Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells
Abstract
1. Introduction
2. Results
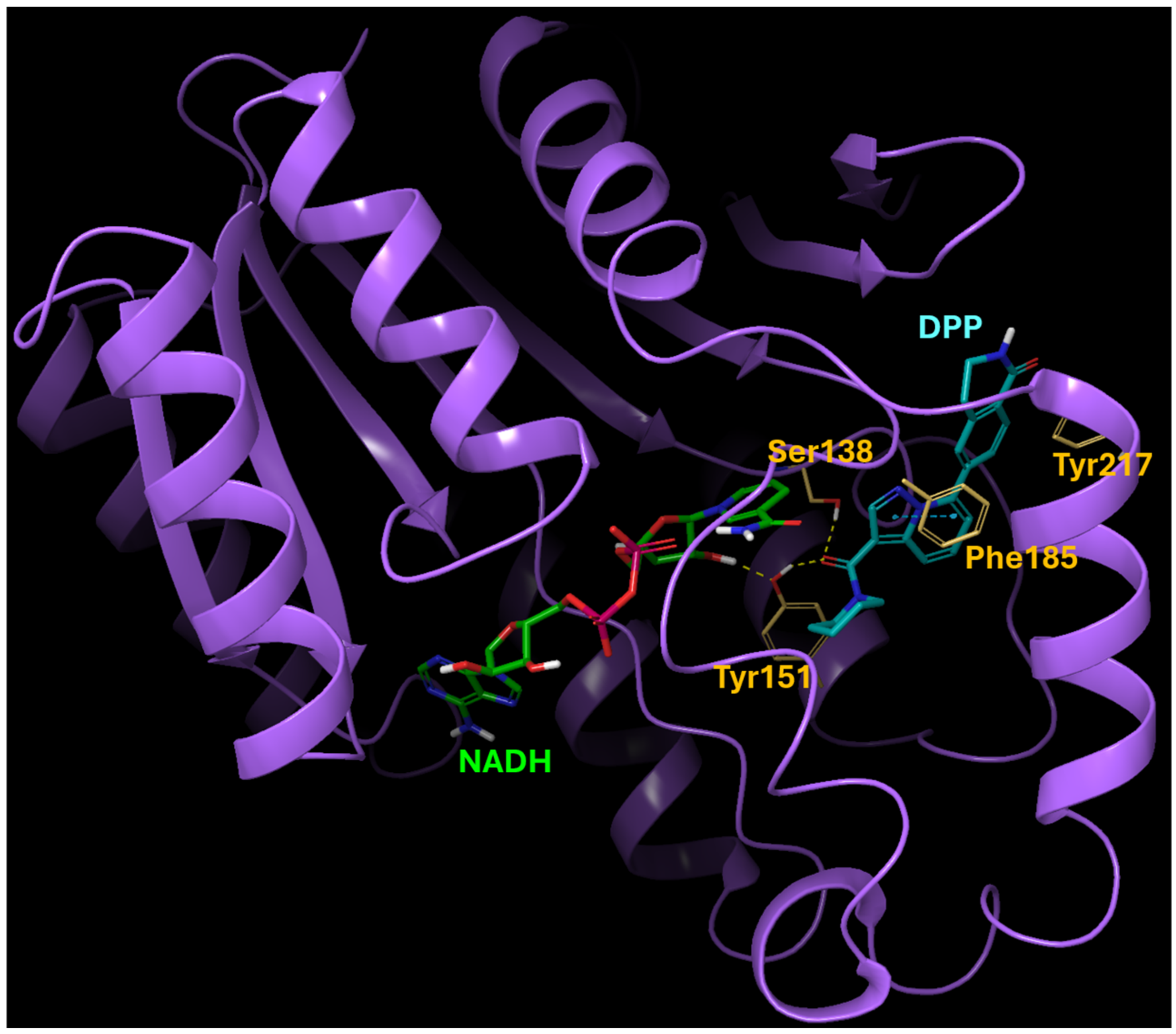
2.1. The Binding Modeling of the Interaction between DPP and 15-PGDH
2.2. DPP Enhanced the Migration of DHT-Damaged HFDPCs
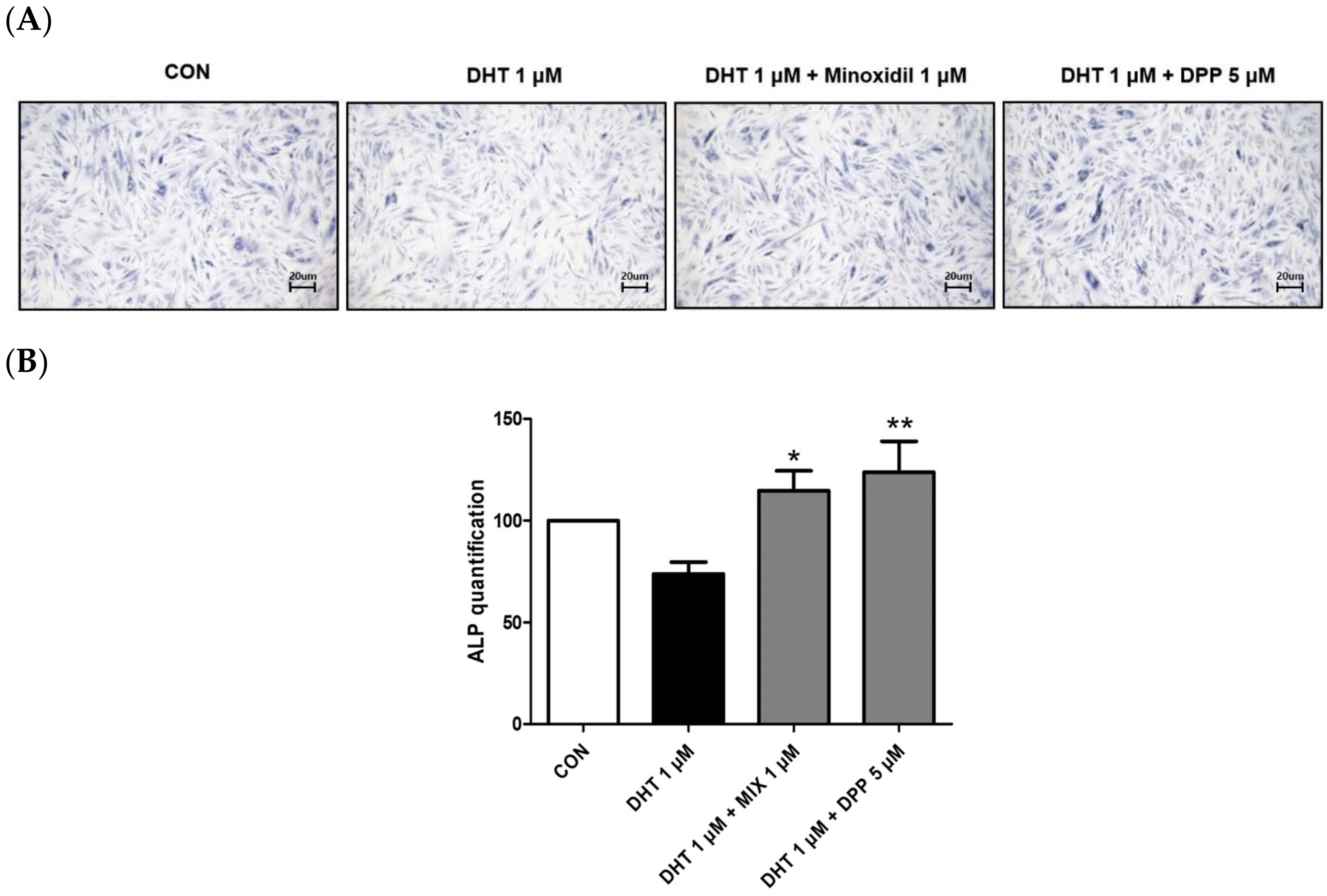
2.3. DPP Increased the Expression Level of Alkaline Phosphatase in DHT-Damaged HFDPCs
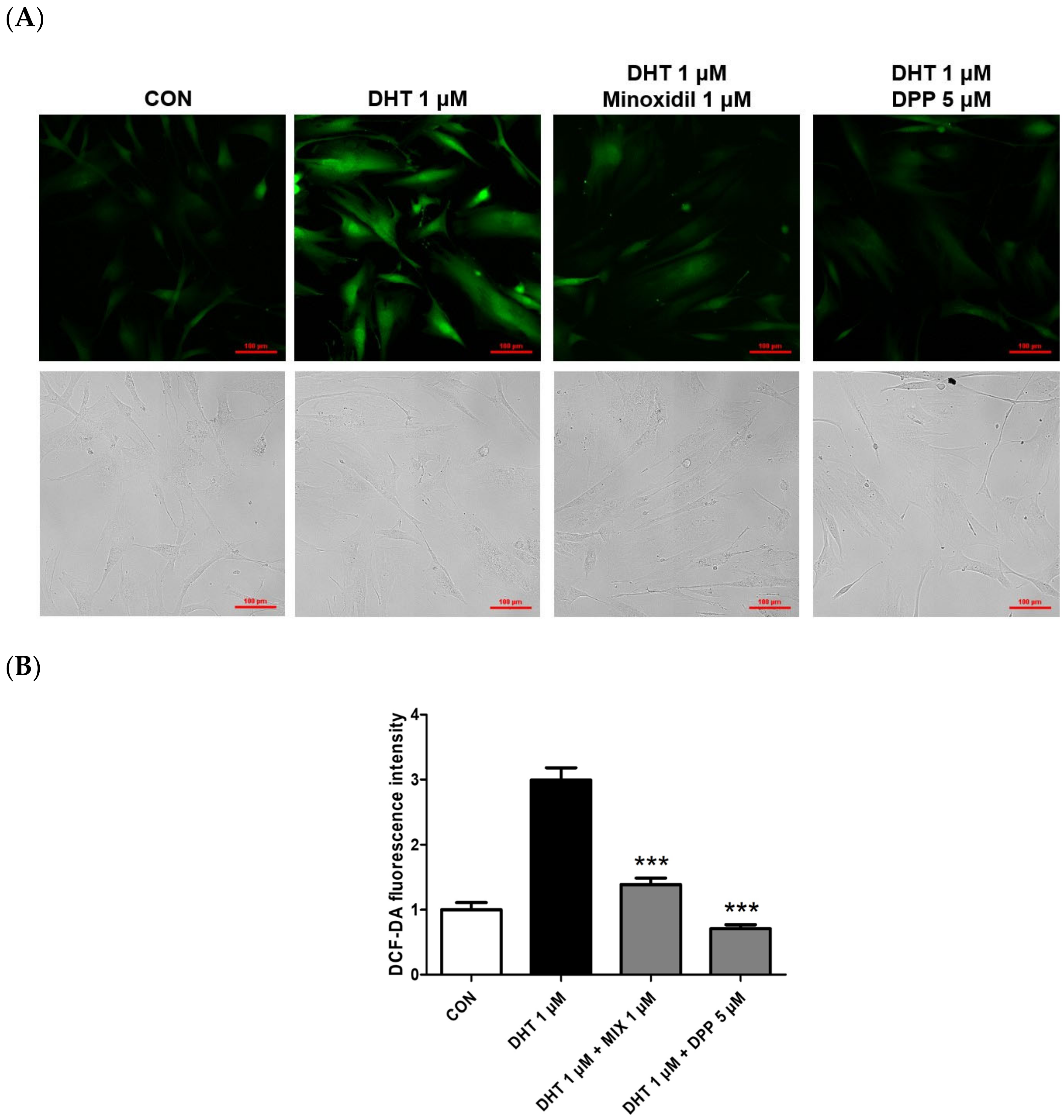
2.4. DPP Reduced the Reactive Oxygen Level in DHT-Damaged HFDPCs
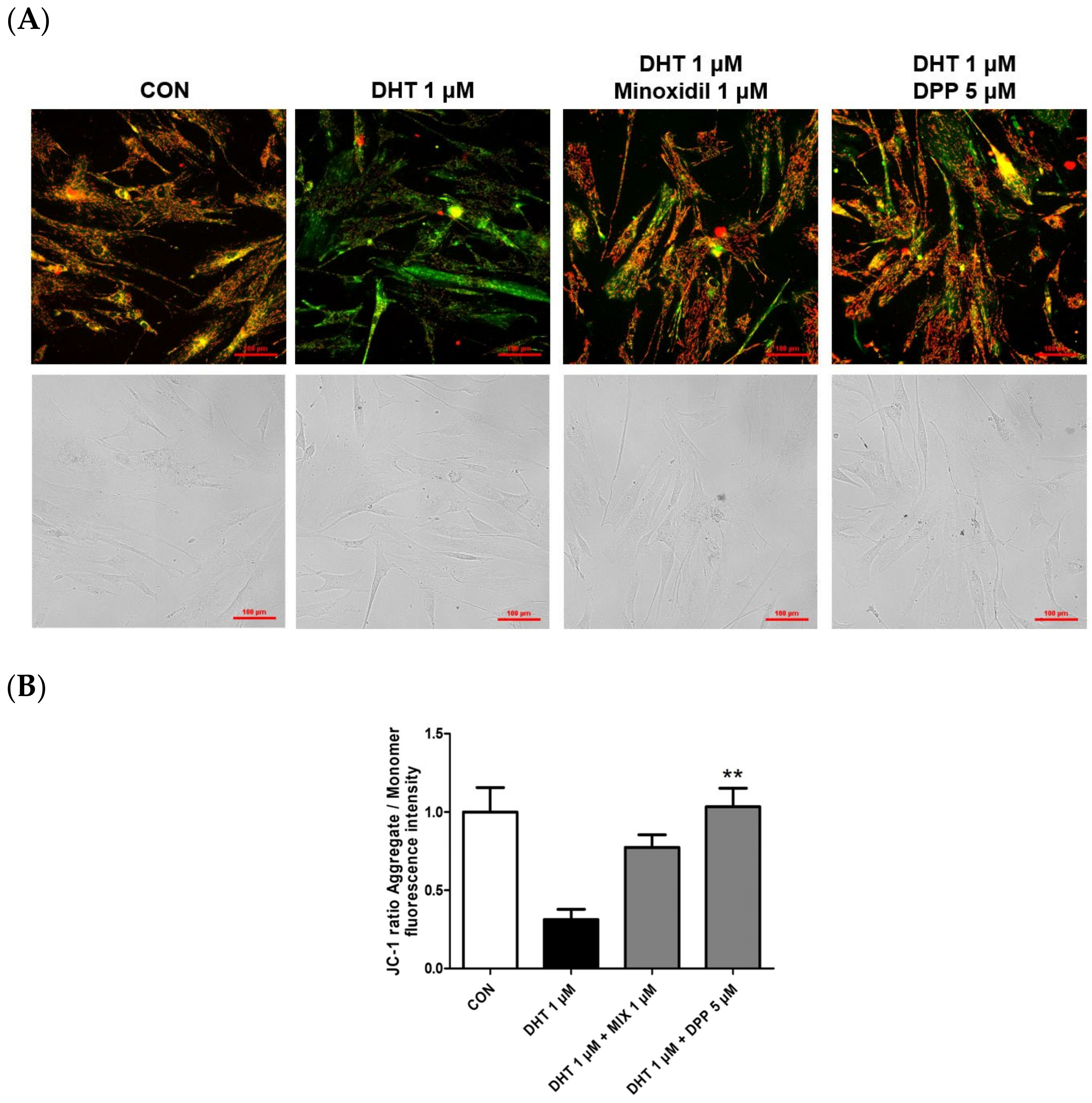
2.5. DPP Recovered the Mitochondrial Potential in DHT-Damaged HFDPCs
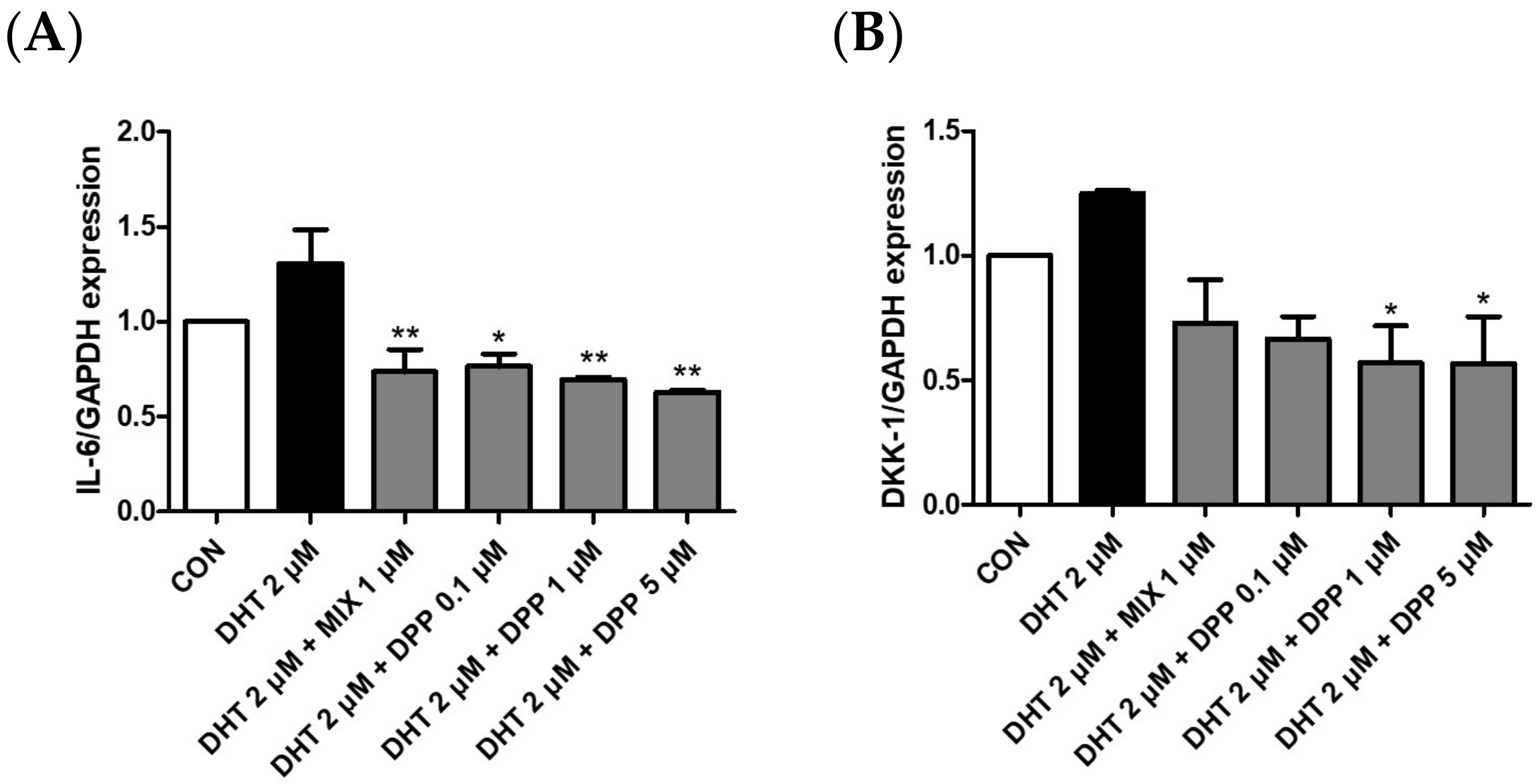
2.6. DPP Decreased the mRNA Expression Level of Dickkopf-Related Protein 1 (DKK-1), and Interleukin 6 (IL-6) in DHT-Damaged HFDPCs
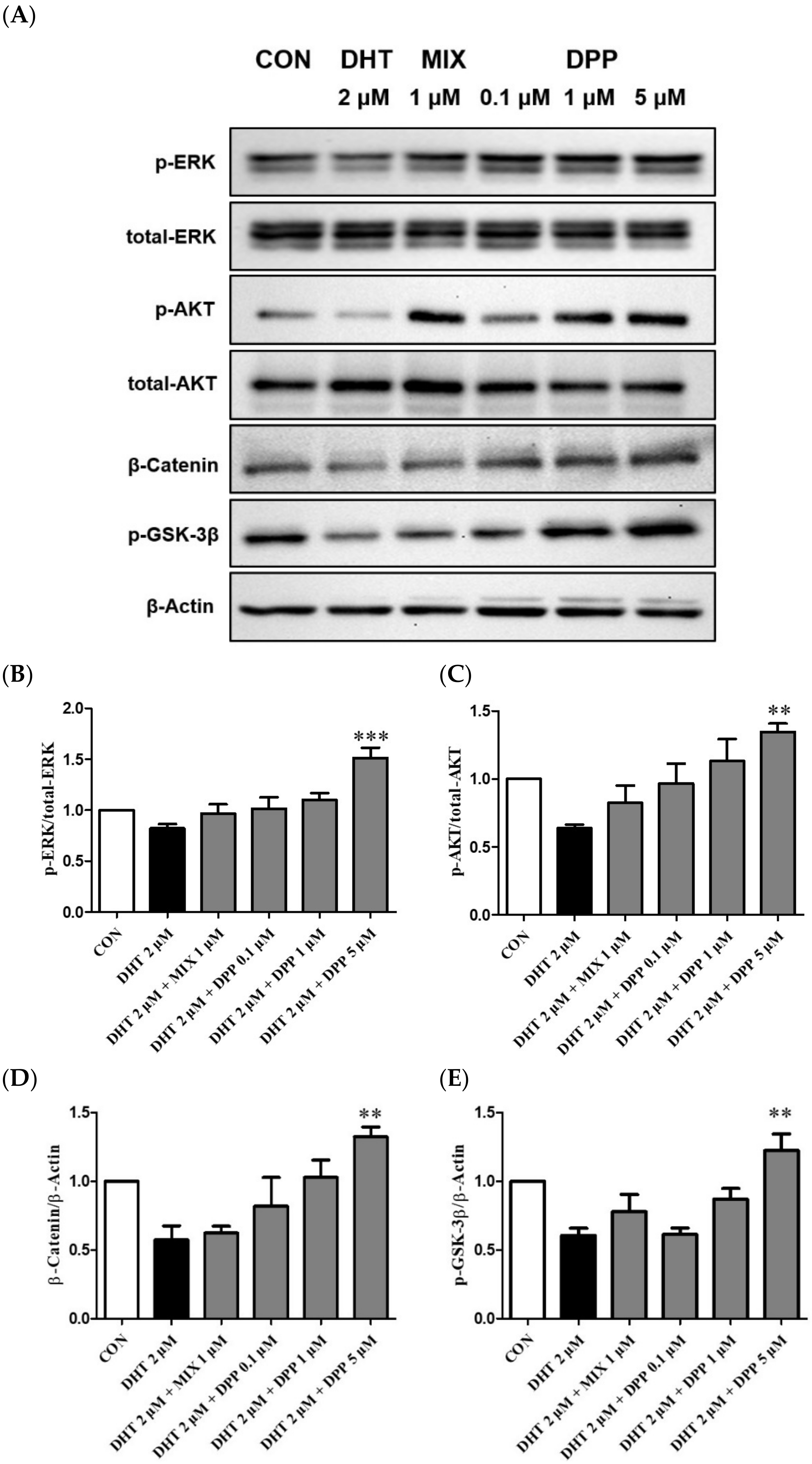
2.7. DPP Increased the Phosphorylation Levels of ERK, AKT β-Catenin, and GSK-3β in DHT-Damaged HFDPCs
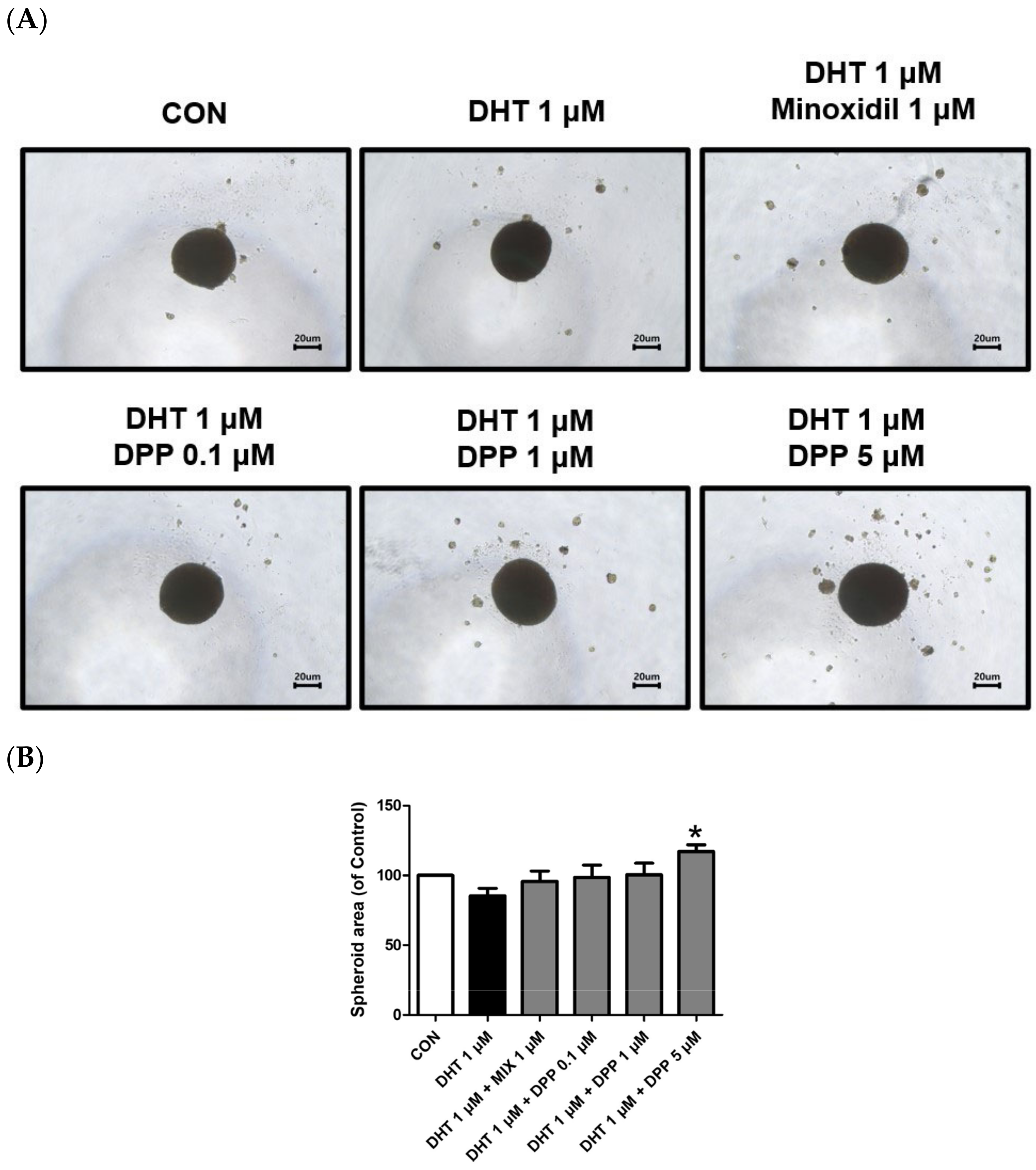
2.8. DPP Increased the Size of the 3-Dimensional (3D) Spheroid in DHT-Damaged HFDPCs
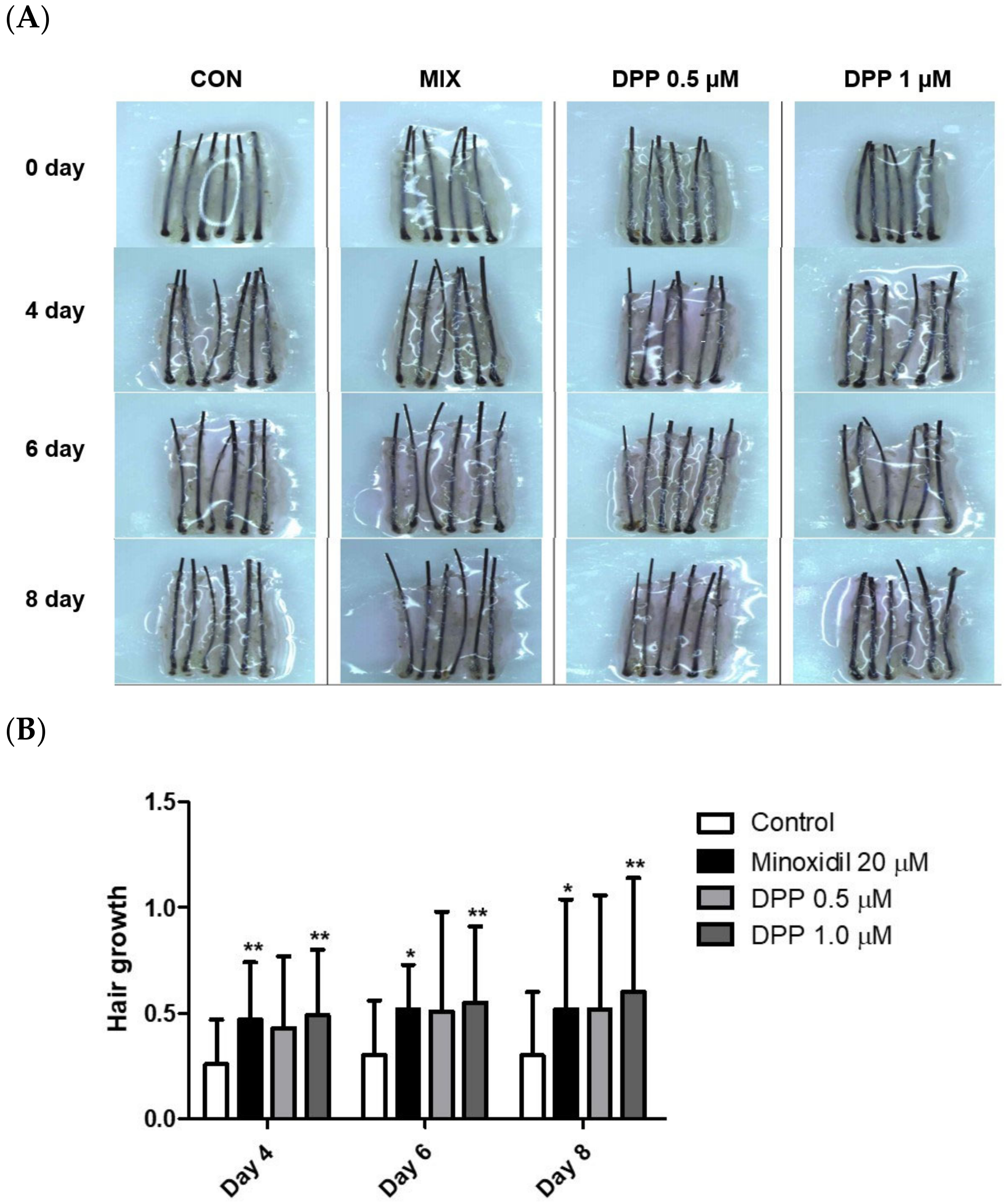
2.9. DPP Stimulated Hair Growth Ex Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Modeling of DPP
4.3. Cell Culture
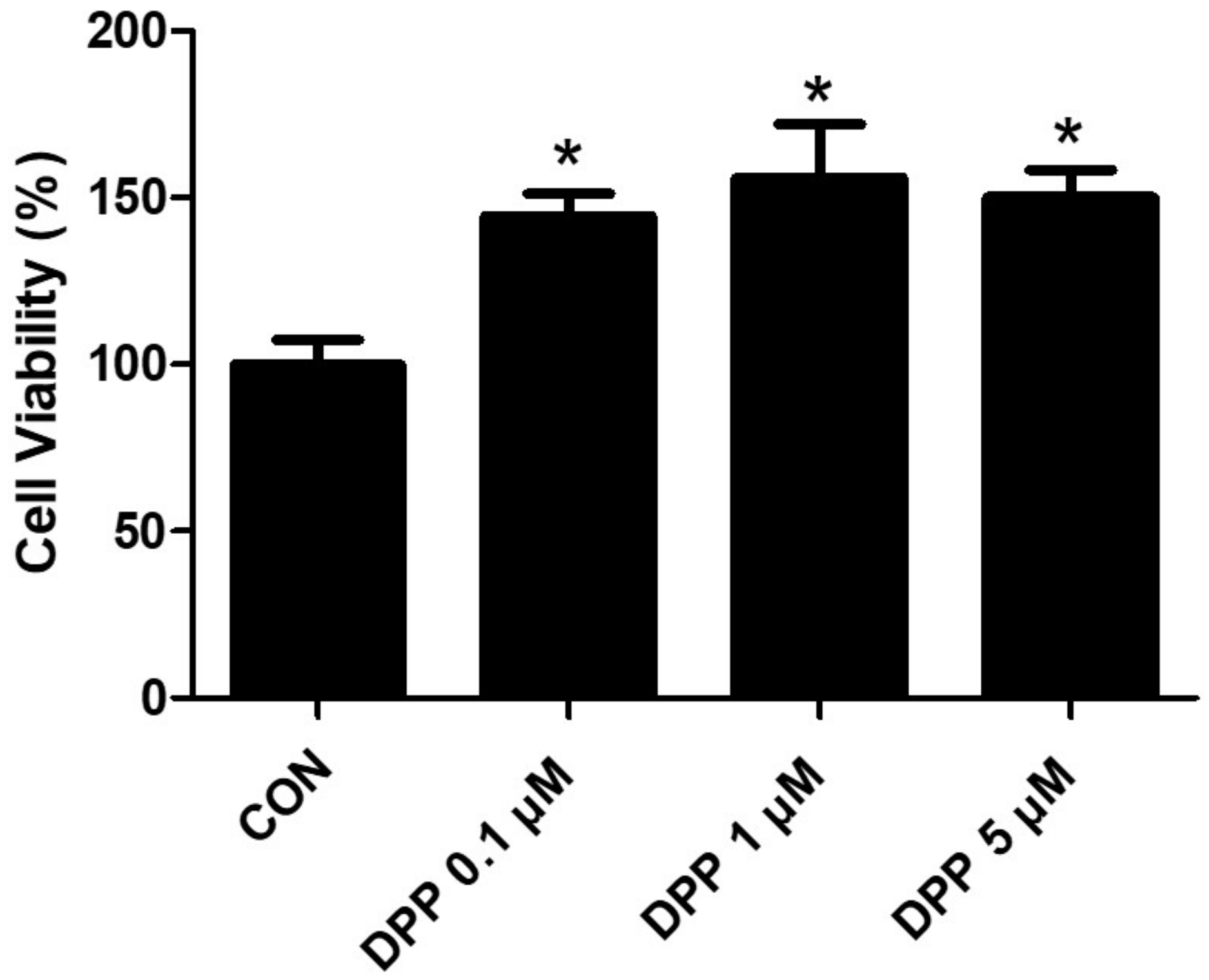
4.4. Cell Viability Assay
4.5. Wound Healing Assay
4.6. Alkaline Phosphatase Staining (ALP) Assay
4.7. DCF-DA ROS Assay
4.8. Measurement of Mitochondrial Membrane Potential
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Western Blot Analysis
4.11. Three-Dimensional Spheroid Culture of HDPCs
4.12. Human Hair Follicle Organ Culture
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazemi, A.; Cline, A.; Gomolin, T.A.; Safai, B. Hair-Loss Perceptions and Treatment Expectations in Young People of Color. J. Drugs Dermatol. 2021, 20, 746–750. [Google Scholar] [PubMed]
