Mechanism of Abnormal Activation of MEK1 Induced by Dehydroalanine Modification
Abstract
1. Introduction
2. Results and Discussion
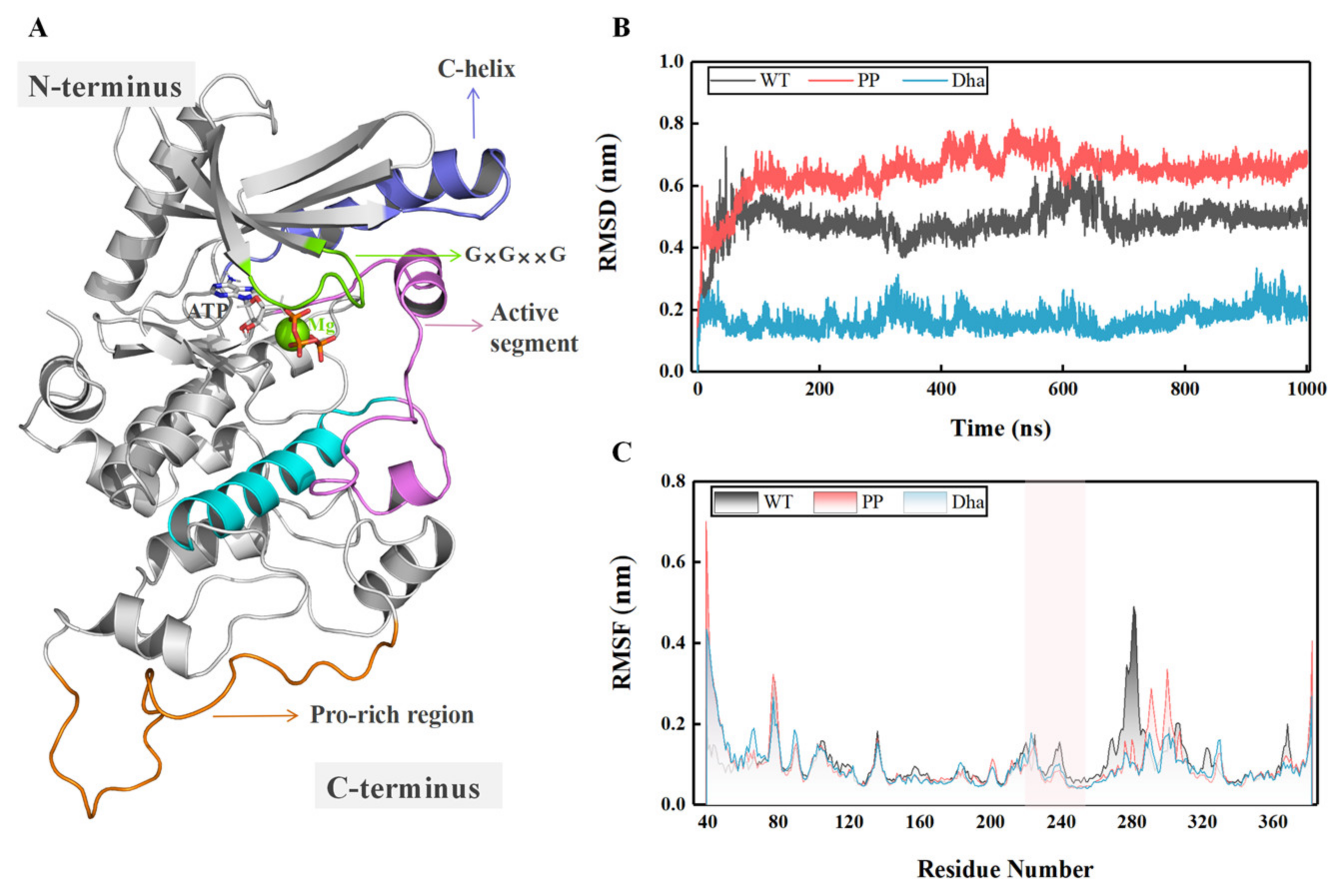
2.1. Mechanism of MEK1 Aberrant Activation Induced by Dehydroalanine Modification
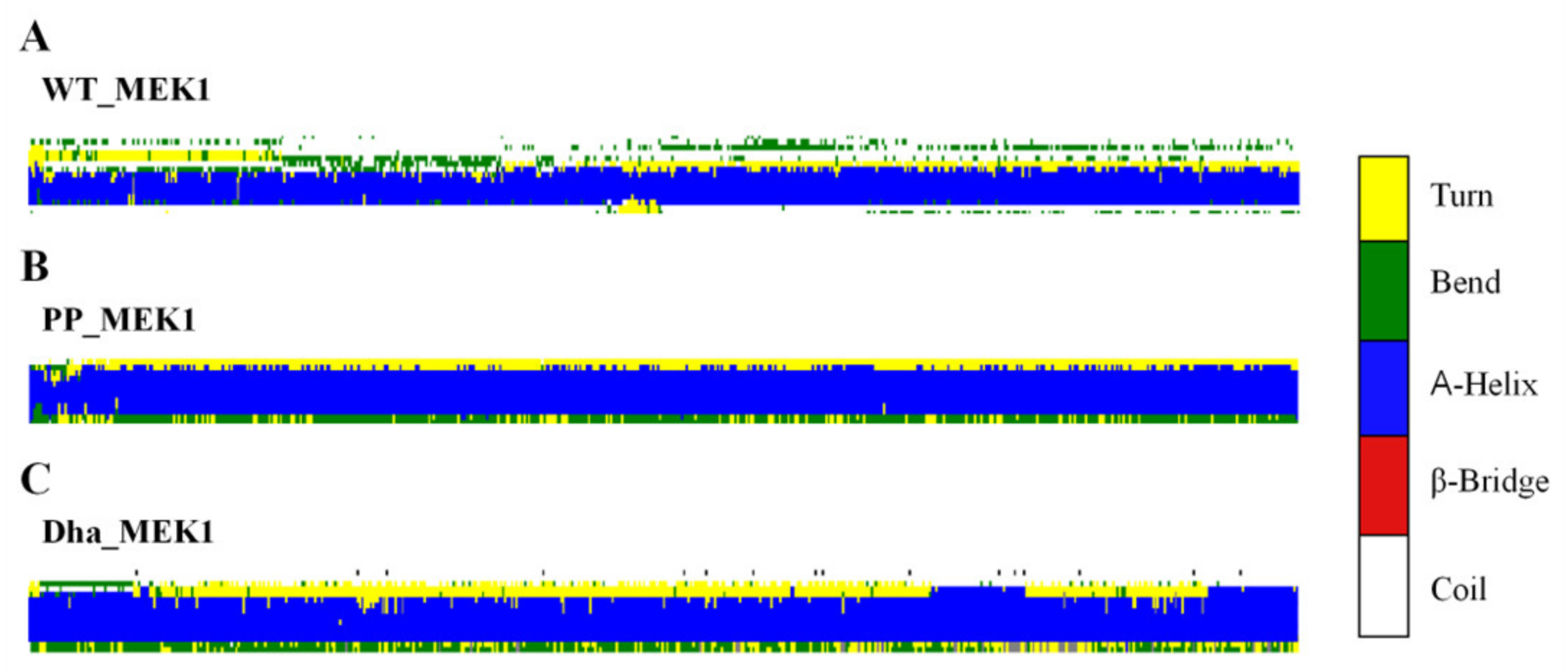
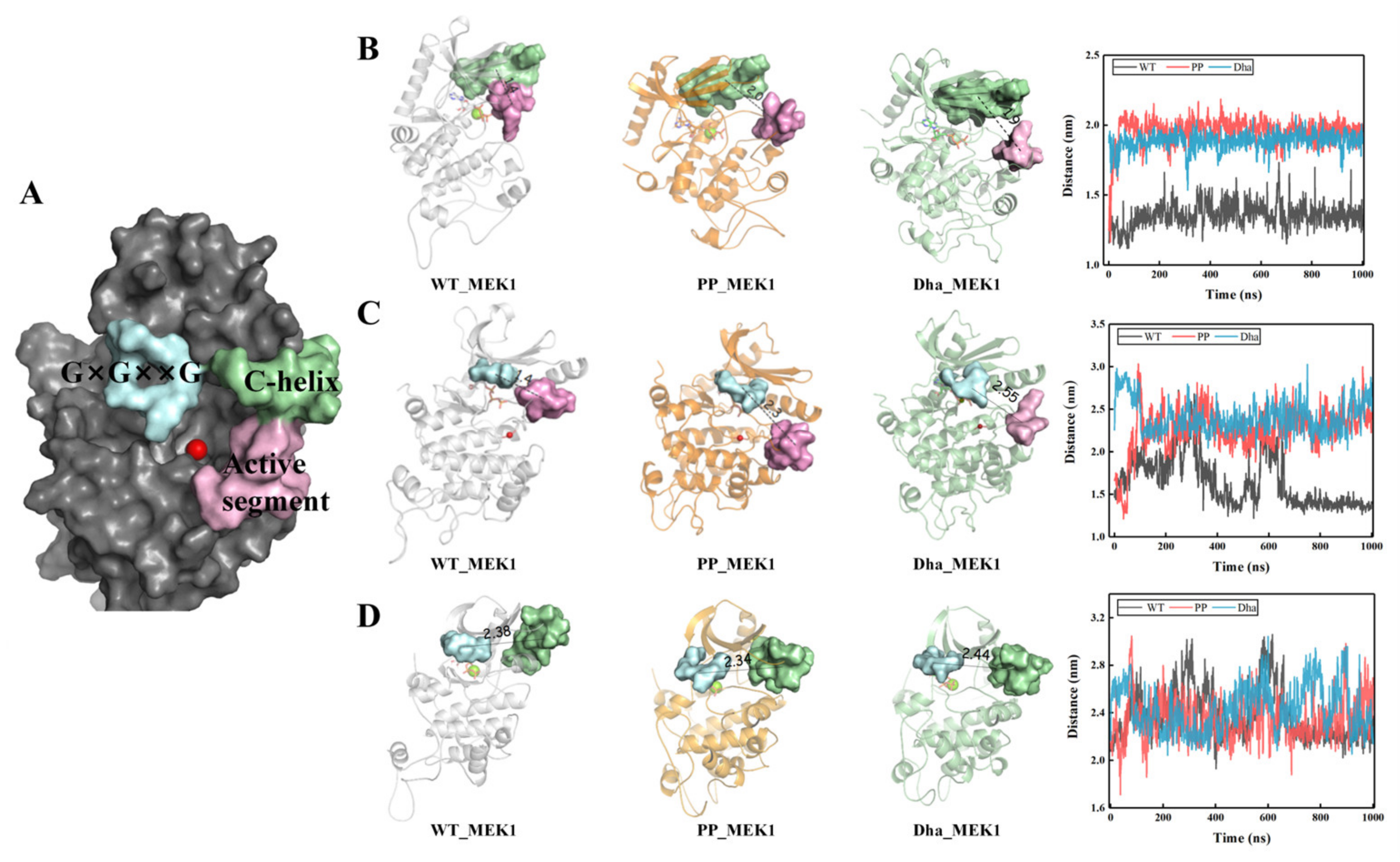
2.1.1. Dha Modifications Mainly Affect Conformational Change of Active Segment
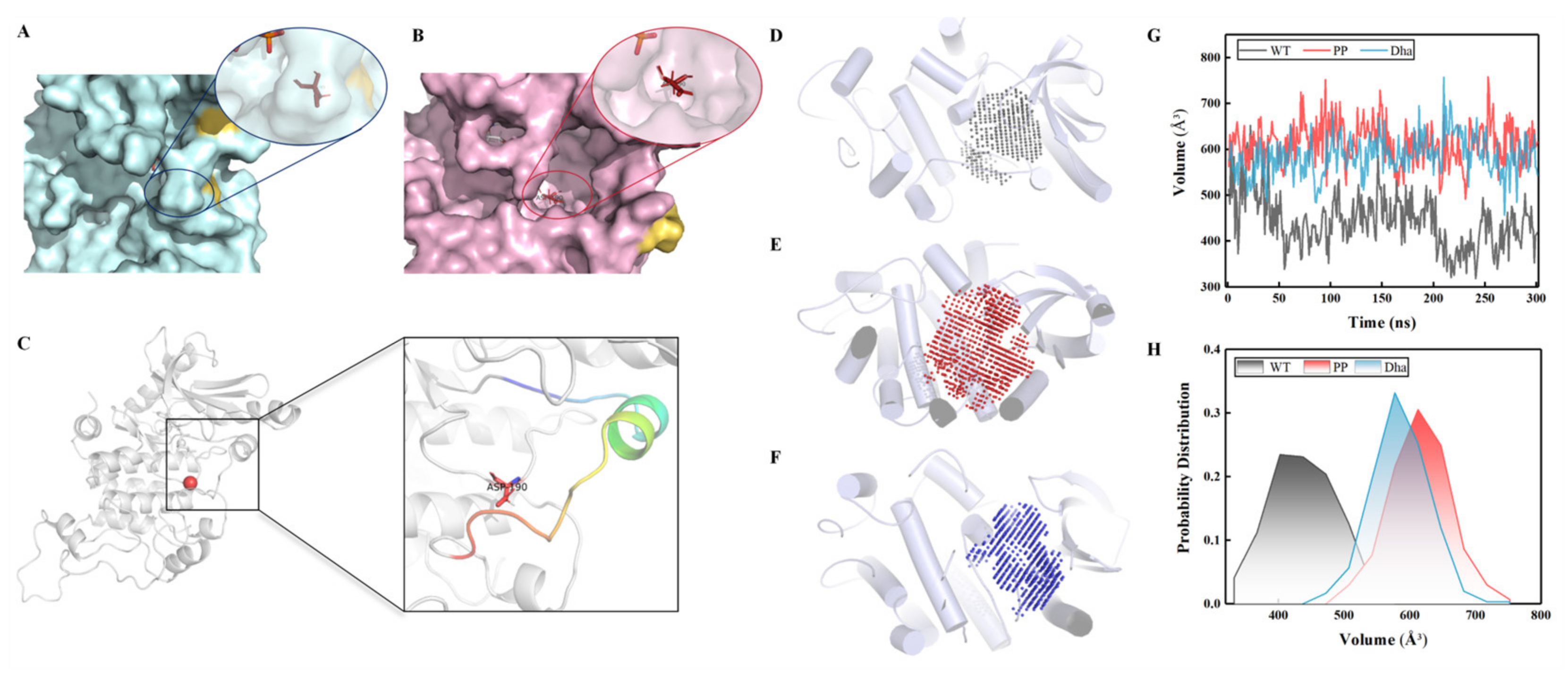
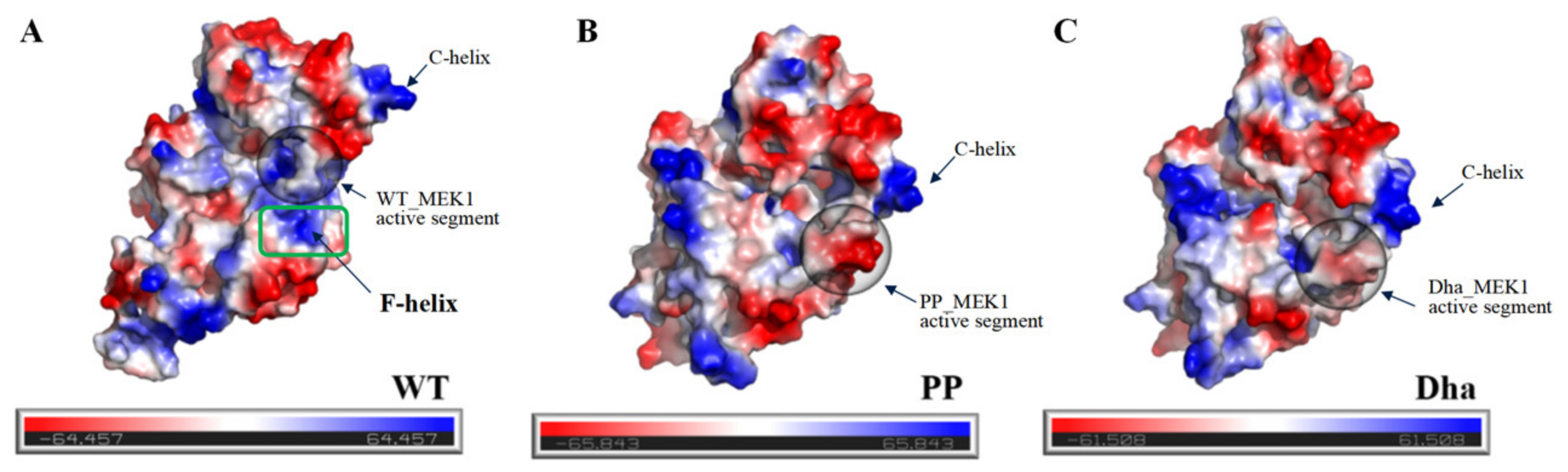
2.1.2. Characteristics of the Active Pocket
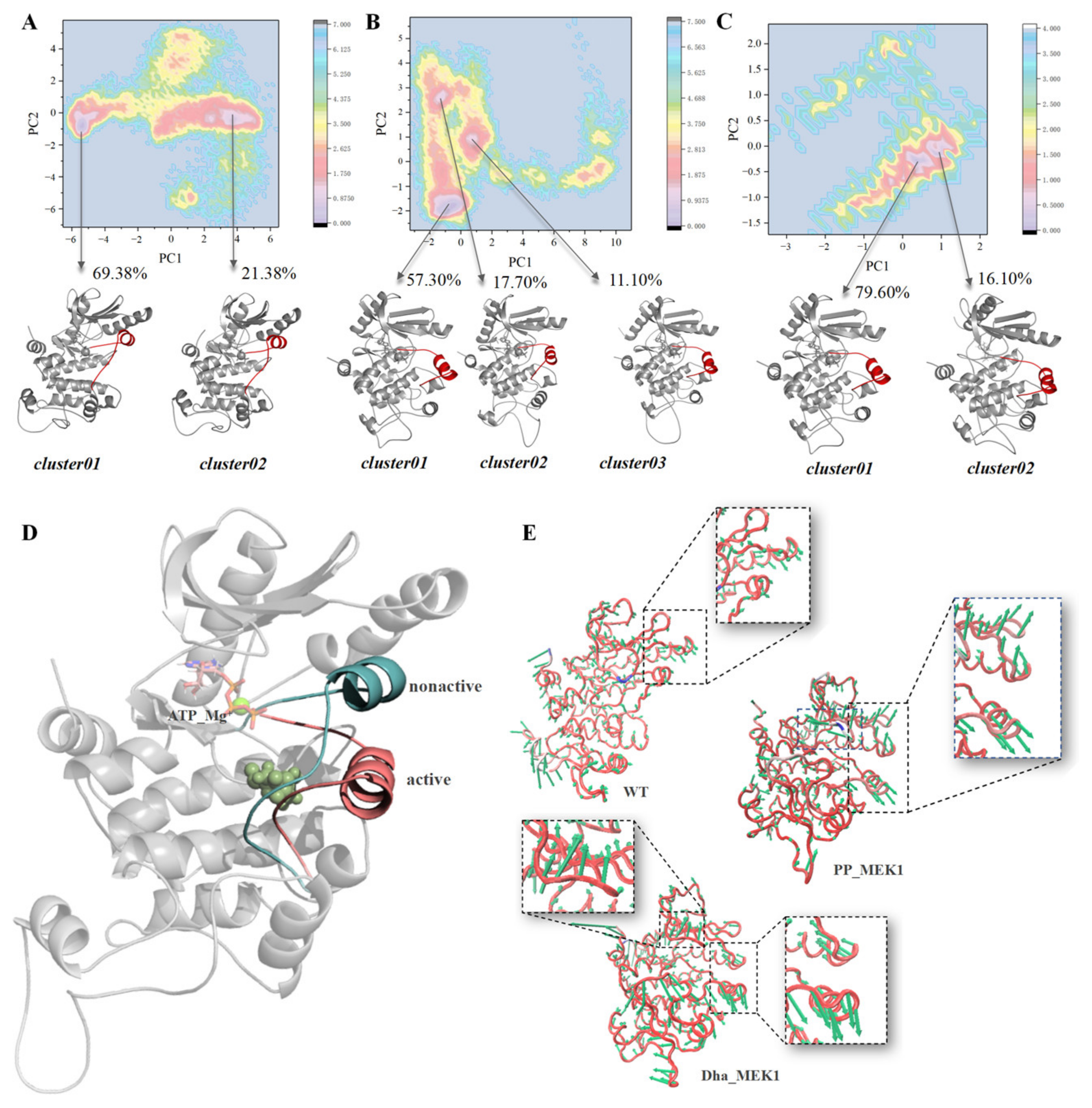
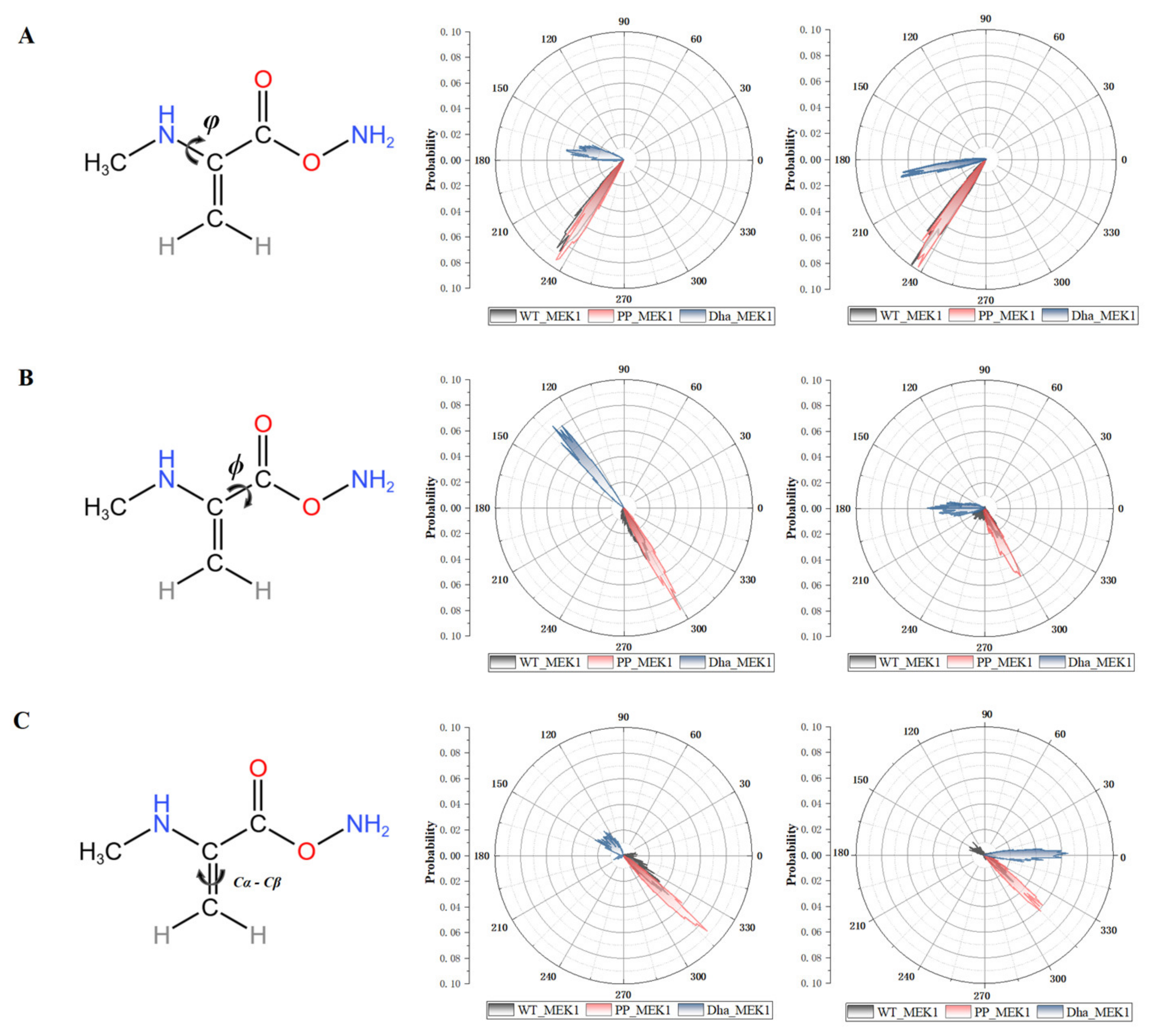
2.1.3. Driving Forces behind the Active Segment Conformational Changes
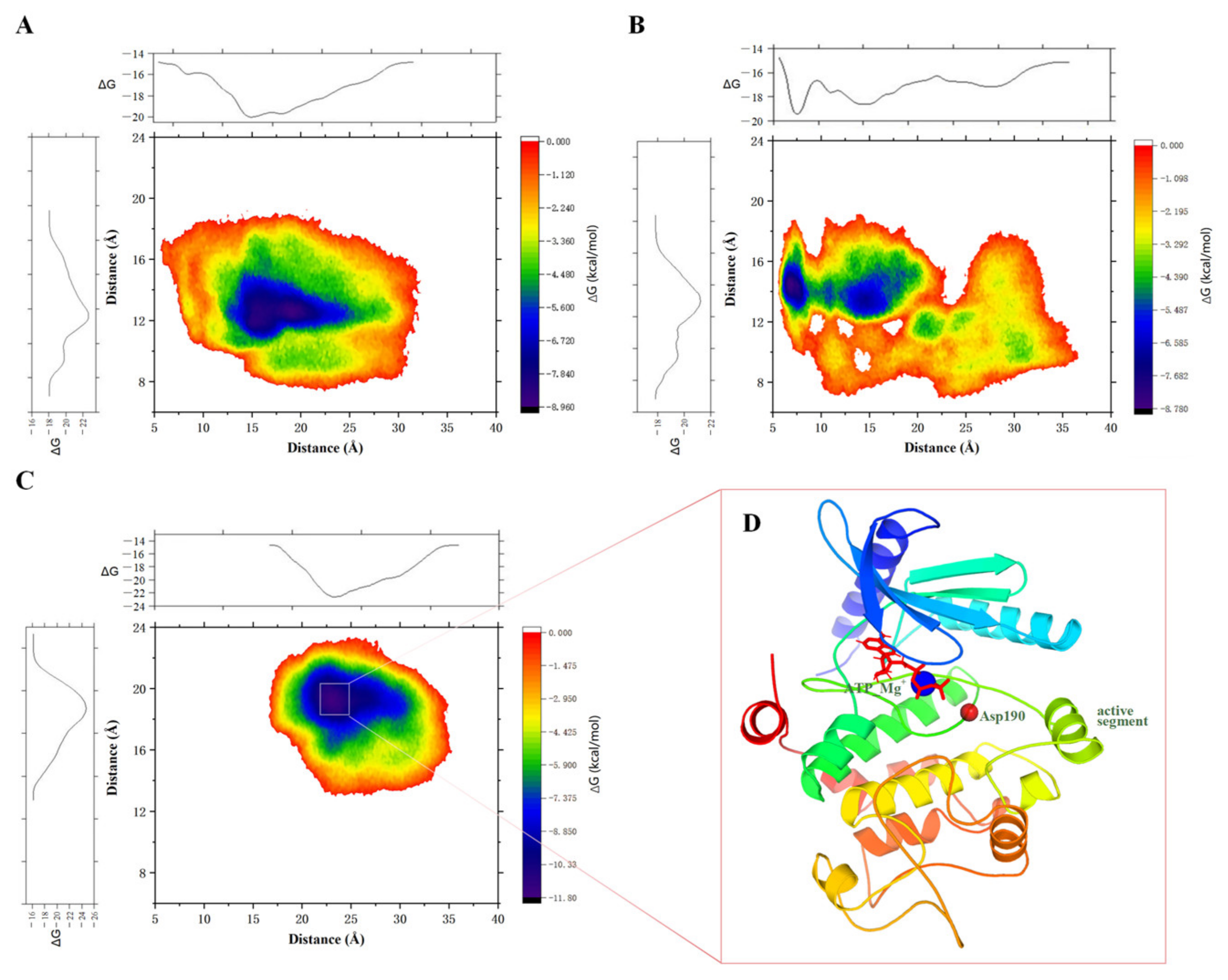
2.1.4. Metadynamic Simulations Indicate That Dha Modifications Cause MEK1 in a Continuous Activated State
2.2. Prediction of the Inhibitory Effects of FDA-Approved Drugs on the Catalytic Activity of Dha-Modified MEK1
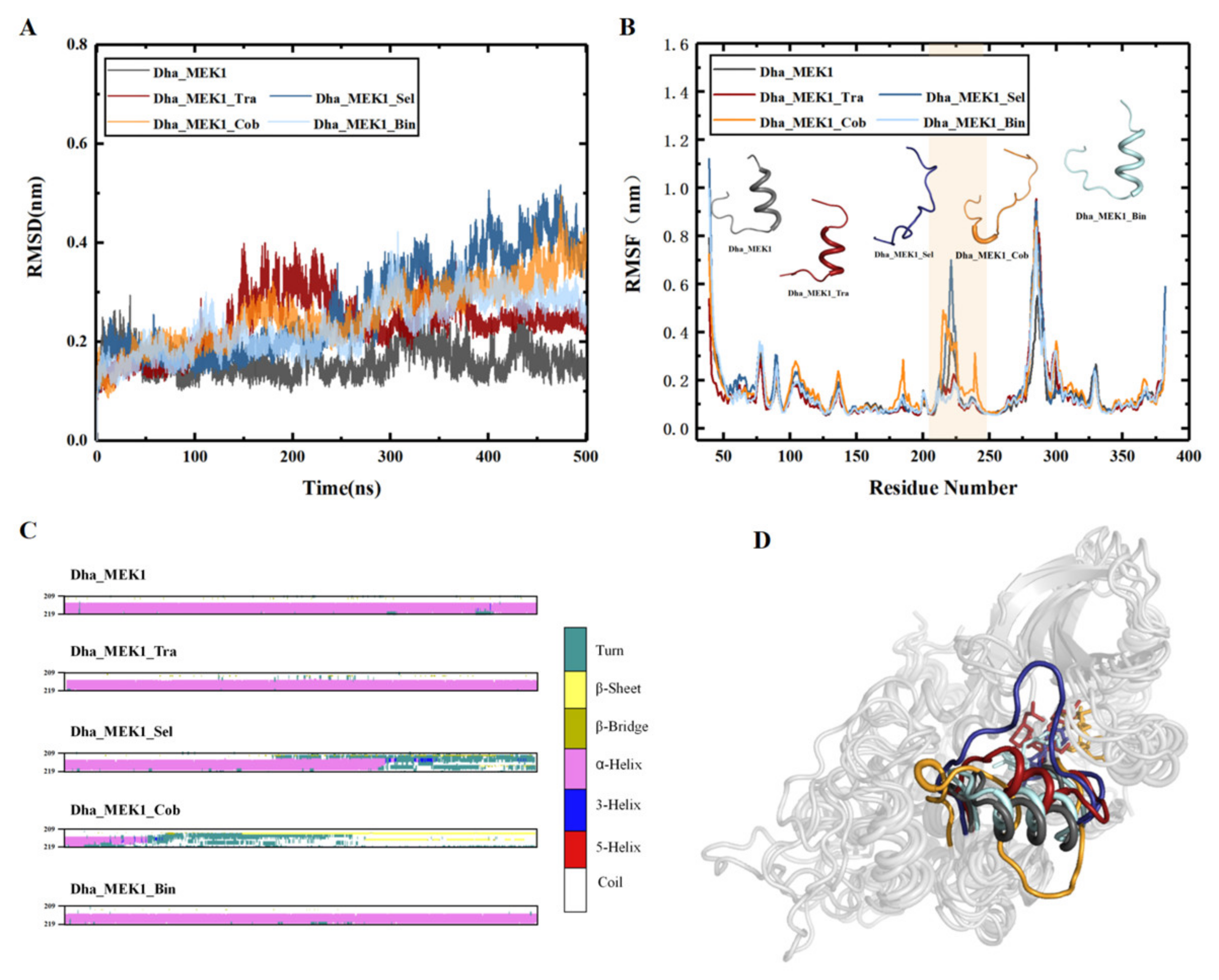
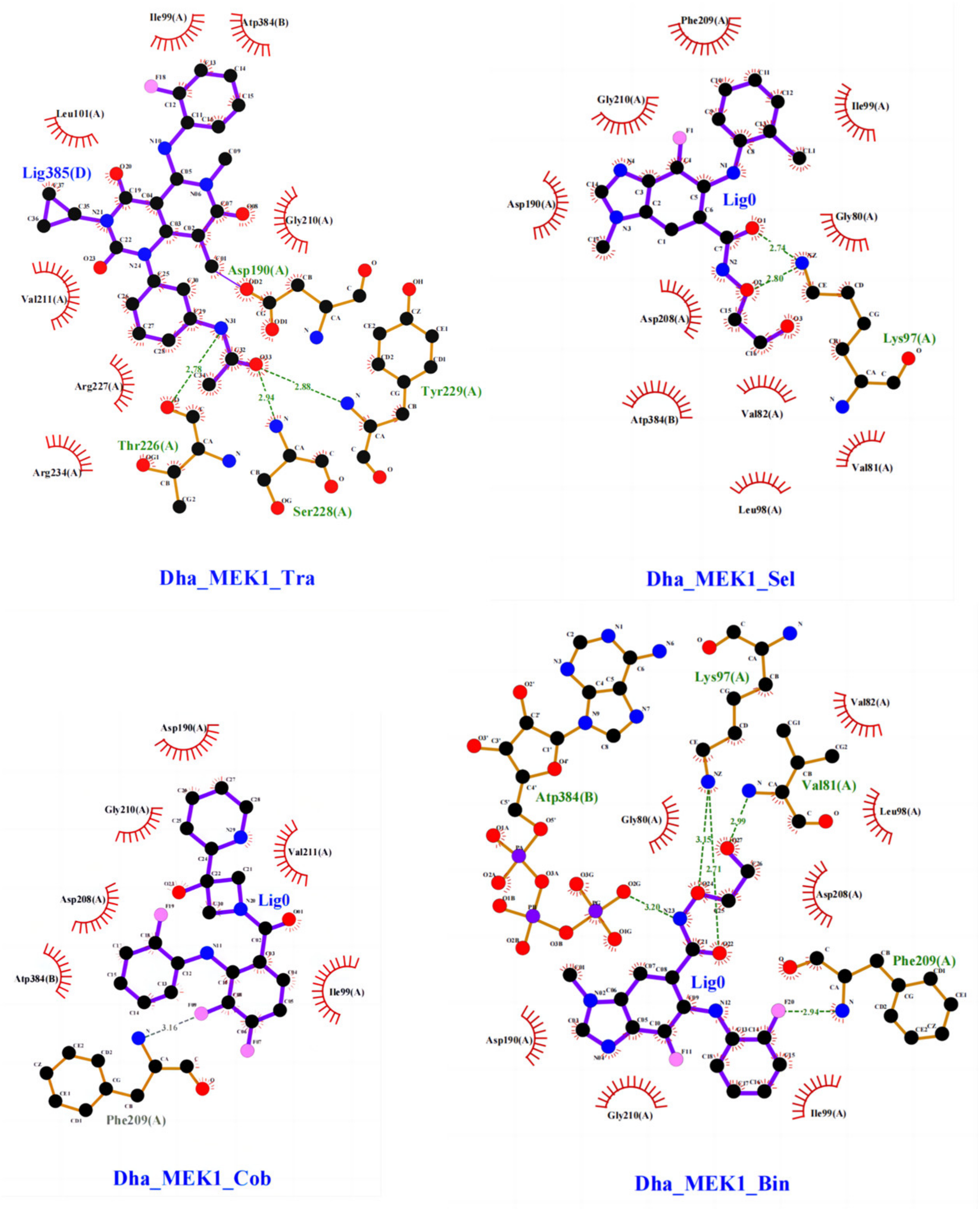
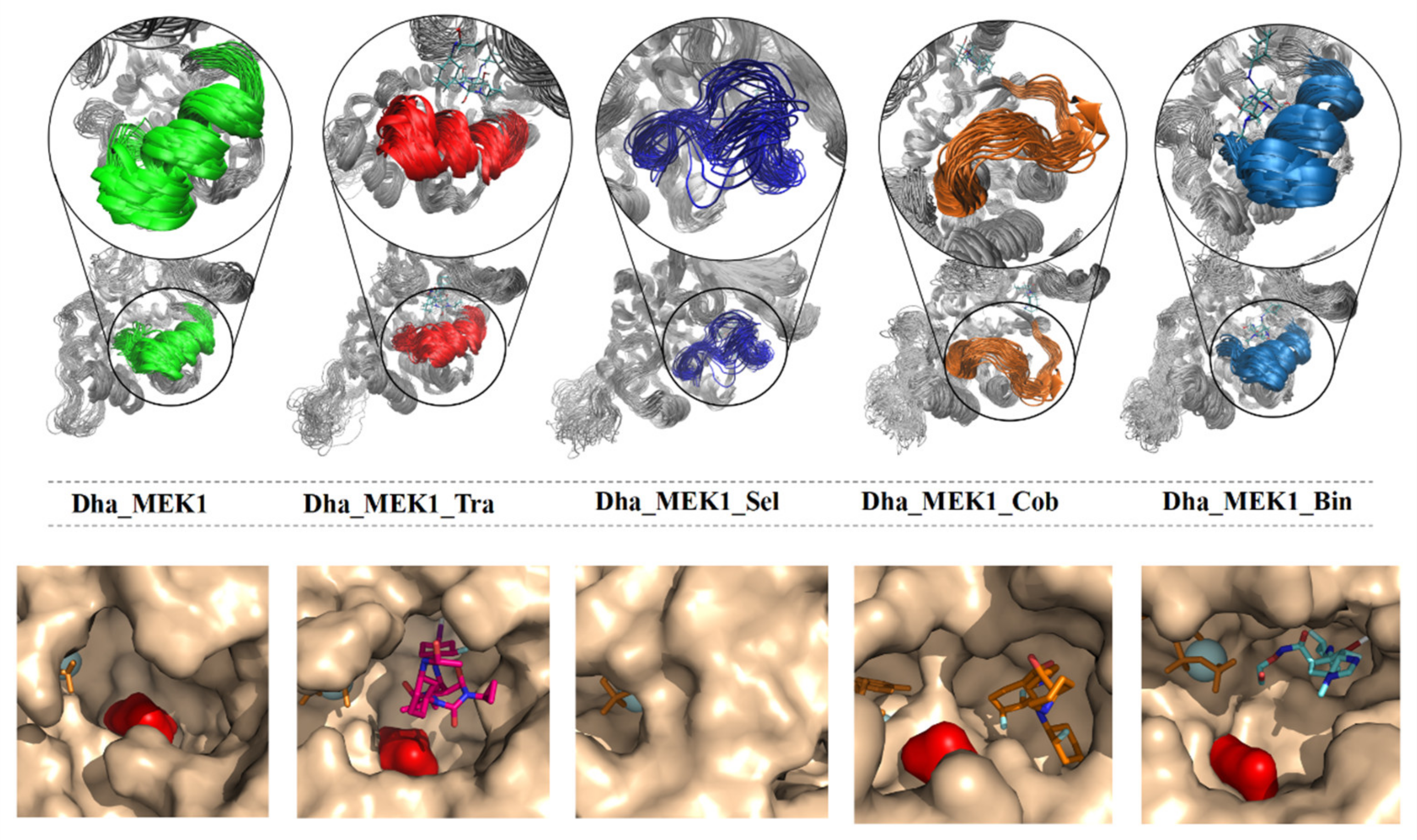
2.2.1. Stability Analysis of Docking Structures
2.2.2. The Inhibitory Effect of Selumetinib on MEK1 Abnormal Activation
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhanasekaran, N.; Premkumar Reddy, E. Signaling by dual specificity kinases. Oncogene 1998, 17, 1447–1455. [Google Scholar] [CrossRef]
- Maloney, R.C.; Zhang, M.; Liu, Y.; Jang, H.; Nussinov, R. The mechanism of activation of MEK1 by B-Raf and KSR1. Cell. Mol. Life Sci. 2022, 79, 218–247. [Google Scholar] [CrossRef] [PubMed]
