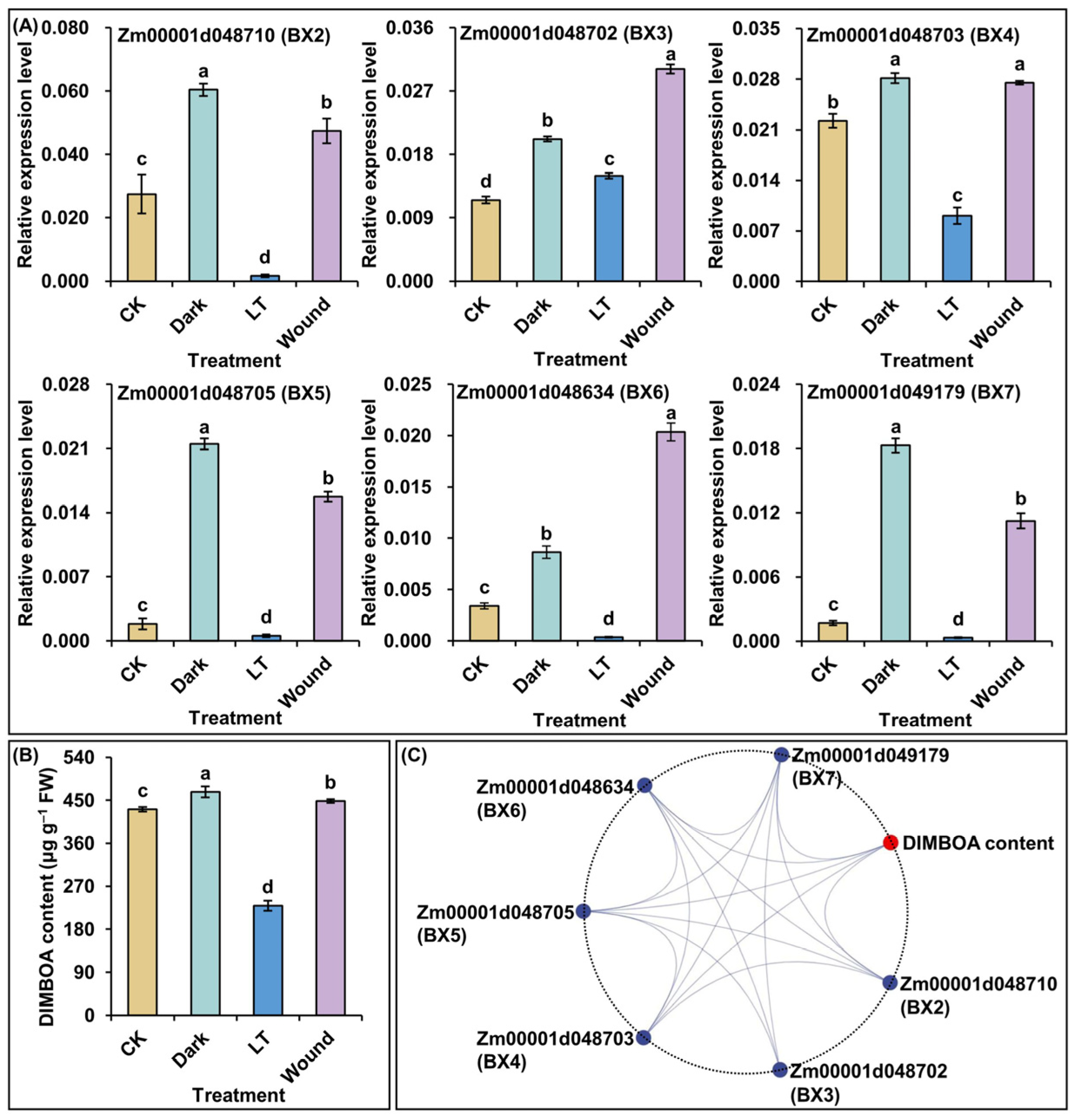
2.6. Effect of Multiple Stress Presences on Expression Levels of Core Conserved DEGs in BGC Genes and DIMBOA Accumulation
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification and Mapping of BGC Gene Members in Maize
4.2. Sequence Analysis, Structural Characterization, Subcellular Localization, and Phylogenetic Tree of BGC Gene Members in Maize
4.3. Expression Patterns of BGC Gene Members in Different Abiotic/Biotic-Induced Tissues by RNA-Seq
4.4. Core Conserved BGC Gene Members Identification
4.5. qRT-PCR Analysis of Core Conserved BGC Gene Members
4.6. Quantification of DIMBOA Contents
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.Q.; Richter, A.; Jander, G. Beyond defense: Multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Batyrshina, Z.S.; Yaakov, B.; Florean, M.; Köllner, T.G.; Tzin, V. The wheat diosygenase BX6 is involved in the formation of benzoxazinoids in planta and contributes to plant defense against insect herbivores. Plant Sci. 2022, 316, 111171. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.P.; Otte, B.A.; Kramer, M.; Schomberg, H.H.; Mirsky, S.B.; Tully, K.L. Benzoxazinoids in roots and shoots of cereal rye (Secale cereal) and their fates in soil after cover crop termination. Chemoecology 2022, 32, 117–128. [Google Scholar] [CrossRef]
- Adhikari, K.B.; Tanwir, F.; Gregersen, P.L.; Steffensen, S.K.; Jensen, B.M.; Poulsen, L.K.; Nielsen, C.H.; Hoyer, S.; Borre, M.; Fomsgaard, I.S. Benzoxazinoids: Ceral phytochemicals with putative therapeutic and health-protecting properties. Mol. Nutr. Food Res. 2015, 59, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Mwendwa, J.M.; Weston, P.A.; Weidenhamer, J.D.; Fomsgaard, I.S.; Wu, H.W.; Gurusinghe, S.; Weston, L.A. Metabolic profiling of benzoxazinoids in the roots and rhizosphere of commercial winter wheat genotypes. Plant Soil 2021, 466, 467–489. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef]
- Tzin, V.; Lindsay, P.L.; Christensen, S.A.; Meihls, L.N.; Blue, L.B.; Jander, G. Genetic mapping shows intraspecific variation and transgressive segregation for caterpillar-induced aphid resistance in maize. Mol. Ecol. 2015, 24, 5739–5750. [Google Scholar] [CrossRef] [PubMed]
- Dafoe, N.J.; Huffaker, A.; Vaughan, M.M.; Duehl, A.J.; Teal, P.E.; Schmelz, E.A. Rapidly induced chemical defenses in maize stems and their effects on short-term growth of Ostrinia nubilalis. J. Chem. 2011, 37, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Marti, G.; Villard, N.; Doyen, G.A.; Wolfender, J.L.; Turlings, T.C.J.; Erb, M. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011, 68, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Handrick, V.; Robert, C.A.M.; Ahern, K.R.; Zhou, S.Q.; Machado, R.A.R.; Maag, D.; Glauser, G.; Fernandez-Penny, F.E.; Chandran, J.N.; Rodgers-Melnik, E.; et al. Biosynthesis of 8-O-methylated benzoxazinoid defense compounds in maize. Plant Cell 2016, 28, 1682–1700. [Google Scholar] [CrossRef] [PubMed]