- Marks, D.H.; Penzi, L.R.; Ibler, E.; Manatis-Lornell, A.; Hagigeorges, D.; Yasuda, M.; Drake, L.A.; Senna, M.M. The Medical and Psychosocial Associations of Alopecia: Recognizing Hair Loss as More than a Cosmetic Concern. Am. J. Clin. Dermatol. 2019, 20, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Toussi, A.; Barton, V.R.; Le, S.T.; Agbai, O.N.; Kiuru, M. Psychosocial and Psychiatric Comorbidities and Health-Related Quality of Life in Alopecia Areata: A Systematic Review. J. Am. Acad. Dermatol. 2021, 85, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R. Male Pattern Androgenetic Alopecia. BMJ 1998, 317, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.E.; Keane, R.W.; Blume-Peytavi, U.; Mullins, D.L.; Nusbaum, B.P.; Whiting, D.; Nicholson, D.W. Androgen Responsive Genes as they Affect Hair Growth. Eur. J. Dermatol. 2001, 11, 304–308. [Google Scholar] [PubMed]
- Kaufman, K.D. Androgens and Alopecia. Mol. Cell. Endocrinol. 2002, 198, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Skin Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Kang, D.; Zhou, Y.; Du, L.; Qu, Q.; Wang, J.; Wen, L.; Fu, D.; Hu, Z.; et al. Microenvironmental Reprogramming of Human Dermal Papilla Cells for Hair Follicle Tissue Engineering. Acta Biomater. 2023, 165, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Nam, Y.J.; Kang, S.; Choi, E.J.; Han, I.; Kim, J.; Kim, D.H.; An, J.H.; Lee, S.; Lee, M.H.; et al. The Local Hypothalamic-Pituitary-Adrenal Axis in Cultured Human Dermal Papilla Cells. BMC Mol. Cell. Biol. 2020, 21, 42-w. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, H. Prostanoids and Hair Follicles: Implications for Therapy of Hair Disorders. Acta Derm. Venereol. 2018, 98, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chovarda, E.; Sotiriou, E.; Lazaridou, E.; Vakirlis, E.; Ioannides, D. The Role of Prostaglandins in Androgenetic Alopecia. Int. J. Dermatol. 2021, 60, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W. The Physiological and Pharmacological Roles of Prostaglandins in Hair Growth. Korean J. Physiol. Pharmacol. 2022, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Yagami, T.; Koma, H.; Yamamoto, Y. Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System. Mol. Neurobiol. 2016, 53, 4754–4771. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution of Platelet and Vascular Arachidonic Acid Metabolism to the Pathophysiology of Atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Colombe, L.; Vindrios, A.; Michelet, J.; Bernard, B.A. Prostaglandin Metabolism in Human Hair Follicle. Exp. Dermatol. 2007, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, S.; Cheng, H.; Cao, J.; Du, W.; Zhang, J.; Chang, Y.; Shen, X.; Guo, Z.; Han, Z.; et al. The Sustained PGE(2) Release Matrix Improves Neovascularization and Skeletal Muscle Regeneration in a Hindlimb Ischemia Model. J. Nanobiotechnol. 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 Mediates Sensory Nerve Regulation of Bone Homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of Prostaglandin E2 in Tissue Repair and Regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef]
- Hezam, K.; Wang, C.; Fu, E.; Zhou, M.; Liu, Y.; Wang, H.; Zhu, L.; Han, Z.; Han, Z.; Chang, Y.; et al. Superior Protective Effects of PGE2 Priming Mesenchymal Stem Cells Against LPS-Induced Acute Lung Injury (ALI) through Macrophage Immunomodulation. Stem Cell. Res. Ther. 2023, 14, 48. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Zhang, C.; Chen, H.; Liu, L.; Chen, S. Effects of SW033291 on the Myogenesis of Muscle-Derived Stem Cells and Muscle Regeneration. Stem Cell. Res. Ther. 2020, 11, 76. [Google Scholar] [CrossRef]
- Hanson, W.R.; Pelka, A.E.; Nelson, A.K.; Malkinson, F.D. Subcutaneous or Topical Administration of 16,16 Dimethyl Prostaglandin E2 Protects from Radiation-Induced Alopecia in Mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Diehl, J.; Levins, P.C. Promising Alternative Clinical Uses of Prostaglandin F2alpha Analogs: Beyond the Eyelashes. J. Am. Acad. Dermatol. 2015, 72, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Smith, J.N.P.; Park, Y.S.; Hadiono, M.; Christo, K.; Jogasuria, A.; Zhang, Y.; Broncano, A.V.; Kasturi, L.; Dawson, D.M.; et al. 15-PGDH Regulates Hematopoietic and Gastrointestinal Fitness during Aging. PLoS ONE 2022, 17, e0268787. [Google Scholar] [CrossRef] [PubMed]
