Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum)
Abstract
:1. Introduction
2. Results
2.1. Identification and Distribution of Aux/IAA Genes in Saccharum spontaneum Genome
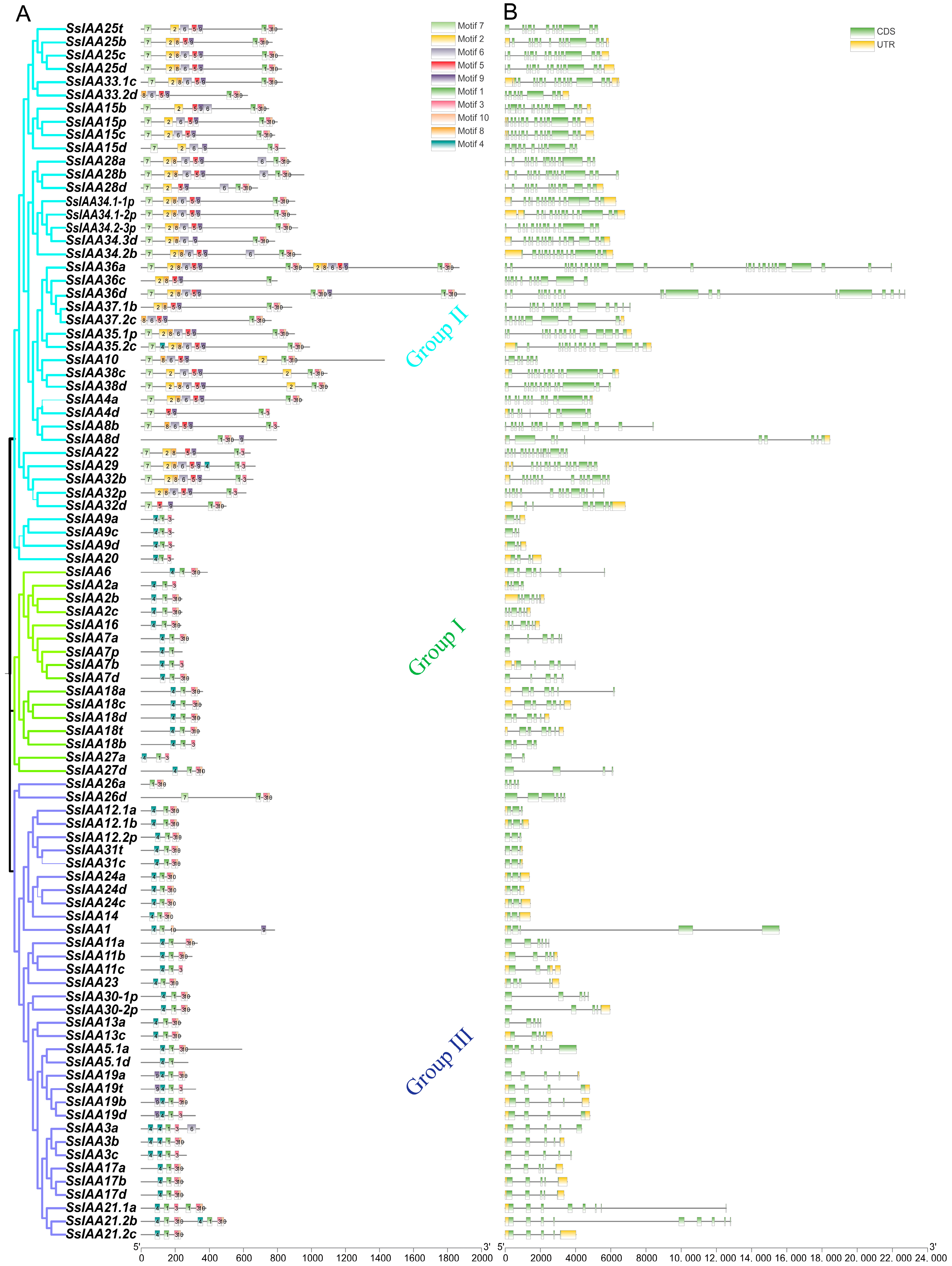
2.2. Phylogenetic and Chromosomal Distribution of IAA Genes in Saccharum spontaneum
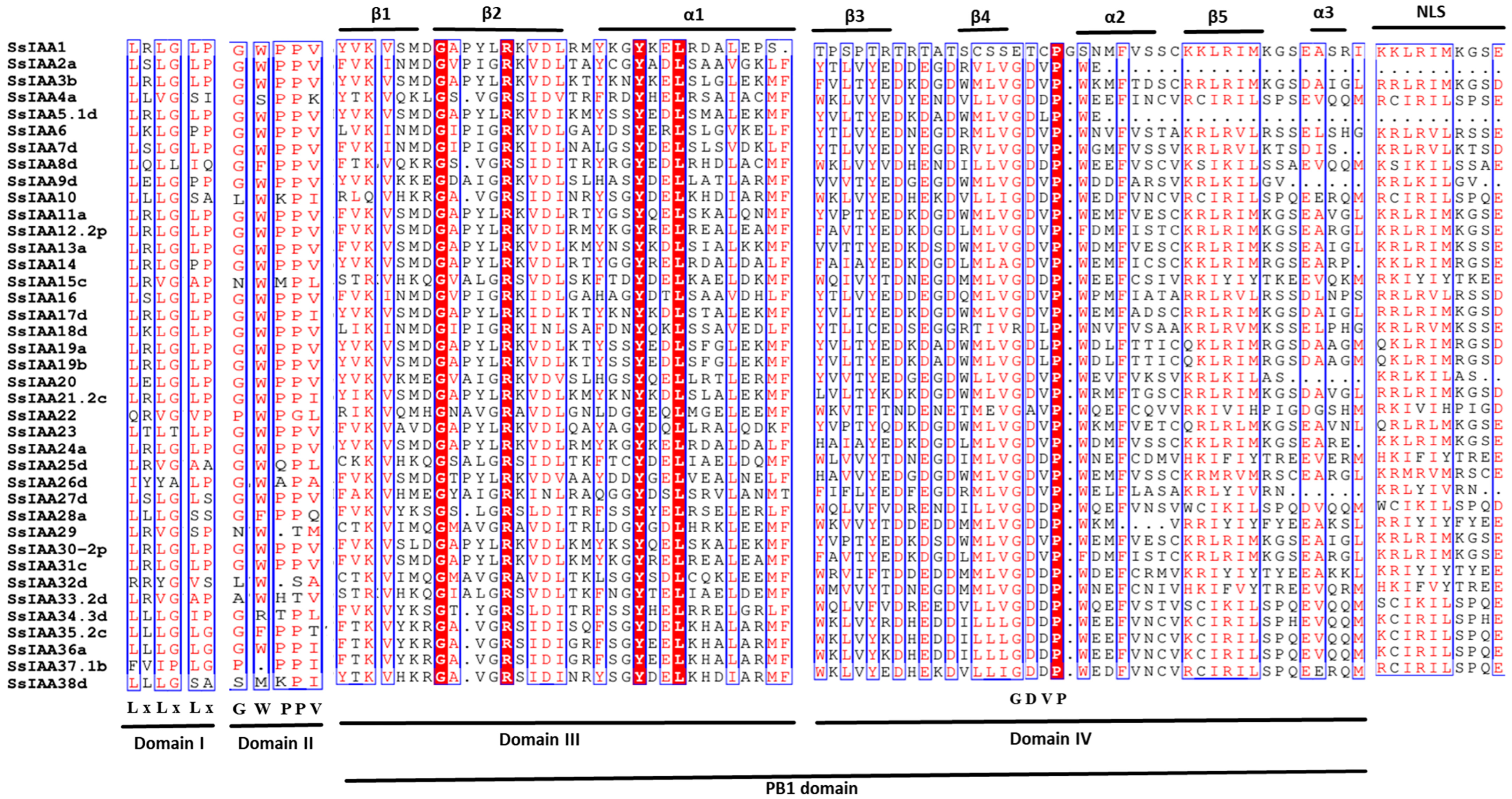
2.3. Motifs, Conserved Domains, and Gene Structure of the SsIAA Gene Family
2.4. Evolutionary and Collinearity Analysis of SsIAA Genes
2.5. Prediction of Cis-Acting Elements in the Promoters of Sugarcane IAA Gene Family
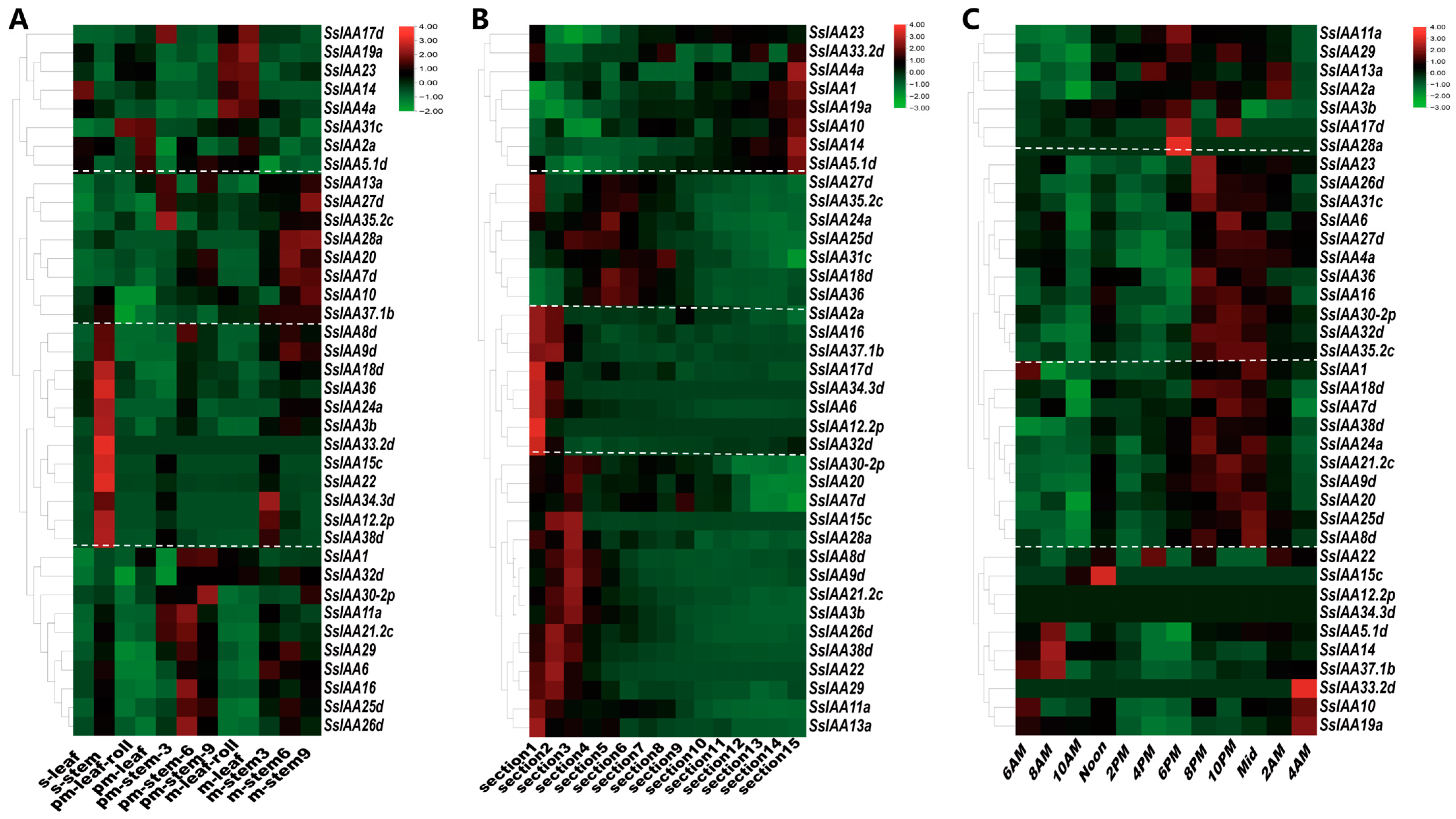
2.6. IAA Gene Family Expression in Sugarcane Growth and Development and Stress Responses
2.7. Subcellular Localization of SsIAA23 and SsIAA12.1a
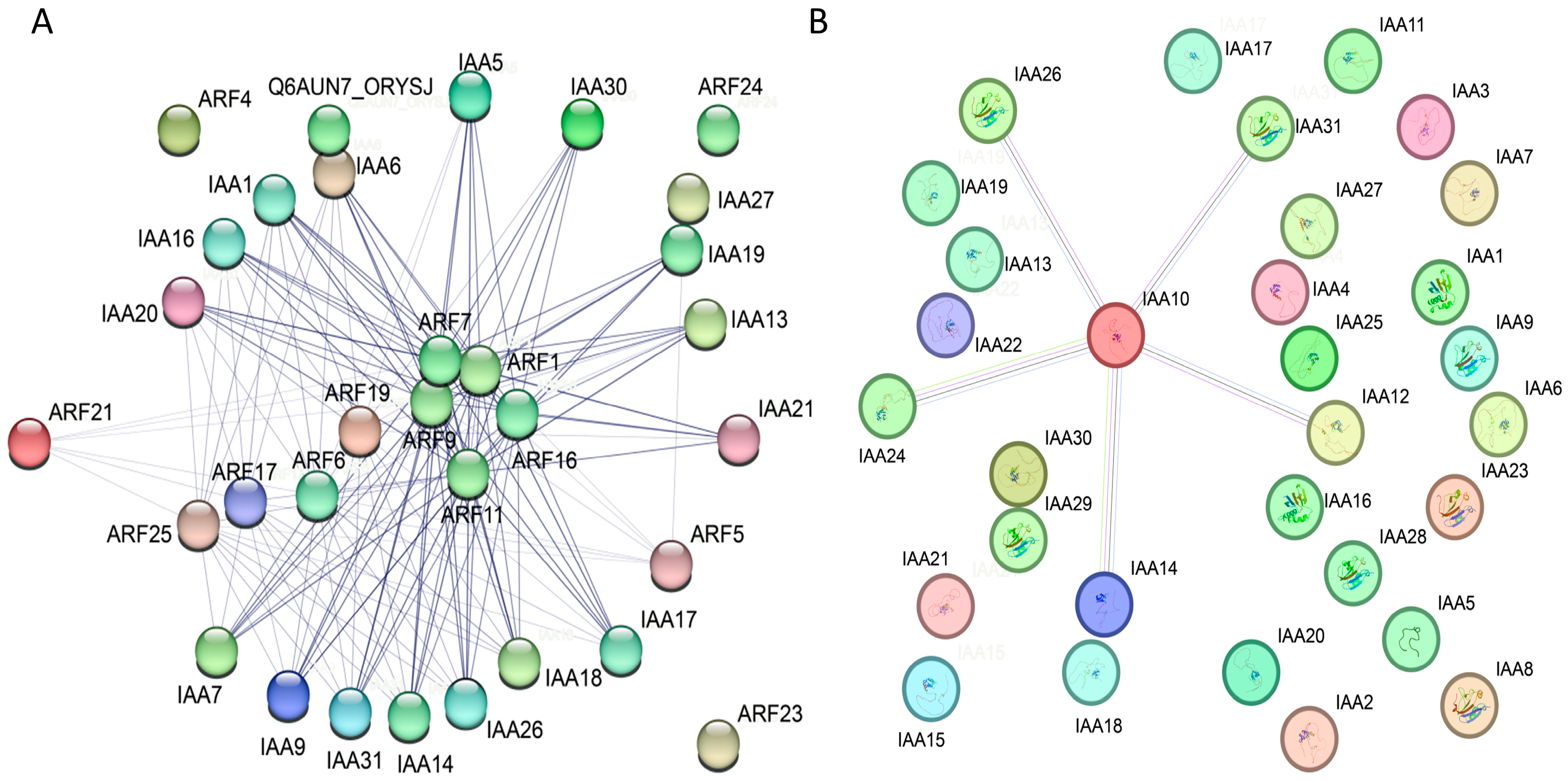
2.8. The Protein–Protein Interaction Prediction of the IAA Gene Family
3. Discussions
4. Materials and Methods
4.1. Identification of IAAs in Saccharum spontaneum
4.2. Phylogenetic Analyses of SsIAAs
4.3. Chromosomal Localization and Synteny Analysis of SsIAA Genes
4.4. Gene Structure, Protein Conserved Motif, Domains, 3D Modeling, and Promoter Cis-Elements Analysis
4.5. Hierarchial Clustering of RNA-Seq Gene Expression
4.6. RNA Extraction and qPCR
4.7. Construction of IAA-GFP Vectors and Subcellular Localization Observations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Han, S.; Qi, Y. Advances in Structure and Function of Auxin Response Factor in Plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Zhang, Q.; Wang, J.; King, G.J.; Liu, K. Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Allotetraploid Rapeseed (Brassica Napus L.). BMC Plant Biol. 2017, 17, 204. [Google Scholar] [CrossRef]
- Szemenyei, H.; Hannon, M.; Long, J.A. TOPLESS Mediates Auxin-Dependent Transcriptional Repression during Arabidopsis Embryogenesis. Science 2008, 319, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Zeng, D.; da Silva, J.A.T.; Qiu, S.; Duan, J.; Bai, S.; He, C. Genome-Wide Identification of Aux/IAA and ARF Gene Families Reveal Their Potential Roles in Flower Opening of Dendrobium Officinale. BMC Genom. 2023, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Q.; Ge, S.; Tang, M.; He, L.; Zou, Y.; Yu, J.; Zhou, Y. SlIAA23-SlARF6 Module Controls Arbuscular Mycorrhizal Symbiosis by Regulating Strigolactone Biosynthesis in Tomato. Plant. Cell Environ. 2023, 46, 1921–1934. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Cai, K.; Zhao, Q.; Zhang, J.; Yuan, H.; Li, H.; Han, L.; Li, X.; Li, K.; Jiang, T.; Zhao, X. Unraveling the Guardians of Growth: A Comprehensive Analysis of the Aux/IAA and ARF Gene Families in Populus Simonii. Plants 2023, 12, 3566. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Sui, C.; Wang, S.; Liu, X.; Zhang, G.; Sun, H.; Wan, K.; Yan, J.; Guo, S. Genome-Wide Evolutionary Analysis of AUX/IAA Gene Family in Wheat Identifies a Novel Gene TaIAA15-1A Regulating Flowering Time by Interacting with ARF. Int. J. Biol. Macromol. 2023, 227, 285–296. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-Sensitive Aux/IAA Proteins Mediate Drought Tolerance in Arabidopsis by Regulating Glucosinolate Levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, J.P. Transcript Profiling Reveals Diverse Roles of Auxin-Responsive Genes during Reproductive Development and Abiotic Stress in Rice. FEBS J. 2009, 276, 3148–3162. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.-K.; Do Choi, Y.; Kim, J.-K. OsIAA6, a Member of the Rice Aux/IAA Gene Family, Is Involved in Drought Tolerance and Tiller Outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA Protein, Mediates Abiotic Stress Tolerance in Rice through an ABA Pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rivera, N.; Khan, M.T.; Khan, I.A.; Iqbal, R.; Aslam, M.M. Sustainable Conversion of Wastes into Green Bioproducts to Introduce Diversification and Green Economy in the Sugar Industry. A Review. Sugar Tech. 2022, 24, 1198–1211. [Google Scholar] [CrossRef]
- Shearman, J.R.; Pootakham, W.; Sonthirod, C.; Naktang, C.; Yoocha, T.; Sangsrakru, D.; Jomchai, N.; Tongsima, S.; Piriyapongsa, J.; Ngamphiw, C.; et al. A Draft Chromosome-Scale Genome Assembly of a Commercial Sugarcane. Sci. Rep. 2022, 12, 20474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-Defined Genome of the Autopolyploid Sugarcane Saccharum Spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.; Robinson-Rechavi, M. Special Care Is Needed in Applying Phylogenetic Comparative Methods to Gene Trees with Speciation and Duplication Nodes. Mol. Biol. Evol. 2021, 38, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Wendel, J.F. Gene Duplication and Evolutionary Novelty in Plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sforça, D.A.; Vautrin, S.; Cardoso-Silva, C.B.; Mancini, M.C.; Romero-da Cruz, M.V.; Pereira, G.d.S.; Conte, M.; Bellec, A.; Dahmer, N.; Fourment, J.; et al. Gene Duplication in the Sugarcane Genome: A Case