- Houseman, J.G.; Campos, F.; Thie, N.M.R.; Philogene, B.J.R.; Atkinson, J.; Morand, P.; Arnason, J.T. Effect of the maize derived compounds DIMBOA and MBOA on growth and digestive processes of European corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1992, 85, 669–674. [Google Scholar] [CrossRef]
- Yan, F.; Xu, C.; Li, S.; Lin, C.; Li, J. Effects of DIMBOA on several enzymatic system in Asian corn borer, Ostrinia furnacalis (Guenee). J. Chem. Ecol. 1995, 21, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Castanera, P.; Torres, V. Wound-induced changes in DIMBOA (2,4-dihydroxy-7-methoxy-2H-1,4 benzoxazin-3 (4H)-one concentration in maize plants caused by Sesamia nonagrioides (Lepidoptera: Noctuidae). Ann. Appl. Biol. 1988, 113, 447–454. [Google Scholar] [CrossRef]
- Varsani, S.; Grover, S.; Zhou, S.; Koch, K.G.; Huang, P.C.; Kolomiets, M.V.; Williams, W.P.; Heng-Moss, T.; Sarath, G.; Luthe, D.S.; et al. 12-Oxo-phytodienoic acid acts as a regulator of maize defense against corn leaf aphid. Plant Physiol. 2019, 179, 1402–1415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Cao, M.; Xie, L.J.; Liang, X.T.; Zeng, R.S.; Su, Y.J.; Huang, J.H.; Wang, R.L.; Luo, S.M. Induction of DIMBOA accumulation and systemic defense responses as a mechanism of enhanced resistance of mycorrhizal corn (Zea mays L.) to sheath blight. Mycorrhiza 2011, 21, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, H.; Fomsgaard, I.S.; Kudsk, P. Root-exuded benzoxazinoids: Uptake and translocation in neighboring plants. J. Agric. Food Chem. 2020, 68, 10609–10617. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Hurle, K. Differential exudation of two benzoxazinoids one of the determining factors for seedling allelopathy of triticeae species. J. Agric. Food Chem. 2005, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.R.; Luo, S.M.; Zeng, R.S.; Wang, J.W.; Huang, J.H.; Li, M. Research advance in cyclic hydroxamic acids, main allelochemicals of Zea mays. Chin. J. Appl. Ecol. 2004, 15, 1079–1082. [Google Scholar]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3 (4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Li, P.; Li, S.; Ma, C.R.; Liu, H.; Wang, L.; Qi, J.F.; Wu, J.Q. ZmMPK6 and ethylene signaling negatively regulate the accumulation of anti-insect metabolites DIMBOA and DIMBOA-Glc in maize inbred line A188. New Phytol. 2021, 229, 2273–2287. [Google Scholar] [CrossRef]
- Gao, C.N.; Song, P.F.; Yao, S.Y.; Sheng, Z.Y.; Li, W.Z.; Luo, M.H.; Yuan, G.H. Stusy on Ostrinia furnacalis larvae inhibit DIMBOA biosynthesis in maize leaves using regurgitant. J. Henan Agric. Univ. 2020, 54, 248–253. [Google Scholar]
- Chandra, A.; Singh, S.B.; Kumar, P. Effect of light on DIMBOA synthesis in maize leaves as revealed by modified cost-effective extraction method. Environ. Monit. Assess. 2013, 185, 9917–9924. [Google Scholar] [CrossRef] [PubMed]