- Antczak, M.I.; Zhang, Y.; Wang, C.; Doran, J.; Naidoo, J.; Voruganti, S.; Williams, N.S.; Markowitz, S.D.; Ready, J.M. Inhibitors of 15-Prostaglandin Dehydrogenase to Potentiate Tissue Repair. J. Med. Chem. 2017, 60, 3979–4001. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Zhang, Y.; Park, Y.; Dawson, D.M.; Larusch, G.A.; Kasturi, L.; Wald, D.; Ready, J.M.; Gerson, S.L.; Markowitz, S.D. A Second-Generation 15-PGDH Inhibitor Promotes Bone Marrow Transplant Recovery Independently of Age, Transplant Dose and Granulocyte Colony-Stimulating Factor Support. Haematologica 2018, 103, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.; Dawson, D.M.; Christo, K.F.; Jogasuria, A.P.; Cameron, M.J.; Antczak, M.I.; Ready, J.M.; Gerson, S.L.; Markowitz, S.D.; Desai, A.B. 15-PGDH Inhibition Activates the Splenic Niche to Promote Hematopoietic Regeneration. JCI Insight 2021, 6, e143658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Desai, A.; Yang, S.Y.; Bae, K.B.; Antczak, M.I.; Fink, S.P.; Tiwari, S.; Willis, J.E.; Williams, N.S.; Dawson, D.M.; et al. Inhibition of the Prostaglandin-Degrading Enzyme 15-PGDH Potentiates Tissue Regeneration. Science 2015, 348, aaa2340. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Jang, Y.J.; Won, G.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Overexpression of Alkaline Phosphatase Improves the Hair-Inductive Capacity of Cultured Human Dermal Papilla Spheres. J. Dermatol. Sci. 2019, 95, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair Cycle-Dependent Changes of Alkaline Phosphatase Activity in the Mesenchyme and Epithelium in Mouse Vibrissal Follicles. Dev. Growth Differ. 