Quantitative Proteomic Analysis of Macrophages Infected with Trypanosoma cruzi Reveals Different Responses Dependent on the SLAMF1 Receptor and the Parasite Strain
Abstract
:1. Introduction
2. Results
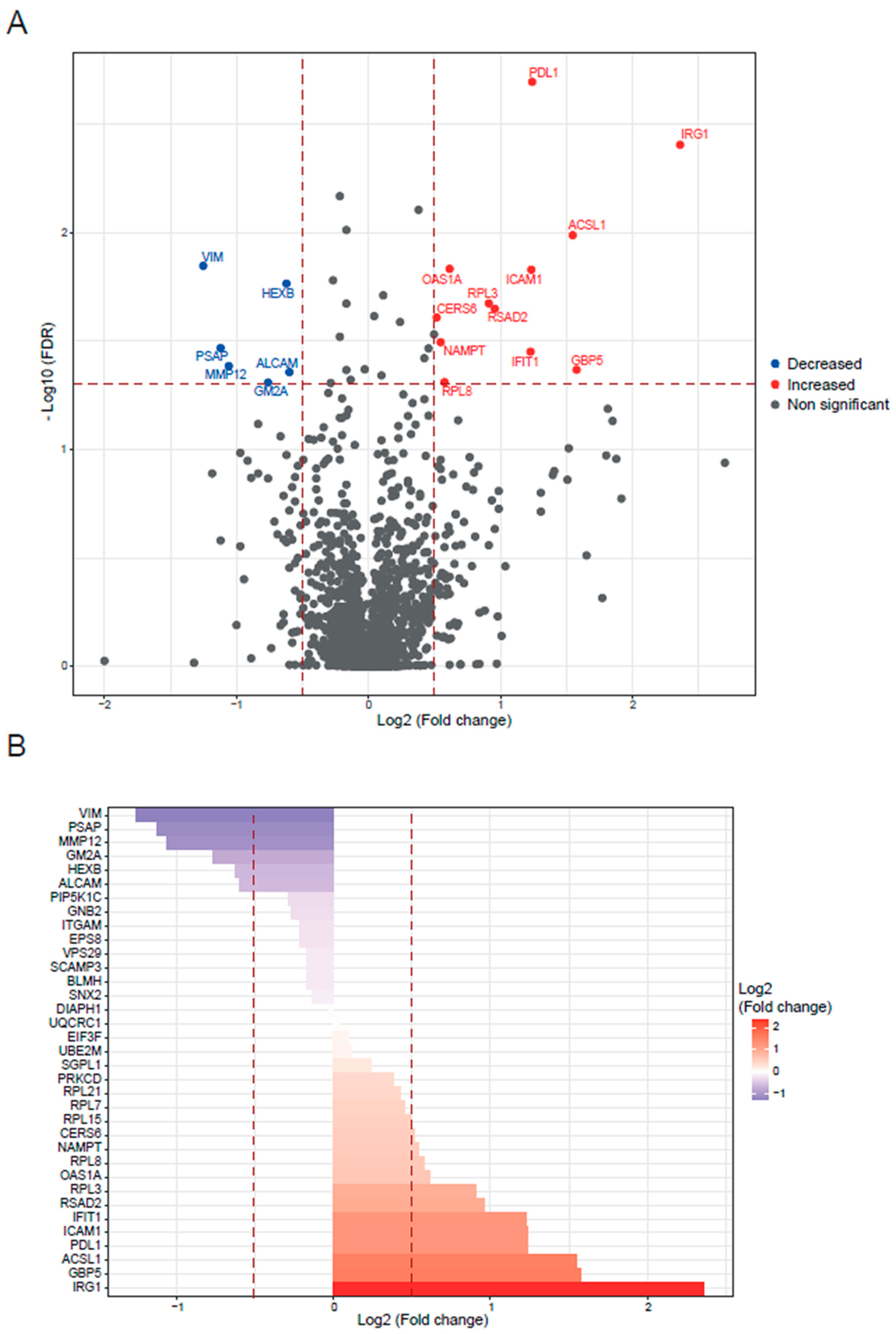
2.1. Quantitative Proteomics Analysis of BALB/c and Slamf1-/- Macrophages Infected with the Y Strain of T. cruzi