Study of Allele Interactions and Evolutionary Patterns in Two Genic Regions. Front. Plant Sci. 2019, 10, 553. [Google Scholar] [CrossRef]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. Cis-Regulatory Elements in Plant Development, Adaptation, and Evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and Its Role in Plant Development: Structure, Signalling, Regulation and Response Mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Cancé, C.; Martin-Arevalillo, R.; Boubekeur, K.; Dumas, R. Auxin Response Factors Are Keys to the Many Auxin Doors. New Phytol. 2022, 235, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia Polymorpha Genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Gao, X.; Liu, Y.; Fu, B. Genome-Wide Identification and Expression Pattern Analysis of the Aux/IAA (Auxin/Indole-3-Acetic Acid) Gene Family in Alfalfa (Medicago Sativa) and the Potential Functions under Drought Stress. BMC Genom. 2024, 25, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Feng, S.; Zhong, S.; Li, H.; Zhong, J.; Shen, C.; Liu, J. Genome-Wide Analysis and Characterization of Aux/IAA Family Genes Related to Fruit Ripening in Papaya (Carica papaya L.). BMC Genom. 2017, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Zhang, S.; Shui, D.; Xia, Z.; Sun, J. Genome-Wide Identification and Expression Analysis of the AUX/IAA Gene Family in Turnip (Brassica rapa ssp. rapa). BMC Plant Biol. 2023, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Jain, M. Genome-Wide Survey and Comprehensive Expression Profiling of Aux/IAA Gene Family in Chickpea and Soybean. Front. Plant Sci. 2015, 6, 918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, M.; Cheng, T.; Wang, J.; Zhang, Q. Corrigendum: Genome-Wide Identification of Aux/IAA Gene Family and Their Expression Analysis in Prunus Mume. Front. Genet. 2023, 14, 1208488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, M.; Li, J.; Zhao, H.; Ge, W.; Zhang, K. Identification and Analysis of Aux/IAA Family in Acer Rubrum. Evol. Bioinform. Online 2021, 17, 1176934321994127. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Wang, Y.; Li, H.; Liu, J.; Tan, J.; He, J.; Bai, J.; Ma, H. Evolution Analysis of the Aux/IAA Gene Family in Plants Shows Dual Origins and Variable Nuclear Localization Signals. Int. J. Mol. Sci. 2017, 18, 2107. [Google Scholar] [CrossRef]