2007, 49, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.; Min, D.S.; Kim, H.; Choi, K. Valproic Acid Induces Hair Regeneration in Murine Model and Activates Alkaline Phosphatase Activity in Human Dermal Papilla Cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxid Med. Cell. Longev 2022, 2022, 3618806. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.I.; Khashaba, R.A.; Fawzy, E.; Baghdady, S.M.A.; Rezk, S.M. Cross Talk between Oxidative Stress and Inflammation in Alopecia Areata. J. Cosmet. Dermatol. 2021, 20, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Yenin, J.Z.; Serarslan, G.; Yonden, Z.; Ulutas, K.T. Investigation of Oxidative Stress in Patients with Alopecia Areata and its Relationship with Disease Severity, Duration, Recurrence and Pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, K.; Lee, M.J.; Lee, J.; Choi, S.; Kim, K.; Ko, J.; Han, H.; Kim, S.Y.; Youn, H.J.; et al. Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5alpha-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann. Dermatol. 2016, 28, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Tavernarakis, N. Mitochondrial Homeostasis: The Interplay between Mitophagy and Mitochondrial Biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Chawla, R.; Mah, T.; Vivekanandan, R.; Tan, S.Y.; Sato, P.Y.; Mallilankaraman, K. Mitochondrial Dysfunction in Age-Related Metabolic Disorders. Proteomics 2020, 20, e1800404. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jedrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell. Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Shin, J.Y.; Choi, Y.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum Multiflorum Extract Support Hair Growth by Elongating Anagen Phase and Abrogating the Effect of Androgen in Cultured Human Dermal Papilla Cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Elgarhy, L.H.; Hasby, E.A.; Mohammad, L. Dickkopf-1 Expression in Androgenetic Alopecia and Alopecia Areata in Male Patients. Am. J. Dermatopathol. 2019, 41, 122–127. [Google Scholar] [CrossRef]