2.2. Quantitative Proteomics Analysis of BALB/c and Slamf1-/- Macrophages Infected with the VFRA Strain of T. cruzi
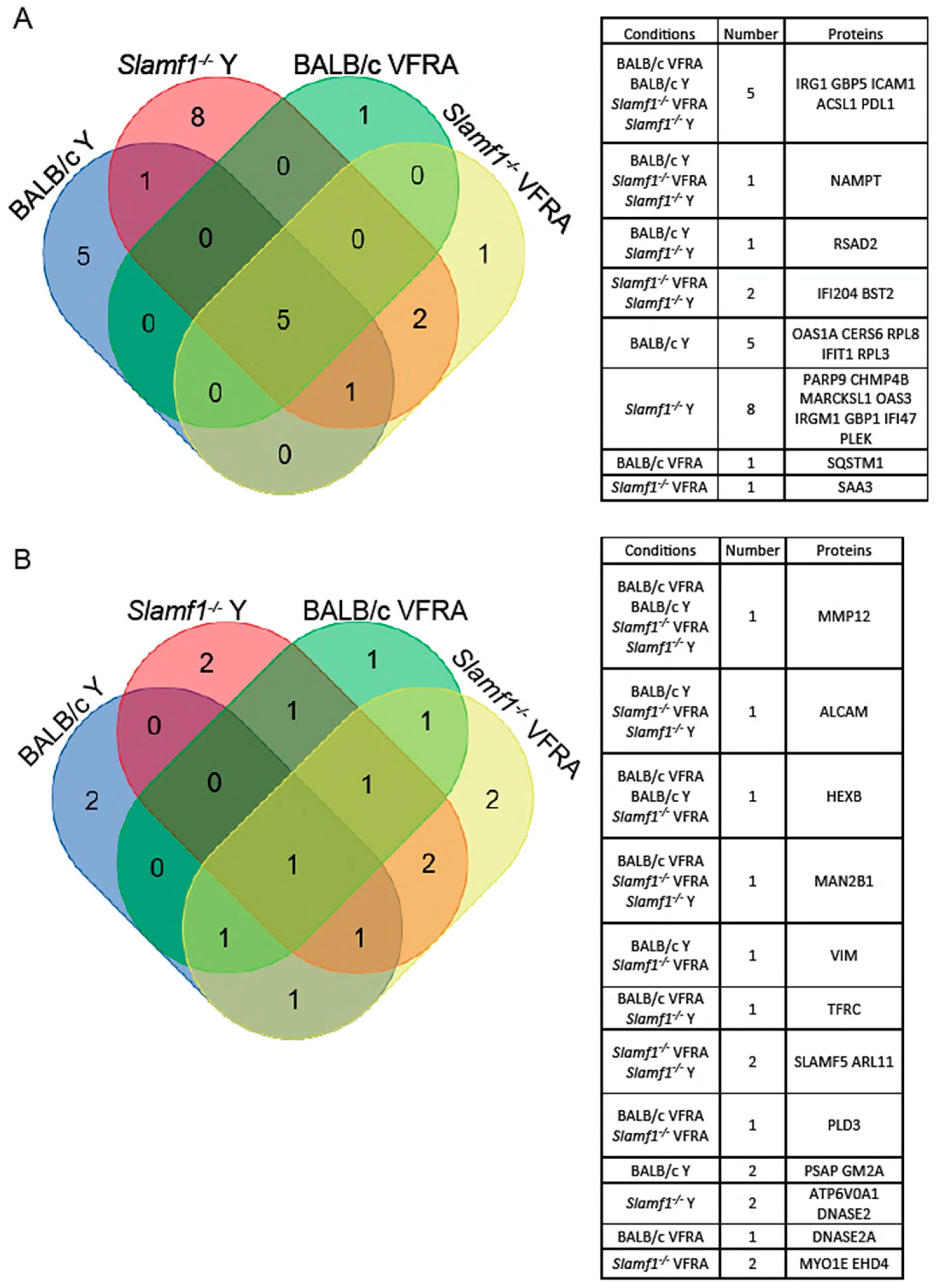
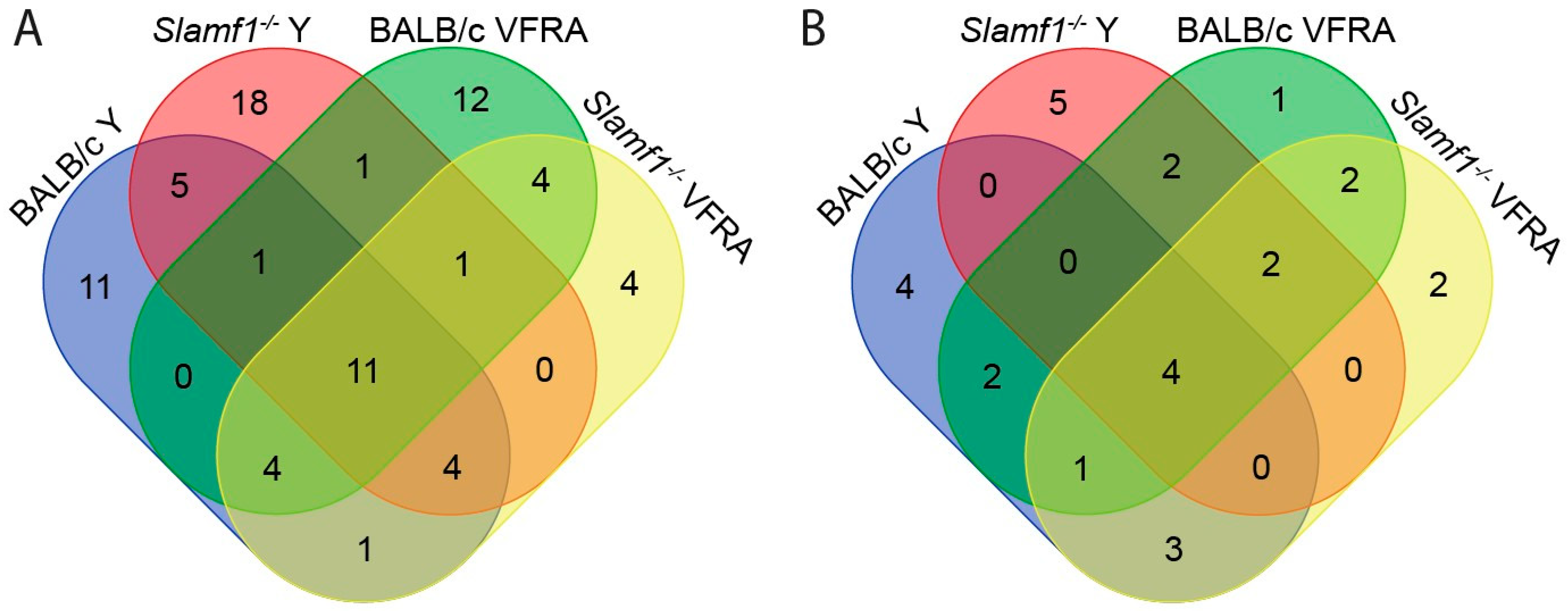
2.3. Comparison between the Experimental Conditions and Protein–Protein Interaction Networks