- Remington, D.L.; Vision, T.J.; Guilfoyle, T.J.; Reed, J.W. Contrasting Modes of Diversification in the Aux/IAA and ARF Gene Families. Plant Physiol. 2004, 135, 1738–1752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Di, P.; Wang, Y. Genome-Wide Identification and Analysis of the Aux/IAA Gene Family in Panax Ginseng: Evidence for the Role of PgIAA02 in Lateral Root Development. Int. J. Mol. Sci. 2024, 25, 3470. [Google Scholar] [CrossRef]
- Guilfoyle, T.J. The PB1 Domain in Auxin Response Factor and Aux/IAA Proteins: A Versatile Protein Interaction Module in the Auxin Response. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, Y.; Pan, J.; Li, Z.; Wang, Q.; Mastouri, F.; Li, Y.; Yang, S.; Liu, M.; Dai, S.; Liu, W. Genome-Wide Identification of PTI1 Family in Setaria Italica and Salinity-Responsive Functional Analysis of SiPTI1-5. BMC Plant Biol. 2021, 21, 319. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Z.; Lu, S. Genome-Wide Identification and Expression Analysis of the Aux/IAA and Auxin Response Factor Gene Family in Medicago Truncatula. Int. J. Mol. Sci. 2021, 22, 494. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Li, F.; Wu, M.; Liu, H.; Gao, Y.; Xiang, Y. Systematic Identification and Expression Pattern Analysis of the Aux/IAA and ARF Gene Families in Moso Bamboo (Phyllostachys Edulis). Plant Physiol. Biochem. PPB 2018, 130, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Babu, K.S.; Janakiraman, V.; Palaniswamy, H.; Kasirajan, L.; Gomathi, R.; Ramkumar, T.R. A Short Review on Sugarcane: Its Domestication, Molecular Manipulations and Future Perspectives. Genet. Resour. Crop Evol. 2022, 69, 2623–2643. [Google Scholar] [CrossRef]
- Wang, L.; Czedik-Eysenberg, A.; Mertz, R.A.; Si, Y.; Tohge, T.; Nunes-Nesi, A.; Arrivault, S.; Dedow, L.K.; Bryant, D.W.; Zhou, W.; et al. Comparative Analyses of C4 and C3 Photosynthesis in Developing Leaves of Maize and Rice. Nat. Biotechnol. 2014, 32, 1158–1165. [Google Scholar] [CrossRef]
- Li, P.; Ponnala, L.; Gandotra, N.; Wang, L.; Si, Y.; Tausta, S.L.; Kebrom, T.H.; Provart, N.; Patel, R.; Myers, C.R.; et al. The Developmental Dynamics of the Maize Leaf Transcriptome. Nat. Genet. 2010, 42, 1060–1067. [Google Scholar] [CrossRef]
- Hu, W.; Hua, X.; Zhang, Q.; Wang, J.; Shen, Q.; Zhang, X.; Wang, K.; Yu, Q.; Lin, Y.-R.; Ming, R.; et al. New Insights into the Evolution and Functional Divergence of the SWEET Family in Saccharum Based on Comparative Genomics. BMC Plant Biol. 2018, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.T.; Berleth, T. The Identification and Characterization of Specific ARF-Aux/IAA Regulatory Modules in Plant Growth and Development. Plant Signal. Behav. 2015, 10, e992748. [Google Scholar] [CrossRef] [PubMed]