- Yuval, B.A.; Avigdor, B.; Dvir, F.; Eviatar, N. Adaptive evolution of benzoxazinoids in wild emmer wheat, Triticum dicoccoides, at “Evolution Canyon”, Mount Carmel, Israel. PLoS ONE 2018, 13, e0190424. [Google Scholar]
- Jabeen, R.; Yamada, K.; Hasegawa, T.; Minami, E.; Shigemori, H.; Hasegawa, K. Direct involvement of benzoxazinoids in the growth suppression induced by phototropic stimulation in maize coleoptiles. Heterocycles 2007, 71, 523–529. [Google Scholar]
- Betsiashvili, M.; Ahern, K.R.; Jander, G. Additive effects of two quantitative trait loci that confer Rhopalosiphum maidis (corn leaf aphid) resistance in maize inbred line Mo17. J. Exp. Bot. 2015, 66, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totawa, NJ, USA, 2005. [Google Scholar]
- Wu, D.Y.; Jiang, B.W.; Ye, C.Y.; Timko, M.P.; Fan, L.J. Horizontal transfer and evolution of the biosynthetic gene cluster for benzoxazinoids in plants. Plant Commun. 2022, 3, 100320. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.N.; Zhao, X.Q.; Chao, W.; Lu, P.N.; Bai, X.D.; Mao, T.T. Genetic variation, DIMBOA accumulation, and candidate gene identification in maize multiple insect-resistance. Int. J. Mol. Sci. 2023, 24, 2138. [Google Scholar] [CrossRef] [PubMed]
- Bakera, B.; Święcicka, M.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Benzoxazinoids biosynthesis in rye (Secale cereal L.) is affected by low temperature. Agronomy 2020, 10, 1260. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, X.Y.; Wang, R.J.; Xue, K.; Yan, F.M.; Xu, C.R. Distribution and variations of three 1,4-benzoxazin-3-ones in maize induced by the Asian corn borer, Ostrinia furnacalis (Guenée). Z. Naturforschung C 2006, 61, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Li, J.; Chen, H.; Shen, C.S.; Wen, Y.Z. Phytoxicity alleviation of imazethapyr to non-target plant wheat: Active regulation between auxin and DIMBOA. Environ. Sci. Pollut. Res. 2023, 30, 116004–116017. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, S.; Singh, R.; Kumar, S.; Singh, S.K.; Singh, I.K. Dynamics of Zea mays transcriptome in response to polyphagous herbivore, Spodoptera litura. Funct. Integr. Genom. 2021, 21, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.M.; Schauberger, D.; Prinz, S.; Kopp, B. Determination of aneugenic 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and 2,4-dihydroxy-1,4-benzoxazin-3-one (DIBOA) contents in sprouts of various Triticum species. Planta Med. 2008, 74, 95. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.G.; Dong, F.S.; Xu, J.; Guo, L.Q.; Kong, Z.Q.; Tian, Y.Y.; Wu, Y.B.; Zheng, Y.Q. A simple method for the isolation and purification of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) from maize (Zea mays L.). J. Integr. Agric. 2013, 12, 95–102. [Google Scholar] [CrossRef]