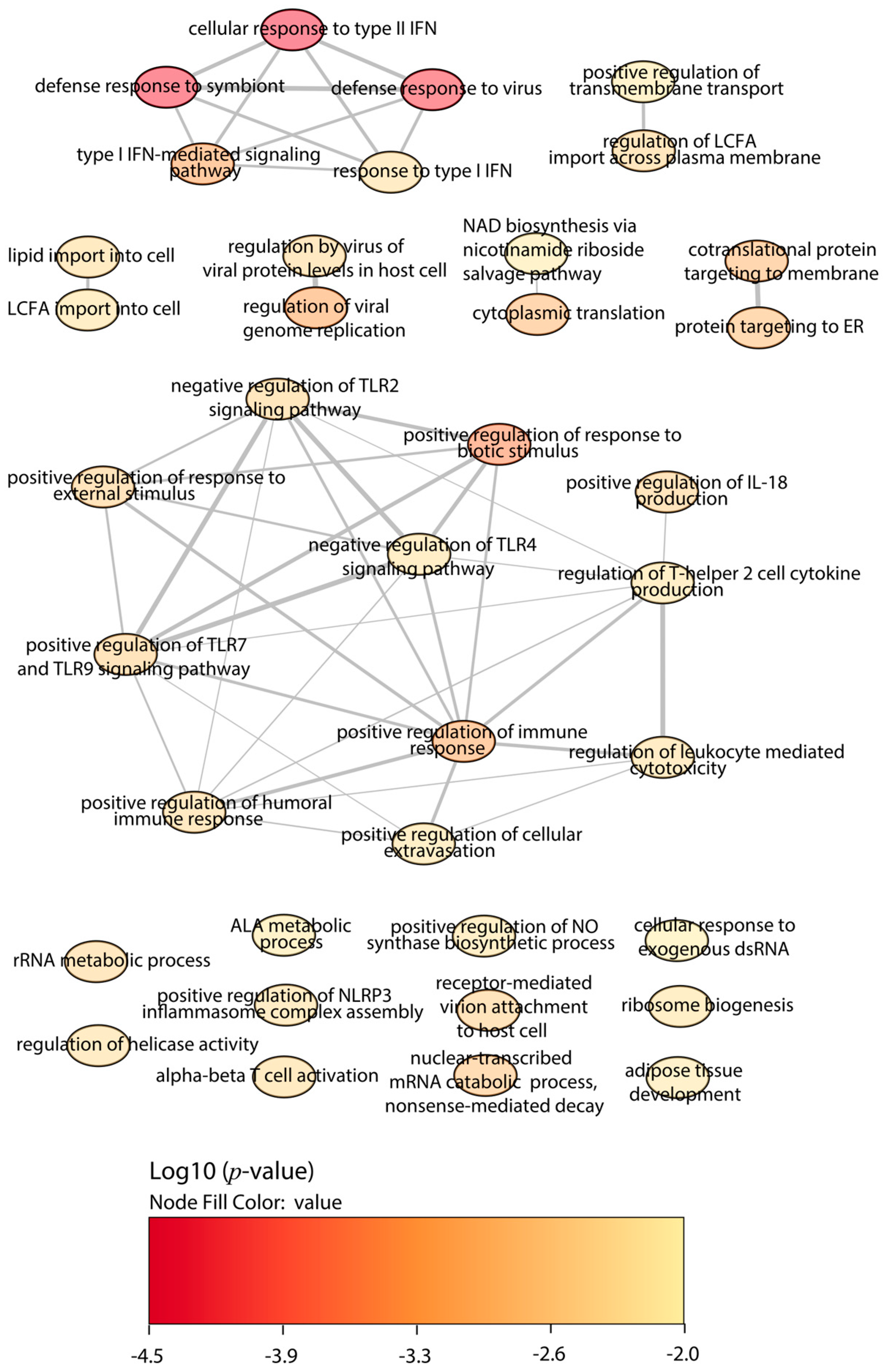
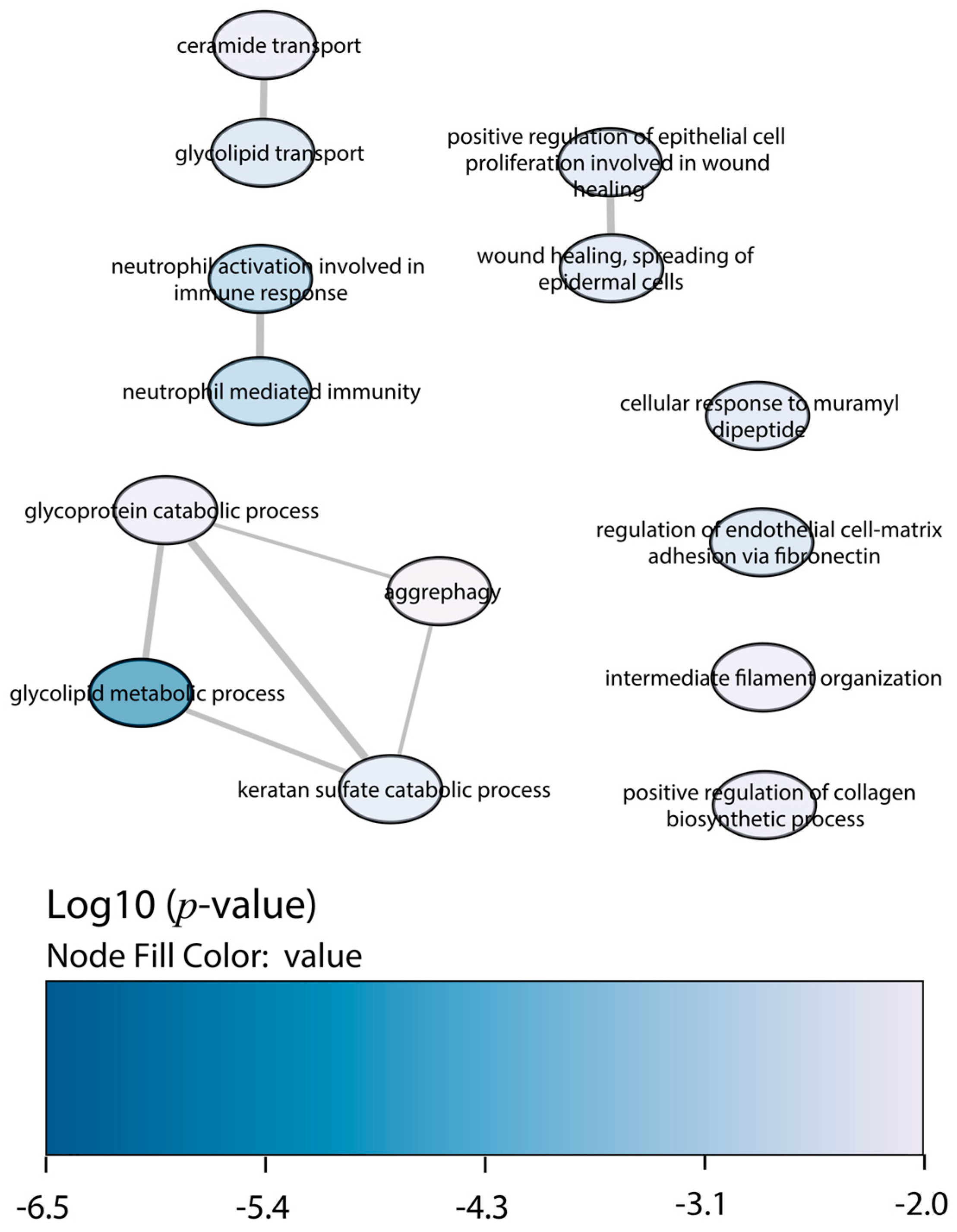
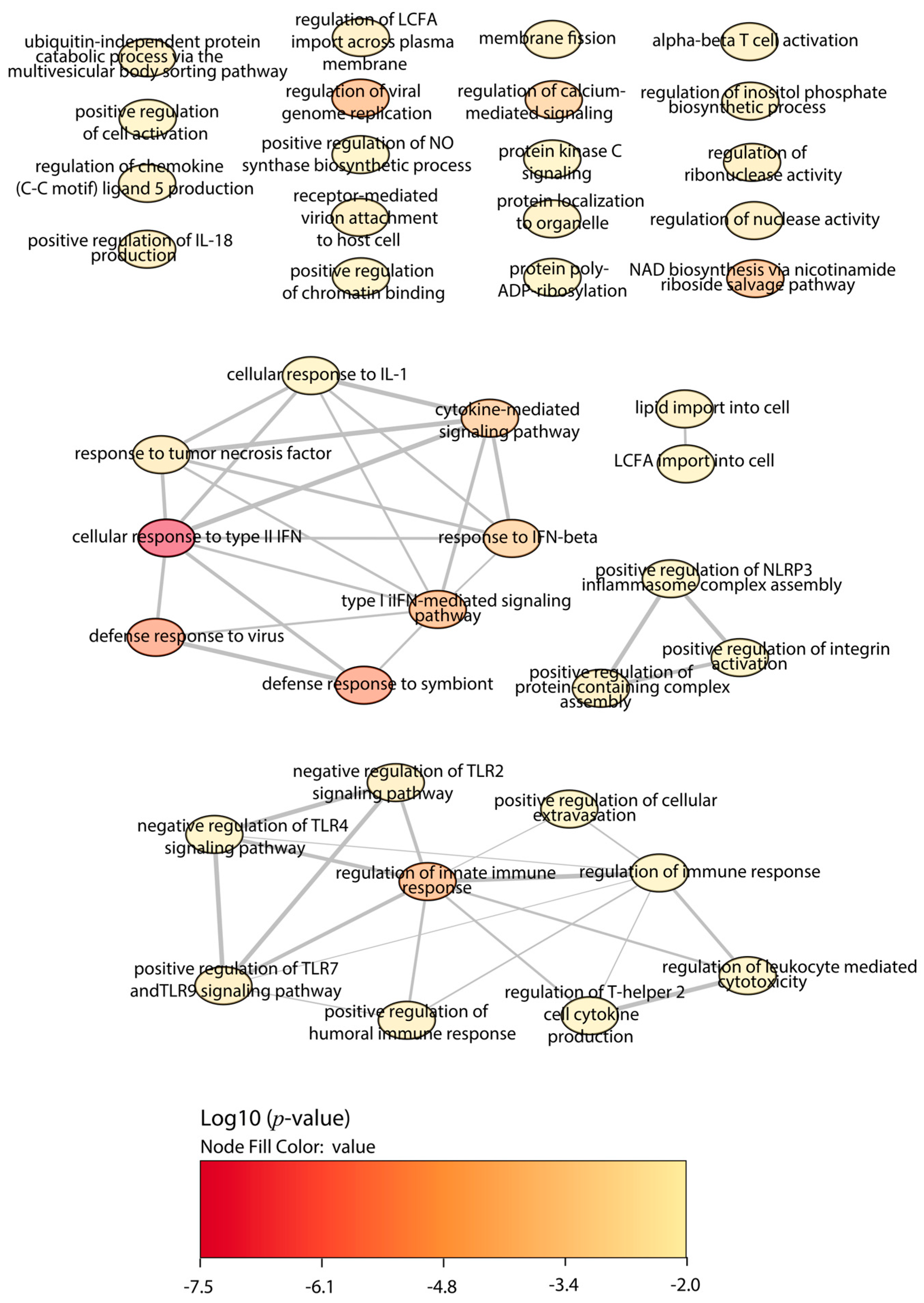
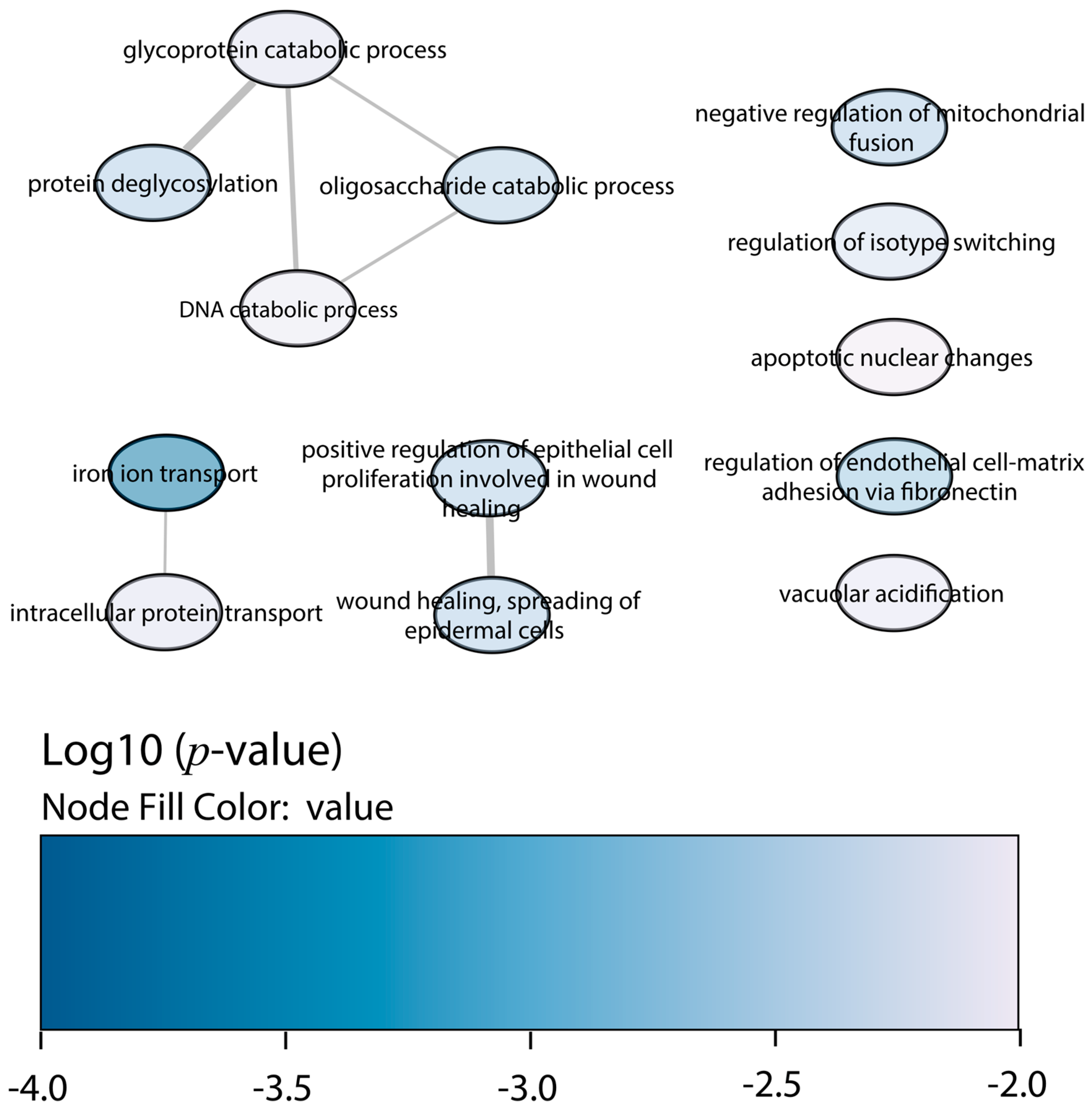
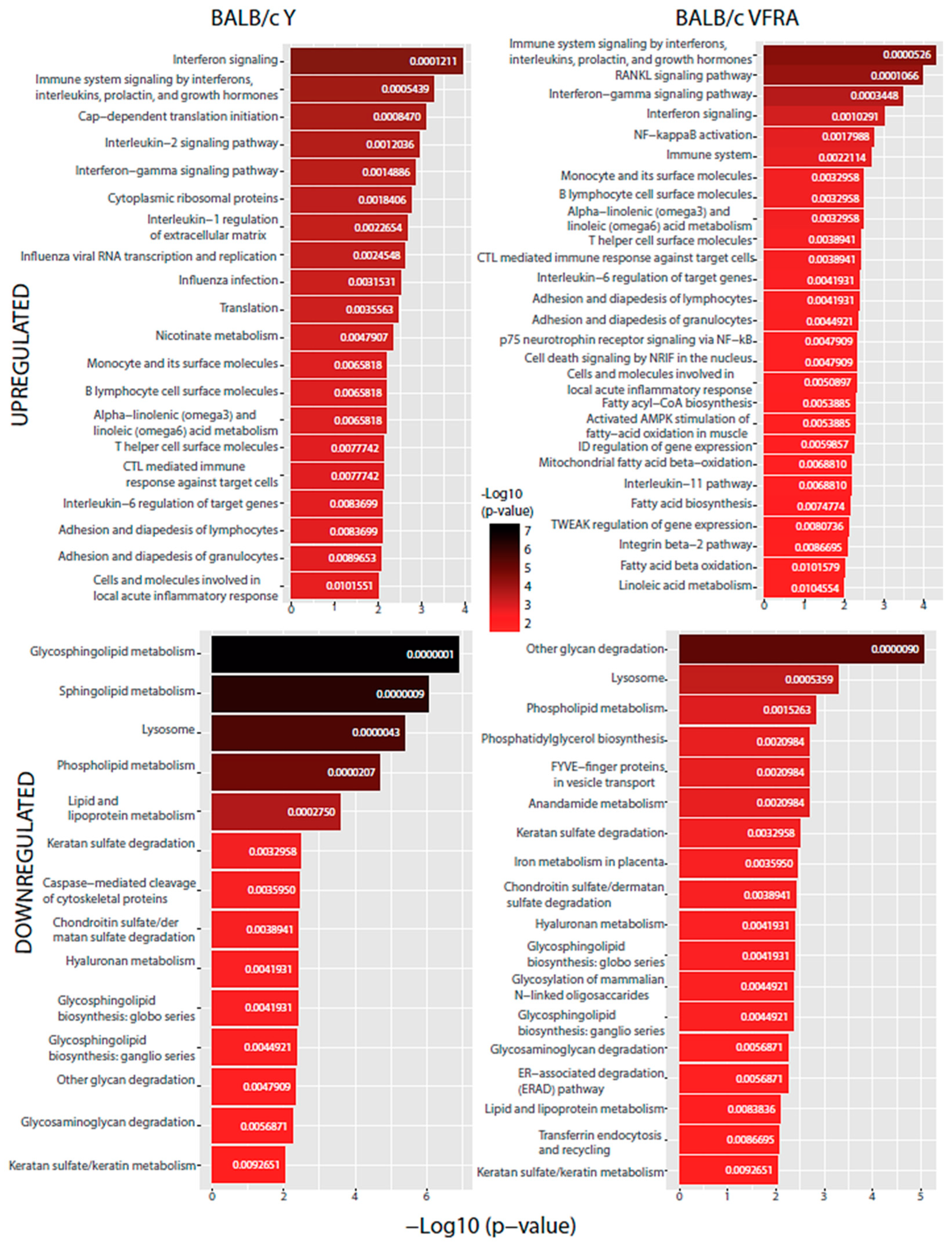
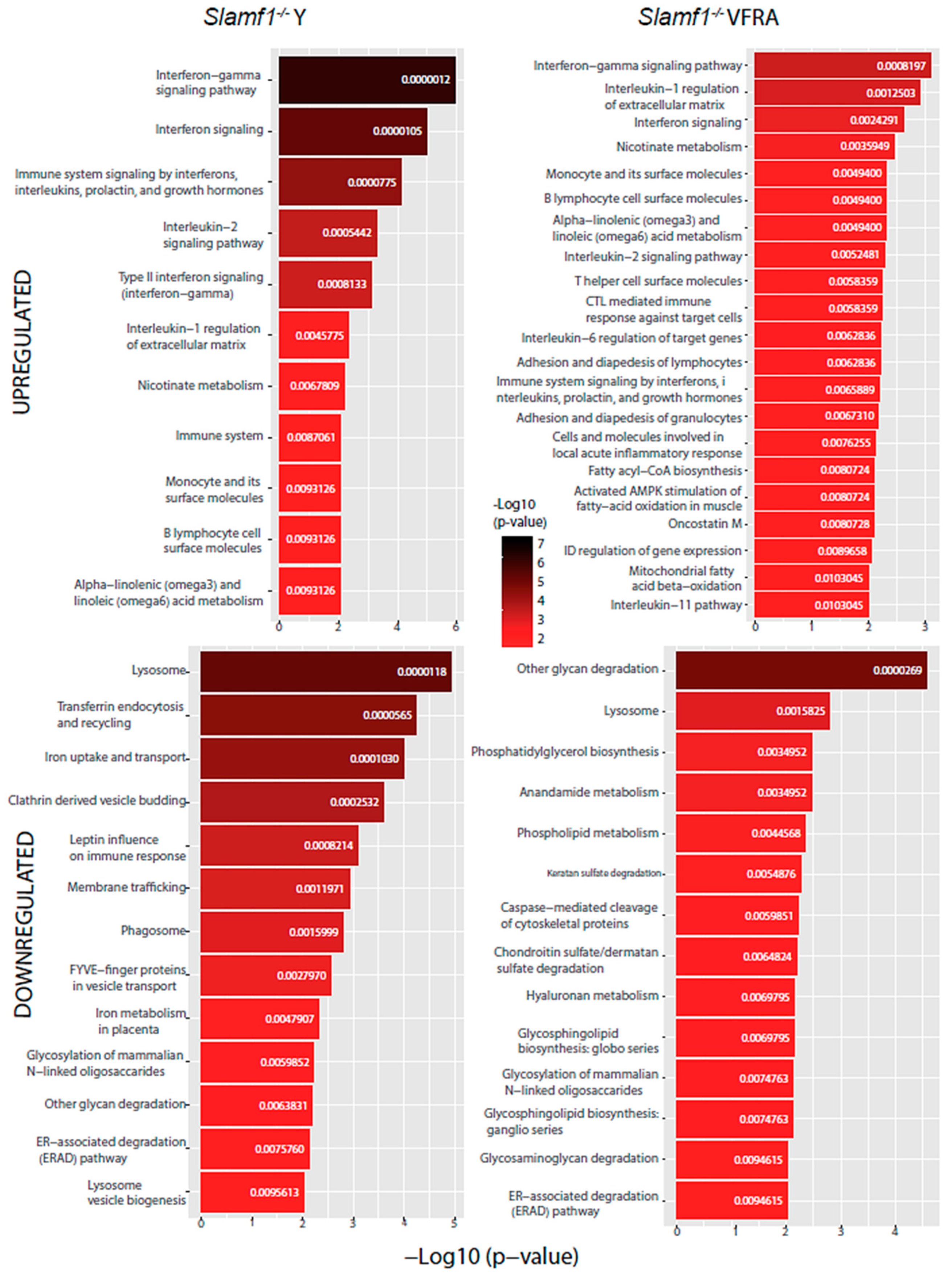
2.4. Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Parasite Culture
4.2. Mice and Ethic Statement
4.3. Isolation of Peritoneal Macrophages and In Vitro Infection
4.4. Protein Extraction and Digestion for the Proteomic Analysis
4.5. TMT Labeling and Fractionation
4.6. Analysis by RP-LC-MS/MS
4.7. Quantitative Proteomic Data Analysis and Representation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guhl, F.; Jaramillo, C.; Vallejo, G.A.; Yockteng, R.; Cárdenas-Arroyo, F.; Fornaciari, G.; Arriaza, B.; Aufderheide, A.C. Isolation of Trypanosoma Cruzi DNA in 4000-Year-Old Mummified Human Tissue from Northern Chile. Am. J. Phys. Anthropol. 1999, 108, 401–407. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease (accessed on 6 May 2024).