- Çakir, B.; Kiliçkaya, O.; Olcay, A.C. Genome-Wide Analysis of Aux/IAA Genes in Vitis Vinifera: Cloning and Expression Profiling of a Grape Aux/IAA Gene in Response to Phytohormone and Abiotic Stresses. Acta Physiol. Plant. 2013, 35, 365–377. [Google Scholar] [CrossRef]
- Kumar, R.; Agarwal, P.; Tyagi, A.K.; Sharma, A.K. Genome-Wide Investigation and Expression Analysis Suggest Diverse Roles of Auxin-Responsive GH3 Genes during Development and Response to Different Stimuli in Tomato (Solanum lycopersicum). Mol. Genet. Genom. 2012, 287, 221–235. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xu, C.; Fu, X.; Shen, Y.; Guo, L.; Leng, M.; Luo, K. The MicroRNA390/Trans-Acting Short Interfering RNA3 Module Mediates Lateral Root Growth under Salt Stress via the Auxin Pathway. Plant Physiol. 2018, 177, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Villano, C.; Esposito, S.; D’Amelia, V.; Garramone, R.; Alioto, D.; Zoina, A.; Aversano, R.; Carputo, D. WRKY Genes Family Study Reveals Tissue-Specific and Stress-Responsive TFs in Wild Potato Species. Sci. Rep. 2020, 10, 7196. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 Regulates MicroRNA-Mediated Cold Stress Response in Arabidopsis Roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef]
- Irfan, M.; Kumar, P.; Ahmad, I.; Datta, A. Unraveling the Role of Tomato Bcl-2-Associated Athanogene (BAG) Proteins during Abiotic Stress Response and Fruit Ripening. Sci. Rep. 2021, 11, 21734. [Google Scholar] [CrossRef] [PubMed]
- Laksana, C.; Sophiphun, O.; Chanprame, S. Lignin Reduction in Sugarcane by Performing CRISPR/Cas9 Site-Direct Mutation of SoLIM Transcription Factor. Plant Sci. 2024, 340, 111987. [Google Scholar] [CrossRef]
- Oz, M.T.; Altpeter, A.; Karan, R.; Merotto, A.; Altpeter, F. CRISPR/Cas9-Mediated Multi-Allelic Gene Targeting in Sugarcane Confers Herbicide Tolerance. Front. Genome Ed. 2021, 3, 673566. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]







| Gene Name | Gene ID | Chromosome | Number of Amino Acids (aa) | Molecular Weight (kD) | Isoelectric Point | Instability Index | Subcellular Localization | Gene Duplication | Physical Position on the Genome | Strand Orientation | Biological Process (GO Term) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SsIAA1 | Sspon.02G0041760-1B | Chr2B | 783 | 82.65 | 9.44 | 64.91 | Nucleus | Dispersed | 77885470-77900915 | Negative | AUX/IAA domain |