- Shou, J.; Ali-Osman, F.; Multani, A.S.; Pathak, S.; Fedi, P.; Srivenugopal, K.S. Human Dkk-1, a Gene Encoding a Wnt Antagonist, Responds to DNA Damage and its Overexpression Sensitizes Brain Tumor Cells to Apoptosis Following Alkylation Damage of DNA. Oncogene 2002, 21, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Chen, Y.; Chen, Y.; Lee, T.; Peng, Y.; Chen, S.; Yen, Y.; Chien, C.; Hsieh, J.; Chen, Y. Interleukin-6 from Adipose-Derived Stem Cells Promotes Tissue Repair by the Increase of Cell Proliferation and Hair Follicles in Ischemia/Reperfusion-Treated Skin Flaps. Mediators Inflamm. 2019, 2019, 2343867. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, H.; Huang, M. Lactoferrin Promotes Hair Growth in Mice and Increases Dermal Papilla Cell Proliferation through Erk/Akt and Wnt Signaling Pathways. Arch. Dermatol. Res. 2019, 311, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.I.; Bae, S.; Ahn, K.J. Flavonoid Silibinin Increases Hair-Inductive Property Via Akt and Wnt/Beta-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2019, 29, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.; Kim, H.; An, I.; An, S. Monoterpenoid Loliolide Regulates Hair Follicle Inductivity of Human Dermal Papilla Cells by Activating the Akt/Beta-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, Z.; Chu, X.; Bi, Z.; Fan, W. An Investigation of Crosstalk between Wnt/Beta-Catenin and Transforming Growth Factor-Beta Signaling in Androgenetic Alopecia. Medicine 2016, 95, e4297. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, H.; Chung, K.B.; Lee, H.J.; Kim, J.; Song, K.; Kim, D. Non-Thermal Atmospheric Pressure Plasma Activates Wnt/Beta-Catenin Signaling in Dermal Papilla Cells. Sci. Rep. 2021, 11, 16125-y. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Choi, Y.K.; Koh, Y.; Hyun, J.; Kang, J.; Lee, K.S.; Lee, C.M.; Yoo, E.; Kang, H. Vanillic Acid Stimulates Anagen Signaling Via the PI3K/Akt/ Beta-Catenin Pathway in Dermal Papilla Cells. Biomol. Ther. 2020, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair Follicle Dermal Papilla Cells at a Glance. J. Cell. Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Topouzi, H.; Logan, N.J.; Williams, G.; Higgins, C.A. Methods for the Isolation and 3D Culture of Dermal Papilla Cells from Human Hair Follicles. Exp. Dermatol. 2017, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Yin, G.; Ye, J.; Pan, X.; Zhu, J.; Lin, B. RNA Sequence Analysis of Dermal Papilla Cells’ Regeneration in 3D Culture. J. Cell. Mol. Med. 2020, 24, 13421–13430. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Ex Vivo Organ Culture of Human Hair Follicles: A Model Epithelial-Neuroectodermal-Mesenchymal Interaction System. Methods Mol. Biol. 2011, 695, 213–227. [Google Scholar] [PubMed]
- Wang, H.; Pan, L.; Wu, Y. Epidemiological Trends in Alopecia Areata at the Global, Regional, and National Levels. Front. Immunol. 2022, 13, 874677. [Google Scholar] [CrossRef] [PubMed]
- Abdin, R.; Zhang, Y.; Jimenez, J.J. Treatment of Androgenetic Alopecia using PRP to Target Dysregulated Mechanisms and Pathways. Front. Med. 2022, 9, 843127. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Hozumi, Y.; Kondo, S. Influence of Prostaglandin F2alpha and its Analogues on Hair Regrowth and Follicular Melanogenesis in a Murine Model. Exp. Dermatol. 2005, 14, 323–328. [Google Scholar] [CrossRef]
- Fawzi, M.M.T.; Mahmoud, S.B.; Shaker, O.G.; Saleh, M.A. Assessment of Tissue Levels of Dickkopf-1 in Androgenetic Alopecia and Alopecia Areata. J. Cosmet. Dermatol. 2016, 15, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hao, Z.; Qi, W.; Wang, Z.; Zhou, M.; Guo, N. The Efficacy of Topical Prostaglandin Analogs for Hair Loss: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 1130623. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Larsson, N. Mitochondrial Dysfunction as a Cause of Ageing. J. Intern. Med. 2008, 263, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Papukashvili, D.; Liu, C.; Rcheulishvili, N.; Xie, F.; Wang, X.; Feng, S.; Sun, X.; Zhang, C.; Li, Y.; He, Y.; et al. DKK1-Targeting Cholesterol-Modified siRNA Implication in Hair Growth Regulation. Biochem. Biophys. Res. Commun. 