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marcondes de Rezende, J. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Schmunis, G.A.; Yadon, Z.E. Chagas Disease: A Latin American Health Problem Becoming a World Health Problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.; Bottazzi, M.E.; Strub-Wourgaft, N.; Sosa-Estani, S.; Torrico, F.; Pajín, L.; Abril, M.; Sancho, J. A New Patient Registry for Chagas Disease. PLoS Negl. Trop. Dis. 2020, 14, e0008418. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L. Immune System Recognition of Trypanosoma Cruzi. Curr. Opin. Immunol. 2007, 19, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, M.; Garg, N.J. P47phox-/- Mice Are Compromised in Expansion and Activation of CD8+ T Cells and Susceptible to Trypanosoma Cruzi Infection. PLoS Pathog. 2014, 10, e1004516. [Google Scholar] [CrossRef]
- Muñoz-Fernández, M.A.; Fernández, M.A.; Fresno, M. Synergism between Tumor Necrosis Factor-Alpha and Interferon-Gamma on Macrophage Activation for the Killing of Intracellular Trypanosoma Cruzi through a Nitric Oxide-Dependent Mechanism. Eur. J. Immunol. 1992, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.M.; Oliveira, A.C.; Bellio, M. The Immune Response to Trypanosoma Cruzi: Role of Toll-Like Receptors and Perspectives for Vaccine Development. J. Parasitol. Res. 2012, 2012, 507874. [Google Scholar] [CrossRef]
- Sintes, J.; Engel, P. SLAM (CD150) Is a Multitasking Immunoreceptor: From Cosignalling to Bacterial Recognition. Immunol. Cell Biol. 2011, 89, 161–163. [Google Scholar] [CrossRef]
- van Driel, B.J.; Liao, G.; Engel, P.; Terhorst, C. Responses to Microbial Challenges by SLAMF Receptors. Front. Immunol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.; Maganto-Garcia, E.; Punzón, C.; Carrión, J.; Terhorst, C.; Fresno, M. The Receptor Slamf1 on the Surface of Myeloid Lineage Cells Controls Susceptibility to Infection by Trypanosoma Cruzi. PLoS Pathog. 2012, 8, e1002799. [Google Scholar] [CrossRef]
- Poveda, C.; Herreros-Cabello, A.; Callejas-Hernández, F.; Osuna-Pérez, J.; Maza, M.C.; Chillón-Marinas, C.; Calderón, J.; Stamatakis, K.; Fresno, M.; Gironès, N. Interaction of Signaling Lymphocytic Activation Molecule Family 1 (SLAMF1) Receptor with Trypanosoma Cruzi Is Strain-Dependent and Affects NADPH Oxidase Expression and Activity. PLoS Negl. Trop. Dis. 2020, 14, e0008608. [Google Scholar] [CrossRef]
- Rodriguez, H.O.; Guerrero, N.A.; Fortes, A.; Santi-Rocca, J.; Gironès, N.; Fresno, M. Trypanosoma Cruzi Strains Cause Different Myocarditis Patterns in Infected Mice. Acta Trop. 2014, 139, 57–66. [Google Scholar] [CrossRef]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-Responsive Gene 1 Protein Links Metabolism to Immunity by Catalyzing Itaconic Acid Production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [PubMed]
- Tallam, A.; Perumal, T.M.; Antony, P.M.; Jäger, C.; Fritz, J.V.; Vallar, L.; Balling, R.; Del Sol, A.; Michelucci, A. Gene Regulatory Network Inference of Immunoresponsive Gene 1 (IRG1) Identifies Interferon Regulatory Factor 1 (IRF1) as Its Transcriptional Regulator in Mammalian Macrophages. PLoS ONE 2016, 11, e0149050. [Google Scholar] [CrossRef]
- Gironès, N.; Carbajosa, S.; Guerrero, N.A.; Poveda, C.; Chillón-Marinas, C.; Fresno, M. Global Metabolomic Profiling of Acute Myocarditis Caused by Trypanosoma Cruzi Infection. PLoS Negl. Trop. Dis. 2014, 8, e3337. [Google Scholar] [CrossRef]
- Dutra, W.O.; Menezes, C.a.S.; Magalhães, L.M.D.; Gollob, K.J. Immunoregulatory Networks in Human Chagas Disease. Parasite Immunol. 2014, 36, 377–387. [Google Scholar] [CrossRef]
- Ferreira, L.R.P.; Frade, A.F.; Baron, M.A.; Navarro, I.C.; Kalil, J.; Chevillard, C.; Cunha-Neto, E. Interferon-γ and Other Inflammatory Mediators in Cardiomyocyte Signaling during Chagas Disease Cardiomyopathy. World J. Cardiol. 2014, 6, 782–790. [Google Scholar] [CrossRef]
- Kirkby, M.; Enosi Tuipulotu, D.; Feng, S.; Lo Pilato, J.; Man, S.M. Guanylate-Binding Proteins: Mechanisms of Pattern Recognition and Antimicrobial Functions. Trends Biochem. Sci. 2023, 48, 883–893. [Google Scholar] [CrossRef]
- Kutsch, M.; Coers, J. Human Guanylate Binding Proteins: Nanomachines Orchestrating Host Defense. FEBS J. 2021, 288, 5826–5849. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Calderon, T.M.; Berman, J.W.; Braunstein, V.L.; Weiss, L.M.; Wittner, M.; Tanowitz, H.B. Infection of Endothelial Cells with Trypanosoma Cruzi Activates NF-kappaB and Induces Vascular Adhesion Molecule Expression. Infect. Immun. 1999, 67, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Laucella, S.; Salcedo, R.; Castaños-Velez, E.; Riarte, A.; De Titto, E.H.; Patarroyo, M.; Orn, A.; Rottenberg, M.E. Increased Expression and Secretion of ICAM-1 during Experimental Infection with Trypanosoma Cruzi. Parasite Immunol. 1996, 18, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Michailowsky, V.; Celes, M.R.N.; Marino, A.P.; Silva, A.A.; Vieira, L.Q.; Rossi, M.A.; Gazzinelli, R.T.; Lannes-Vieira, J.; Silva, J.S. Intercellular Adhesion Molecule 1 Deficiency Leads to Impaired Recruitment of T Lymphocytes and Enhanced Host Susceptibility to Infection with Trypanosoma Cruzi. J. Immunol. 2004, 173, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, R.; Du, R.; Song, C.; Xie, F.; Ren, L.; Li, J. Platelet-Derived Microvesicles Mediate Cardiomyocyte Ferroptosis by Transferring ACSL1 During Acute Myocardial Infarction. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, Y.; Wang, J.; Gu, J. Defects in Macrophage Reprogramming in Cancer Therapy: The Negative Impact of PD-L1/PD-1. Front. Immunol. 2021, 12, 690869. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, J.; Wherry, E.J. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity 2016, 44, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Gigley, J.P.; Bhadra, R.; Moretto, M.M.; Khan, I.A. T Cell Exhaustion in Protozoan Disease. Trends Parasitol. 2012, 28, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, F.R.S.; Mariano, F.S.; Oliveira, C.J.F.; Pavanelli, W.R.; Guedes, P.M.M.; Silva, G.K.; Campanelli, A.P.; Milanezi, C.M.; Azuma, M.; Honjo, T.; et al. Regulation of Trypanosoma Cruzi-Induced Myocarditis by Programmed Death Cell Receptor 1. Infect. Immun. 2011, 79, 1873–1881. [Google Scholar] [CrossRef]