| SsIAA2a | Sspon.03G0022760-1A | Chr3A | 204 | 21.05 | 5.38 | 48.17 | Nucleus | Dispersed | 69521520-69522442 | Positive | Regulation of transcription, DNA-templated |
| SsIAA3a | Sspon.03G0024820-1A | Chr3A | 342 | 36.48 | 9.34 | 44.68 | Nucleus | Dispersed | 75376437-75380644 | Positive | Response to auxin |
| SsIAA4a | Sspon.05G0001870-1A | Chr5A | 947 | 104.19 | 5.87 | 61.16 | Nucleus | Dispersed | 5939018-5943933 | Positive | Root development |
| SsIAA5.1a | Sspon.03G0011430-1A | Chr3A | 589 | 62.87 | 10.52 | 52.91 | Nucleus | Dispersed | 31092210-31096179 | Positive | Regulation of transcription, DNA-templated |
| SsIAA6 | Sspon.03G0006790-1A | Chr3A | 388 | 42.06 | 9.15 | 44.89 | Nucleus | WGD or segmental | 18637083-18642587 | Negative | Response to heat |
| SsIAA7a | Sspon.04G0014810-1A | Chr4A | 278 | 29.59 | 7.8 | 49.33 | Nucleus | Tandem | 55340442-55343662 | Positive | Response to auxin |
| SsIAA8d | Sspon.08G0022500-3D | Chr8D | 793 | 85.86 | 5.71 | 54.34 | Chloroplast | Unknown | 49305894-49314316 | Negative | Response to heat |
| SsIAA9a | Sspon.04G0001230-1A | Chr4A | 191 | 20.44 | 5.49 | 42.22 | Nucleus | WGD or segmental | 4697296-4698075 | Negative | Regulation of transcription, DNA-templated |
| SsIAA10 | Sspon.06G0002150-1T | Chr6A | 1429 | 155.9 | 5.77 | 57.27 | Nucleus | Singleton | 6930821-6932644 | Negative | Response to heat |
| SsIAA11a | Sspon.01G0023790-1A | Chr1A | 330 | 34.98 | 5.29 | 64.09 | Nucleus | WGD or segmental | 85454695-85457182 | Positive | Regulation of transcription, DNA-templated |
| SsIAA12.1a | Sspon.01G0023800-1A | Chr1A | 217 | 23.1 | 8.7 | 48.53 | Nucleus | Singleton | 85497372-85498224 | Positive | Regulation of transcription, DNA-templated |
| SsIAA13a | Sspon.01G0028160-1A | Chr1A | 234 | 25.18 | 8.86 | 48.73 | Nucleus | WGD or segmental | 90932535-90933387 | Negative | Regulation of transcription, DNA-templated |
| SsIAA14 | Sspon.02G0041760-3D | Chr1D | 175 | 18.8 | 6.73 | 35.71 | Nucleus | Dispersed | 101374456-101375168 | Positive | Regulation of transcription, DNA-templated |
| SsIAA15b | Sspon.03G0027750-1B | Chr3B | 748 | 83.41 | 7.03 | 56.43 | Nucleus | WGD or segmental | 6658010-6662331 | Negative | Regulation of transcription, DNA-templated |
| SsIAA16 | Sspon.03G0022760-1P | Chr7B | 233 | 23.99 | 6.76 | 42.19 | Nucleus | Dispersed | 45460339-45461913 | Negative | Regulation of transcription, DNA-templated |