- Frey, M.; Huber, K.; Park, W.J.; Sicker, D.; Lindberg, P.; Meeley, R.B.; Simmons, C.R.; Yalpani, N.; Gierl, A. A 2-oxoglutarate-dependent dioxygenase is integrated in DIMBOA-biosynthesis. Phytochemistry 2003, 62, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, R.; Schmidt, H.; Osterrieder, A.; Fiesselmann, A.; Schullehner, K.; Haslbeck, M.; Sicker, D.; Hofmann, D.; Yalpani, N.; Simmons, C.; et al. Elucidation of the final reactions of DIMBOA-glucoside biosynthesis in maize: Characterization of Bx6 and Bx7. Plant Physiol. 2008, 146, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lopes, L.D.; Lopez-Guerrero, M.G.; Dijk, K.V.; Alvarez, S.; Riethoven, J.J.; Schachtman, D.P. Natural variation in root exudation of GABA and DIMBOA impacts the maize root endosphere and rhizosphere microbimes. J. Exp. Bot. 2022, 73, erac202. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Chomet, P.; Glawischnig, E.; Stettner, C.; Grun, S.; Winklmair, A.; Eisenreich, W.; Bacher, A.; Meeley, R.B.; Briggs, S.P.; et al. Analysi of a chemical plant defense mechanism in grasses. Science 1997, 277, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Kliem, R.; Saedler, H.; Gierl, A. Expression of a cytochrome P450 gene family in maize. Mol. Gen. Genet. MGG 1995, 246, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.; Rattei, T.; Haslbeck, M.; Schwab, W.; Gierl, A.; Frey, M. Comparative analysis of benoxazinoid biosynthesis in monocots and dicots: Independent recruitment of stabilization and activation functions. Plant Cell 2012, 24, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Von Rad, U.; Huttl, R.; Lottspeich, F.; Gierl, A.; Frey, M. Two glucosyltransferases are involved in detoxification of benzoxxazinoids in maize. Plant J. 2002, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Yan, J.P.; Fu, Z.C.; Yang, T.F.; Zhang, L.; Ren, J.; Li, G.Y.; Zeng, H.M. Genome-wide identification, phylogenomics, and expression analysis of benzoxazinoids gene family in rice (Oryza sativa). Plant Stress 2023, 10, 100214. [Google Scholar] [CrossRef]
- Li, X.; He, K.L.; Wang, Z.Y.; Bai, S.X. Quantitative trait loci for Asian corn borer resistance in maize population. Agric. Sci. China 2010, 9, 77–84. [Google Scholar] [CrossRef]
- Åhman, I.; Johansson, M. Effect of light on DIMBOA-glucoside concentration in wheat (Triticum aestivum L.). Ann. Appl. Biol. 1994, 24, 569–574. [Google Scholar] [CrossRef]
- Feng, Y.J.; Wang, J.W.; Luo, S.M.; Fan, H.Z.; Jin, Q. Costs of jasmonic acid induced defense in aboveground and belowground parts of corn (Zea mays L.). J. Chem. Ecol. 2012, 38, 984–991. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Iwamura, H. Induction of HDMBOA-Glc accumulation and DIMBOA-Glc 4-O-methyltransferase by jasmonic acid in poaceous plants. Phytochemistry 2002, 61, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Phuong, T.T.T.; Yamamoto, M.; Fujii, T.; Kojima, W.; Matsuo, T.; Ishikawa, Y. Comparison of the ability to catabolize DIMBOA, a maize antibiotic, between Ostrinia furnacalis and Ostrinia scapulalis (Lepidoptera: Crambidae), with reference to their hybrids. Appl. Entomol. Zool. 2016, 51, 143–149. [Google Scholar] [CrossRef]