- English, J.M.; Cobb, M.H. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol. Sci. 2002, 23, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sabsay, K.R.; Lee, R.T.; Ravatt, L.M.; Oza, J.P.; McDonald, A.R. Computational Models for Activated Human MEK1: Identification of Key Active Site Residues and Interactions. J. Chem. Inf. Model. 2019, 59, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Caunt, C.J.; Sale, M.J.; Smith, P.D.; Cook, S.J. MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road. Nat. Rev. Cancer 2015, 15, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Ohren, J.F.; Chen, H.F.; Pavlovsky, A.; Whitehead, C.; Zhang, E.L.; Kuffa, P.; Yan, C.H.; McConnell, P.; Spessard, C.; Banotai, C.; et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004, 11, 1192–1197. [Google Scholar] [CrossRef]
- Jindal, G.A.; Goyal, Y.; Humphreys, J.M.; Yeung, E.; Tian, K.; Patterson, V.L.; He, H.; Burdine, R.D.; Goldsmith, E.J.; Shvartsman, S.Y. How activating mutations affect MEK1 regulation and function. J. Biol. Chem. 2017, 292, 18814–18820. [Google Scholar] [CrossRef] [PubMed]
- Catoni, C.; Poggiana, C.; Facchinetti, A.; Pigozzo, J.; Piccin, L.; Chiarion-Sileni, V.; Rosato, A.; Minervini, G.; Scaini, M.C. Investigating the Retained Inhibitory Effect of Cobimetinib against p.P124L Mutated MEK1: A Combined Liquid Biopsy and in Silico Approach. Cancers 2022, 14, 4153. [Google Scholar] [CrossRef]
- Wang, H.; Chi, L.; Yu, F.; Dai, H.; Si, X.; Gao, C.; Wang, Z.; Liu, L.; Zheng, J.; Ke, Y.; et al. The overview of Mitogen-activated extracellular signal-regulated kinase (MEK)-based dual inhibitor in the treatment of cancers. Bioorg. Med. Chem. 2022, 70, 116922. [Google Scholar] [CrossRef]