2023, 668, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/Beta-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Bejaoui, M.; Oliva, A.K.; Ke, M.S.; Ferdousi, F.; Isoda, H. 3D Spheroid Human Dermal Papilla Cell as an Effective Model for the Screening of Hair Growth Promoting Compounds: Examples of Minoxidil and 3,4,5-Tri-O-Caffeoylquinic Acid (TCQA). Cells 2022, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular Docking with Deep Learning. J. Cheminform 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Bakan, A.; Meireles, L.M.; Bahar, I. ProDy: Protein Dynamics Inferred from Theory and Experiments. Bioinformatics 2011, 27, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.W.; Kim, H.J.; Jeon, C.Y.; Lee, Y.; Kim, M.; Kim, J.; Kim, S.R.; Lee, S.; Lim, D.C.; Park, H.D.; et al. Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2024, 25, 7485. https://doi.org/10.3390/ijms25137485
Lim HW, Kim HJ, Jeon CY, Lee Y, Kim M, Kim J, Kim SR, Lee S, Lim DC, Park HD, et al. Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells. International Journal of Molecular Sciences. 2024; 25(13):7485. https://doi.org/10.3390/ijms25137485
Chicago/Turabian StyleLim, Hye Won, Hak Joong Kim, Chae Young Jeon, Yurim Lee, Mujun Kim, Jinsick Kim, Soon Re Kim, Sanghwa Lee, Dong Chul Lim, Hee Dong Park, and et al. 2024. "Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells" International Journal of Molecular Sciences 25, no. 13: 7485. https://doi.org/10.3390/ijms25137485
APA StyleLim, H. W., Kim, H. J., Jeon, C. Y., Lee, Y., Kim, M., Kim, J., Kim, S. R., Lee, S., Lim, D. C., Park, H. D., Park, B. C., & Shin, D. W. (2024). Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells. International Journal of Molecular Sciences, 25(13), 7485. https://doi.org/10.3390/ijms25137485