| SsIAA17a | Sspon.07G0010810-1A | Chr7A | 248 | 26.71 | 7.68 | 41.01 | Nucleus | Unknown | 35935896-35938803 | Positive family | Regulation of transcription, DNA-templated |
| SsIAA18a | Sspon.07G0003900-1A | Chr7A | 359 | 38.42 | 5.84 | 52.94 | Nucleus | Unknown | 9728691-9733926 | Positive | Regulation of transcription, DNA-templated |
| SsIAA19a | Sspon.07G0002380-1A | Chr7A | 267 | 27.95 | 6.2 | 47.95 | Nucleus | Unknown | 5913783-5917916 | Positive | Regulation of transcription, DNA-templated |
| SsIAA20 | Sspon.08G0013920-1A | Chr8A | 189 | 20.54 | 5.99 | 60 | Nucleus | Unknown | 57584849-57586099 | Positive | Regulation of transcription, DNA-templated |
| SsIAA21.1a | Sspon.08G0007190-1A | Chr8A | 379 | 42.07 | 8.99 | 38.47 | Nucleus | Unknown | 22454558-22466916 | Positive | Response to heat |
| SsIAA22 | Sspon.03G0037540-1B | Chr3B | 639 | 71.77 | 5.72 | 58.42 | Nucleus | WGD or segmental | 99443945-99447482 | Positive | Regulation of transcription, DNA-templated |
| SsIAA23 | Sspon.03G0036500-1B | Chr3B | 214 | 23.15 | 7.77 | 48.78 | Nucleus | WGD or segmental | 90332265-90334872 | Negative | Regulation of transcription, DNA-templated |
| SsIAA24a | Sspon.02G0026740-1A | Chr2A | 191 | 20.4 | 6.31 | 38.52 | Nucleus | Dispersed | 94923088-94923850 | Positive | Regulation of transcription, DNA-templated |
| SsIAA25b | Sspon.02G0027120-2B | Chr2B | 768 | 85.17 | 5.97 | 64.77 | Nucleus | WGD or segmental | 95779585-95785095 | Negative | Regulation of transcription, DNA-templated |
| SsIAA26a | Sspon.02G0009510-1A | Chr2A | 139 | 15.24 | 4.62 | 32.78 | Nucleus | WGD or segmental | 26751431-26752199 | Negative | Regulation of transcription, DNA-templated |
| SsIAA27a | Sspon.05G0016400-1A | Chr5A | 162 | 17.22 | 5.87 | 34.82 | Nucleus | Singleton | 66902764-66903857 | Positive | Regulation of transcription, DNA-templated |
| SsIAA28a | Sspon.02G0031340-1A | Chr2A | 878 | 96.97 | 5.94 | 64.59 | Nucleus | WGD or segmental | 114616279-114621376 | Positive | Regulation of transcription, DNA-templated |
| SsIAA29 | Sspon.04G0010200-2B | Chr4B | 668 | 74.42 | 5.89 | 61.13 | Nucleus | WGD or segmental | 26207214-26212018 | Negative | Negative regulation of transcription, DNA-templated |
| SsIAA30-1p | Sspon.01G0023790-1P | Chr2A | 288 | 30.28 | 5.18 | 57.26 | Nucleus | WGD or segmental | 111385647-111390381 | Positive | Regulation of transcription, DNA-templated |