- Fleischmann, J.; Feichtner, A.; DeFalco, L.; Kugler, V.; Schwaighofer, S.; Huber, R.G.; Stefan, E. Allosteric Kinase Inhibitors Reshape MEK1 Kinase Activity Conformations in Cells and In Silico. Biomolecules 2021, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.M.; Real, A.M.; Marsiglia, W.M.; Chow, A.; Duffy, M.E.; Yerabolu, J.R.; Scopton, A.P.; Dar, A.C. Structural basis for the action of the drug trametinib at KSR-bound MEK. Nature 2020, 588, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chang, M.T.; Johnsen, H.C.; Gao, S.P.; Sylvester, B.E.; Sumer, S.O.; Zhang, H.; Solit, D.B.; Taylor, B.S.; Schultz, N.; et al. 3D clusters of somatic mutations in cancer reveal numerous rare mutations as functional targets. Genome Med. 2017, 9, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, S.I.; Rimoldi, D.; Iseli, C.; Valsesia, A.; Robyr, D.; Gehrig, C.; Harshman, K.; Guipponi, M.; Bukach, O.; Zoete, V.; et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat. Genet. 2011, 44, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.N.; Sylvester, B.E.; Won, H.; McDermott, G.; DeLair, D.; Ramirez, R.; Yao, Z.; Shen, R.; Dao, F.; Bogomolniy, F.; et al. Extreme Outlier Analysis Identifies Occult Mitogen-Activated Protein Kinase Pathway Mutations in Patients with Low-Grade Serous Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, K.H.; Smalley, K.S. Making sense of MEK1 mutations in intrinsic and acquired BRAF inhibitor resistance. Cancer Discov. 2012, 2, 390–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, K.Y.; Galan, S.R.G.; Zeng, Y.B.; Zhou, T.H.; He, C.; Raj, R.; Riedl, J.; Liu, S.; Chooi, K.P.; Garg, N.; et al. LanCLs add glutathione to dehydroamino acids generated at phosphorylated sites in the proteome. Cell 2021, 184, 2680–2695. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.F.; Barford, D. Eliminylation: A post-translational modification catalyzed by phosphothreonine lyases. Trends Biochem. Sci. 2009, 34, 108–114. [Google Scholar] [CrossRef]