- Yan, F.; Liang, X.; Zhu, X. The role of DIMBOA on the feeding of Asian corn borer, Ostrinia furnacalis (Guenee) (Lep., Pyralidae). J. Appl. Entomol. 1999, 123, 49–53. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Briesemeister, S.; Rahnenführer, J.; Kohlbacher, O. YLoc-an interpretable web server for predicting subcellular localization. Nucleic Acids Res. 2010, 38, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Gao, J.L.; Wang, Z.G.; Hu, S.P.; Zhang, F.J.; Bao, H.Z.; Fan, Y.F. Maize canopy photosynthetic efficiency, plant growth, and yield response to tillage depth. Agronomy 2019, 9, 3. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Hossain, Z.; Zhao, B.Y.; Bai, X.D.; Mao, T.T. New insights into light spectral quality inhibits the plasticity elongation of maize mesocotyl and coleoptile during seed germination. Front. Plant Sci. 2023, 14, 1152399. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Wen, W.L.; Yang, D.G.; Zhang, Q.; Feng, D.D.; Wang, H.Y. Effects of low temperature and recovery on photosynthetic characteristics of maize. J. Maize Sci. 2014, 22, 92–97. [Google Scholar]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Miao, Y.P.; Ma, J.F.; Zhang, P.P.; Chen, T.; Liu, Y.; Che, Z.; Shahinnia, F.; Yang, D.L. Consensus genomic regions for grain quality traits in wheat revealed by meta-QTL analysis and in silico transcriptome integration. Plant Genome 2023, 16, e20336. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.N.; Butrón, A.; López-Malvar, A.; Rodríguez, V.M.; Sandoya, G.V.; Malvar, R.A. Defensive changes in maize leaves induced by feeding of Mediterranean corn borer larvae. BMC Plant Biol. 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Li, J.Y.; Niu, Y.N.; Hossain, Z.; Gao, X.Q.; Bai, X.D.; Mao, T.T.; Qi, G.X.; He, F.Q. Exogenous serotonin (5-HT) promotes mesocotyl and coleoptile elongation in maize seedlings under deep-seeding stress through enhancing auxin accumulation and inhibiting lignin formation. Int. J. Mol. Sci. 2023, 24, 17061. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Shi, Z.; He, F.; Niu, Y.; Qi, G.; Sun, S.; Li, X.; Gao, X. Benzoxazinoids Biosynthetic Gene Cluster Identification and Expression Analysis in Maize under Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2024, 25, 7460. https://doi.org/10.3390/ijms25137460
Zhao X, Shi Z, He F, Niu Y, Qi G, Sun S, Li X, Gao X. Benzoxazinoids Biosynthetic Gene Cluster Identification and Expression Analysis in Maize under Biotic and Abiotic Stresses. International Journal of Molecular Sciences. 2024; 25(13):7460. https://doi.org/10.3390/ijms25137460
Chicago/Turabian StyleZhao, Xiaoqiang, Zhenzhen Shi, Fuqiang He, Yining Niu, Guoxiang Qi, Siqi Sun, Xin Li, and Xiquan Gao. 2024. "Benzoxazinoids Biosynthetic Gene Cluster Identification and Expression Analysis in Maize under Biotic and Abiotic Stresses" International Journal of Molecular Sciences 25, no. 13: 7460. https://doi.org/10.3390/ijms25137460