- Arana, Y.; Gálvez, R.I.; Jacobs, T. Role of the PD-1/PD-L1 Pathway in Experimental Trypanosoma Cruzi Infection and Potential Therapeutic Options. Front. Immunol. 2022, 13, 866120. [Google Scholar] [CrossRef]
- Fonseca, R.; Salgado, R.M.; Borges da Silva, H.; do Nascimento, R.S.; D’Império-Lima, M.R.; Alvarez, J.M. Programmed Cell Death Protein 1-PDL1 Interaction Prevents Heart Damage in Chronic Trypanosoma Cruzi Infection. Front. Immunol. 2018, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Dulgerian, L.R.; Garrido, V.V.; Stempin, C.C.; Cerbán, F.M. Programmed Death Ligand 2 Regulates Arginase Induction and Modifies Trypanosoma Cruzi Survival in Macrophages during Murine Experimental Infection. Immunology 2011, 133, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Garg, N.J. Trypanosoma Cruzi Induces the PARP1/AP-1 Pathway for Upregulation of Metalloproteinases and Transforming Growth Factor β in Macrophages: Role in Cardiac Fibroblast Differentiation and Fibrosis in Chagas Disease. mBio 2020, 11, e01853-20. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; Rivera Gonzalez, O.J.; Kaminski, A.R.; Moore, E.T.; Lindsey, M.L. Matrix Metalloproteinase-12 as an Endogenous Resolution Promoting Factor Following Myocardial Infarction. Pharmacol. Res. 2018, 137, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; He, J.; Zhou, X.; Pan, H.; He, F.; Du, A.; Yu, B.; Jiang, N.; Li, X.; Yuan, K.; et al. Discovering Common Pathogenetic Processes between COVID-19 and Tuberculosis by Bioinformatics and System Biology Approach. Front. Cell. Infect. Microbiol. 2023, 13, 1280223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Zhang, R.; Liu, L.-L.; Li, H.-J.; Zhu, H. Expression Analysis and Antiviral Activity of Koi Carp (Cyprinus Carpio) Viperin against Carp Edema Virus (CEV). Fish Shellfish Immunol. 2024, 148, 109519. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.L.; Huang, D.; Blane, T.R.; Thinnes, T.C.; Murakami, Y.; Fukui, R.; Miyake, K.; Nemazee, D. Cleavage of DNA and RNA by PLD3 and PLD4 Limits Autoinflammatory Triggering by Multiple Sensors. Nat. Commun. 2021, 12, 5874. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.L.; Huang, D.; Huber, C.; Mårtensson, A.; Tardif, V.; Skog, P.D.; Blane, T.R.; Thinnes, T.C.; Osborn, K.; Chong, H.S.; et al. PLD3 and PLD4 Are Single-Stranded Acid Exonucleases That Regulate Endosomal Nucleic-Acid Sensing. Nat. Immunol. 2018, 19, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Gil-Jaramillo, N.; Rocha, A.P.; Raiol, T.; Motta, F.N.; Favali, C.; Brigido, M.M.; Bastos, I.M.D.; Santana, J.M. The First Contact of Human Dendritic Cells With Trypanosoma Cruzi Reveals Response to Virus as an Unexplored Central Pathway. Front. Immunol. 2021, 12, 638020. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Wang, S.; Du, J. Multi-Functional BST2/Tetherin against HIV-1, Other Viruses and LINE-1. Front. Cell. Infect. Microbiol. 2022, 12, 979091. [Google Scholar] [CrossRef]
- Sintes, J.; Romero, X.; de Salort, J.; Terhorst, C.; Engel, P. Mouse CD84 Is a Pan-Leukocyte Cell-Surface Molecule That Modulates LPS-Induced Cytokine Secretion by Macrophages. J. Leukoc. Biol. 2010, 88, 687–697. [Google Scholar] [CrossRef]
- Arya, S.B.; Kumar, G.; Kaur, H.; Kaur, A.; Tuli, A. ARL11 Regulates Lipopolysaccharide-Stimulated Macrophage Activation by Promoting Mitogen-Activated Protein Kinase (MAPK) Signaling. J. Biol. Chem. 2018, 293, 9892–9909. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Costa-Silva, B.; Barral, D.C.; Pojo, M. Unraveling the Relevance of ARL GTPases in Cutaneous Melanoma Prognosis through Integrated Bioinformatics Analysis. Int. J. Mol. Sci. 2021, 22, 9260. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Goettsch, C.; Sharma, A.; Ricchiuto, P.; Goh, W.W.B.; Halu, A.; Yamada, I.; Yoshida, H.; Hara, T.; Wei, M.; et al. PARP9 and PARP14 Cross-Regulate Macrophage Activation via STAT1 ADP-Ribosylation. Nat. Commun. 2016, 7, 12849. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, S.; Ahmed, M.; Rosa, B.A.; Boothby, M.; Cho, S.H.; Rangel-Moreno, J.; Mbandi, S.K.; Schreiber, V.; Gupta, A.; Zuniga, J.; et al. Poly(ADP-Ribose) Polymerase 9 Mediates Early Protection against Mycobacterium Tuberculosis Infection by Regulating Type I IFN Production. J. Clin. Investig. 2023, 133, e158630. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lu, S.; Garces, J.; Jin, T.; Li, J. Protein Kinase C-Regulated Dynamitin-Macrophage-Enriched Myristoylated Alanine-Rice C Kinase Substrate Interaction Is Involved in Macrophage Cell Spreading. J. Biol. Chem. 2000, 275, 23948–23956. [Google Scholar] [CrossRef] [PubMed]
- Hsin, I.-L.; Sheu, G.-T.; Jan, M.-S.; Sun, H.-L.; Wu, T.-C.; Chiu, L.-Y.; Lue, K.-H.; Ko, J.-L. Inhibition of Lysosome Degradation on Autophagosome Formation and Responses to GMI, an Immunomodulatory Protein from Ganoderma Microsporum. Br. J. Pharmacol. 2012, 167, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Maric-Biresev, J.; Hunn, J.P.; Krut, O.; Helms, J.B.; Martens, S.; Howard, J.C. Loss of the Interferon-γ-Inducible Regulatory Immunity-Related GTPase (IRG), Irgm1, Causes Activation of Effector IRG Proteins on Lysosomes, Damaging Lysosomal Function and Predicting the Dramatic Susceptibility of Irgm1-Deficient Mice to Infection. BMC Biol. 2016, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Buijze, H.; Brinkmann, V.; Hurwitz, R.; Dorhoi, A.; Kaufmann, S.H.E.; Pei, G. Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes. Int. J. Mol. Sci. 2023, 24, 9701. [Google Scholar] [CrossRef] [PubMed]