- Chan, W.C.; Dodd, H.M.; Horn, N.; Maclean, K.; Lian, L.Y.; Bycroft, B.W.; Gasson, M.J.; Roberts, G.C. Structure-activity relationships in the peptide antibiotic nisin: Role of dehydroalanine 5. Appl. Environ. Microbiol. 1996, 62, 2966–2969. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, J.P.; Brunzelle, J.S.; Moll, G.N.; van der Donk, W.A.; Nair, S.K. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science 2006, 311, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L. Reversible and irreversible protein glutathionylation: Biological and clinical aspects. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1183. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.W.; Bowers, A.A. Dehydroamino acids: Chemical multi-tools for late-stage diversification. Org. Biomol. Chem. 2019, 17, 3653–3669. [Google Scholar] [CrossRef]
- Li, H.T.; Xu, H.; Zhou, Y.; Zhang, J.; Long, C.Z.; Li, S.Q.; Chen, S.; Zhou, J.M.; Shao, F. The phosphothreonine lyase activity of a bacterial type III effector family. Science 2007, 315, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, L.Y.; Li, E.M.; Dong, G. Application of molecular dynamics simulation in biomedicine. Chem. Biol. Drug Des. 2022, 99, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z.; Yang, N. Mechanism of the Conformational Change of the Protein Methyltransferase SMYD3: A Molecular Dynamics Simulation Study. Int. J. Mol. Sci. 2021, 22, 7185. [Google Scholar] [CrossRef]