| SsIAA31c | Sspon.01G0023800-3C | Chr2C | 226 | 24.03 | 8.24 | 54.97 | Nucleus | Dispersed | 108456291-108457177 | Negative | Regulation of transcription, DNA-templated |
| SsIAA32b | Sspon.05G0028050-1B | Chr5B | 655 | 72.49 | 5.87 | 59.41 | Nucleus | Dispersed | 60122402-60128086 | Positive | Regulation of transcription, DNA-templated |
| SsIAA33.1c | Sspon.06G0032700-1C | Chr6C | 828 | 90.87 | 6.6 | 52.21 | Nucleus | Proximal | 91761546-91766954 | Positive | Response to abscisic acid |
| SsIAA34.1-1p | Sspon.04G0017640-1P | Chr8A | 901 | 99.99 | 5.93 | 68.72 | Nucleus | Unknown | 4675614-4680912 | Negative | Response to heat |
| SsIAA35.1p | Sspon.04G0018530-1P | Chr8A | 899 | 99.48 | 6.36 | 57.25 | Nucleus | Unknown | 6040168-6047060 | Negative | Regulation of transcription, DNA-templated |
| SsIAA36a | Sspon.04G0018530-1A | Chr4A | 1867 | 207.23 | 6.25 | 57.06 | Nucleus | WGD or segmental | 66589636-66611591 | Negative | Regulation of transcription, DNA-templated |
| SsIAA37.1b | Sspon.04G0030300-1B | Chr4B | 883 | 98.89 | 5.48 | 51.09 | Nucleus | Dispersed | 76690693-76697801 | Negative | Regulation of transcription, DNA-templated |
| SsIAA38c | Sspon.06G0019860-2C | Chr6C | 1091 | 120.82 | 6.14 | 57.95 | Nucleus | Dispersed | 5171377-5177422 | Positive | Response to heat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Shad, M.A.; Shu, Y.; Nong, S.; Li, X.; Wu, S.; Yang, J.; Rao, M.J.; Aslam, M.Z.; Huang, X.; et al. Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum). Int. J. Mol. Sci. 2024, 25, 7473. https://doi.org/10.3390/ijms25137473
Huang X, Shad MA, Shu Y, Nong S, Li X, Wu S, Yang J, Rao MJ, Aslam MZ, Huang X, et al. Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum). International Journal of Molecular Sciences. 2024; 25(13):7473. https://doi.org/10.3390/ijms25137473
Chicago/Turabian StyleHuang, Xiaojin, Munsif Ali Shad, Yazhou Shu, Sikun Nong, Xianlong Li, Songguo Wu, Juan Yang, Muhammad Junaid Rao, Muhammad Zeshan Aslam, Xiaoti Huang, and et al. 2024. "Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum)" International Journal of Molecular Sciences 25, no. 13: 7473. https://doi.org/10.3390/ijms25137473
APA StyleHuang, X., Shad, M. A., Shu, Y., Nong, S., Li, X., Wu, S., Yang, J., Rao, M. J., Aslam, M. Z., Huang, X., Huang, D., & Wang, L. (2024). Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum). International Journal of Molecular Sciences, 25(13), 7473. https://doi.org/10.3390/ijms25137473