- Weigle, A.T.; Feng, J.; Shukla, D. Thirty years of molecular dynamics simulations on posttranslational modifications of proteins. Phys. Chem. Chem. Phys. 2022, 24, 26371–26397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, T.; Wang, S.; Duan, T.; Huang, S.; Xie, Y. The Modulation of Sucrose Nonfermenting 1-Related Protein Kinase 2.6 State by Persulfidation and Phosphorylation: Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2023, 24, 11512. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Yang, H.; Guo, X.; Huang, T.; Han, W. Computational Study on the Effect of Inactivating/Activating Mutations on the Inhibition of MEK1 by Trametinib. Int. J. Mol. Sci. 2020, 21, 2167. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2022 update. Pharmacol. Res. 2022, 175, 106037. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Momin, S.S.; Shaikh, S.; Alafnan, A.; Alanazi, J.; Said Almermesh, M.H.; Anwar, S. Drug Repurposing: A New Hope in Drug Discovery for Prostate Cancer. ACS Omega 2023, 8, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, E.B.; Schultz, S.A.; Chen, J. Using Metadynamics To Explore the Free Energy of Dewetting in Biologically Relevant Nanopores. J. Phys. Chem. B 2022, 126, 6428–6437. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.R.; Sorensen, J.; Hensley, N.; Wong, C.; Zhu, C.; Perison, T.; Amaro, R.E. POVME 3.0: Software for Mapping Binding Pocket Flexibility. J. Chem. Theory Comput. 2017, 13, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, J.X.; Guo, X.Q.; Huang, T.C.; Han, J.R.; Gao, J.J.; Xu, D.; Han, W.W. How oncogenic mutations activate human MAP kinase 1 (MEK1): A molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2020, 38, 3942–3958. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Siodlak, D. α,β-Dehydroamino acids in naturally occurring peptides. Amino Acids 2015, 47, 1–17. [Google Scholar] [CrossRef]
- Zanuy, D.; Casanovas, J.; Aleman, C. The conformation of dehydroalanine in short homopeptides: Molecular dynamics simulations of a 6-residue chain. Biophys. Chem. 2002, 98, 301–312. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Cheng, X.; Lee, J.; Kim, S.; Park, S.-J.; Patel, D.S.; Beaven, A.H.; Lee, K.I.; Rui, H.; Park, S.; et al. CHARMM-GUI 10 Years for Biomolecular Modeling and Simulation. J. Comput. Chem. 2016, 38, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Jo, S.; MacKerell, A.D.; Klauda, J.B.; Im, W. CHARMM-GUI Input Generator for NAMD, Gromacs, Amber, Openmm, and CHARMM/OpenMM Simulations using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 110, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Turpin, E.R.; Mulholland, S.; Teale, A.M.; Bonev, B.B.; Hirst, J.D. New CHARMM force field parameters for dehydrated amino acid residues, the key to lantibiotic molecular dynamics simulations. RSC Adv. 2014, 4, 48621–48631. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Branduardi, D. Free-Energy Calculations with Metadynamics: Theory and Practice. Rev. Comput. Chem. 2015, 28, 1–49. [Google Scholar] [CrossRef]
- Barducci, A.; Bussi, G.; Parrinello, M. Well-tempered metadynamics: A smoothly converging and tunable free-energy method. Phys. Rev. Lett. 2008, 100, 020603. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Tribello, G.A. Analyzing and Biasing Simulations with PLUMED. Methods Mol. Biol. 2019, 2022, 529–578. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Todd, A.K.; Zloh, M.; Gunaratnam, M.; Parkinson, G.N.; Neidle, S. Crystal structure of a promoter sequence in the B-raf gene reveals an intertwined dimer quadruplex. J. Am. Chem. Soc. 2013, 135, 19319–19329. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Keam, S.J. Selumetinib: First Approval. Drugs 2020, 80, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Scholl, F.A.; Dumesic, P.A.; Barragan, D.I.; Harada, K.; Charron, J.; Khavari, P.A. Selective role for Mek1 but not Mek2 in the induction of epidermal neoplasia. Cancer Res. 2009, 69, 3772–3778. [Google Scholar] [CrossRef] [PubMed]




| Hydrogen Bond | WT_MEK1 | PP_MEK1 | Dha_MEK1 | |
|---|---|---|---|---|
| Donor | Acceptor | Occupancy | Occupancy | Occupancy |
| ARG108-Side-NH1 | ASP136-Main-O | 0.25% | 24.25% | 13.75% |
| ARG189-Side-NH2 | SER241-Main-O | 0.00% | 36.00% | 40.75% |
| ARG189-Side-NH2 | ASP245-Side-OD1 | 0.00% | 27.00% | 26.25% |
| GLY213-Main-N | TYR240-Side-OH | 0.00% | 15.00% | 48.50% |
| ARG234-Side-NH2 | ASP217-Side-OD2 | 0.00% | 39.50% | 64.75% |
| ARG234-Side-NE | ASP217-Side-OD1 | 0.00% | 44.75% | 73.50% |
| SER244-Side-OG | GLU233-Side-OE2 | 13.25% | 23.00% | 76.50% |
| ARG305-Side-NE | SER228-Side-OG | 0.00% | 0.00% | 26.00% |
| Systems | Donor | Receptor | Occupancy |
|---|---|---|---|
| Dha_MEK1_Tra | GLU102-Side-OE2 | TRA385-Side-O33 | 27.99% |
| GLU102-Side-OE1 | TRA385-Side-O33 | 21.66% | |
| Dha_MEK1_Sel | ASP190-Side-OD1 | SEL385-Side-O3 | 36.98% |
| MET187-Main-O | SEL385-Side-O3 | 33.33% | |
| ASP190-Side-OD2 | SEL385-Side-O3 | 22.99% | |
| PHE209-Main-O | SEL385-Side-O3 | 14.33% | |
| MET219-Main-N | SEL385-Side-N4 | 11.33% | |
| THR226-Side-OG1 | SEL 385-Side-N4 | 10.33% | |
| THR226-Main-N | SEL 385-Side-F1 | 20.33% | |
| ARG227-Main-N | SEL 385-Side-O3 | 20.33% | |
| Dha_MEK1_Cob | PHE209-Main-N | COB385-Side-F07 | 25.61% |
| ASP190-Side-OD2 | SEL385-Side-LP1 | 12.13% | |
| ILE186-Side-CB | COB385-Side-LP1 | 26.33% | |
| LYS185-Side-CB | COB385-Side-LP1 | 21.33% | |
| GLU114-Side-CG | COB385-Side-LP1 | 11.33% | |
| Dha_MEK1_Bin | ASP217-Side-OD2 | BIN385-Side-O27 | 19.93% |
| ASP217-Side-OD1 | BIN385-Side-O27 | 16.61% | |
| ILE216-Side-CG1 | BIN385-Side-LP1 | 12.33% | |
| ASP190-Side-OD1 | BIN385-Side-O27 | 31.00% | |
| ILE216-Side-CG1 | BIN385-Side-LP1 | 12.33% | |
| VAL211-Side-CG2 | BIN385-Side-LP1 | 11.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Du, S.-S.; Zhao, C.-Y.; Li, T.-L.; Tong, S.-C.; Zhao, L. Mechanism of Abnormal Activation of MEK1 Induced by Dehydroalanine Modification. Int. J. Mol. Sci. 2024, 25, 7482. https://doi.org/10.3390/ijms25137482
Zhao Y, Du S-S, Zhao C-Y, Li T-L, Tong S-C, Zhao L. Mechanism of Abnormal Activation of MEK1 Induced by Dehydroalanine Modification. International Journal of Molecular Sciences. 2024; 25(13):7482. https://doi.org/10.3390/ijms25137482
Chicago/Turabian StyleZhao, Yue, Shan-Shan Du, Chao-Yue Zhao, Tian-Long Li, Si-Cheng Tong, and Li Zhao. 2024. "Mechanism of Abnormal Activation of MEK1 Induced by Dehydroalanine Modification" International Journal of Molecular Sciences 25, no. 13: 7482. https://doi.org/10.3390/ijms25137482
APA StyleZhao, Y., Du, S.-S., Zhao, C.-Y., Li, T.-L., Tong, S.-C., & Zhao, L. (2024). Mechanism of Abnormal Activation of MEK1 Induced by Dehydroalanine Modification. International Journal of Molecular Sciences, 25(13), 7482. https://doi.org/10.3390/ijms25137482