- Collazo, C.M.; Yap, G.S.; Sempowski, G.D.; Lusby, K.C.; Tessarollo, L.; Vande Woude, G.F.; Sher, A.; Taylor, G.A. Inactivation of LRG-47 and IRG-47 Reveals a Family of Interferon Gamma-Inducible Genes with Essential, Pathogen-Specific Roles in Resistance to Infection. J. Exp. Med. 2001, 194, 181–188. [Google Scholar] [CrossRef]
- Macaluso, G.; Grippi, F.; Di Bella, S.; Blanda, V.; Gucciardi, F.; Torina, A.; Guercio, A.; Cannella, V. A Review on the Immunological Response against Trypanosoma Cruzi. Pathogens 2023, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.V.H.; Zamboni, D.S. Inflammasome Activation in Response to Intracellular Protozoan Parasites. Trends Parasitol. 2020, 36, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Misheva, M.; Johnson, J.; McCullagh, J. Role of Oxylipins in the Inflammatory-Related Diseases NAFLD, Obesity, and Type 2 Diabetes. Metabolites 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; de Waard, A.A.; Wuhrer, M.; Spaapen, R.M. The Role of Glycosphingolipids in Immune Cell Functions. Front. Immunol. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol Inhibits Toll-Like Receptor-Mediated Inflammation by Danger-Associated Molecular Patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Nisimura, L.M.; Coelho, L.L.; de Melo, T.G.; Vieira, P.d.C.; Victorino, P.H.; Garzoni, L.R.; Spray, D.C.; Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; et al. Trypanosoma Cruzi Promotes Transcriptomic Remodeling of the JAK/STAT Signaling and Cell Cycle Pathways in Myoblasts. Front. Cell. Infect. Microbiol. 2020, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Caetano, B.C.; Carmo, B.B.; Melo, M.B.; Cerny, A.; dos Santos, S.L.; Bartholomeu, D.C.; Golenbock, D.T.; Gazzinelli, R.T. Requirement of UNC93B1 Reveals a Critical Role for TLR7 in Host Resistance to Primary Infection with Trypanosoma Cruzi. J. Immunol. 2011, 187, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Fresno, M.; Gironès, N. Comparative Proteomic Analysis of Trypomastigotes from Trypanosoma Cruzi Strains with Different Pathogenicity. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 76, 104041. [Google Scholar] [CrossRef]
- Amato Neto, V. Origin of the “Y Strain” of Trypanosoma Cruzi. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 171. [Google Scholar] [CrossRef]
- Sanchiz, Á.; Morato, E.; Rastrojo, A.; Camacho, E.; González-de la Fuente, S.; Marina, A.; Aguado, B.; Requena, J.M. The Experimental Proteome of Leishmania Infantum Promastigote and Its Usefulness for Improving Gene Annotations. Genes 2020, 11, 1036. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Suárez, H.; Andreu, Z.; Mazzeo, C.; Toribio, V.; Pérez-Rivera, A.E.; López-Martín, S.; García-Silva, S.; Hurtado, B.; Morato, E.; Peláez, L.; et al. CD9 Inhibition Reveals a Functional Connection of Extracellular Vesicle Secretion with Mitophagy in Melanoma Cells. J. Extracell. Vesicles 2021, 10, e12082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiao, W.; Su, Z.; Cheng, J.; Zheng, C.; Zhang, Z.; Wang, Y.; Wang, L.; Xu, B.; Li, S.; et al. Hippocampal Proteomic Alteration in Triple Transgenic Mouse Model of Alzheimer’s Disease and Implication of PINK 1 Regulation in Donepezil Treatment. J. Proteome Res. 2019, 18, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Gong, P.; Zhao, W.; Zhang, J.; Wu, X.; Xin, C.; Xiong, Z.; Li, Z.; Wu, X.; Wan, Q.; et al. Quantitative iTRAQ-Based Proteomic Analysis of Piperine Protected Cerebral Ischemia/Reperfusion Injury in Rat Brain. Neurochem. Int. 2019, 124, 51–61. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]

| BALB/c Y | BALB/c VFRA | Slamf1-/- Y | Slamf1-/- VFRA | |
|---|---|---|---|---|
| Interferons | ↑ IFN type I-II responses | ↑ IFN type I-II responses | ↑ IFN type I-II responses | ↑ IFN type I-II responses |
| TLRs | ↓ TLR2/TLR4 signaling | ↓ TLR2/TLR4 signaling | ↓ TLR2/TLR4 signaling | ↓ TLR2/TLR4 signaling |
| ↑ TLR7/TLR9 signaling | ↑TLR7/TLR9 signaling | |||
| Interleukins | ↑ IL-18 production | ↑ IL-18 production | ↑ IL-18 production | ↑ IL-18 production |
| ↑ IL-2 signaling | ↑ IL-2 signaling | ↑ IL-2 signaling | ||
| ↑ IL-11 signaling | ↑ IL-11 signaling | |||
| Lipid metabolism | ↑ LCFA import | ↑ LCFA import | ↑ LCFA import | ↑ LCFA import |
| ↑ ALA metabolism | ↑ ALA metabolism | ↑ ALA metabolism | ↑ ALA metabolism | |
| ↑ LA metabolism | ↑ LA metabolism | ↑ LA metabolism | ↑ LA metabolism | |
| ↓ GSL biosynthesis | ↓ GSL biosynthesis | ↓ GSL biosynthesis | ||
| ↓ Cer and GL transport | ↓ GSL catabolism | ↓ GSL catabolism | ||
| ↓ AEA metabolism | ↓ AEA metabolism | |||
| ↓ PG biosynthesis | ↓ PG biosynthesis | |||
| Carbohydrate metabolism | ↓ GP catabolism | ↓ GP catabolism | ↓ GP catabolism | ↓ GP catabolism |
| ↓ GAG degradation | ↓ GAG degradation | ↓ GAG degradation | ||
| ↓ OS catabolism | ↓ OS catabolism | ↓ OS catabolism | ||
| ↓ protein deglycosylation | ↓ protein deglycosylation | ↓ protein deglycosylation | ||
| Effects on other immune cells | ↑ αβ T cell activation | ↑ αβ T cell activation | ||
| ↓ neutrophil activation | ↓ neutrophil activation | |||
| Phagocytic processes | ↓ vacuolar acidification | |||
| ↓ clathrin-derived vesicle budding | ||||
| ↓ lysosome biogenesis | ||||
| ↓ phagosome processes | ||||
| Other intracellular pathways | ↑ NLRP3 inflammasome | ↑ NLRP3 inflammasome | ↑ NLRP3 inflammasome | ↑ NLRP3 inflammasome |
| ↓ ERAD pathway | ↓ ERAD pathway | ↓ ERAD pathway | ||
| ↑ protein translation | ↑ pexophagy/aggrephagy | ↑ integrin activation | ||
| ↓ aggrephagy | ↑ β2 integrin pathway | ↑ PKC signaling | ||
| ↑ RANKL pathway | ↓ iron uptake & transport | |||
| ↑ TWEAK regulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herreros-Cabello, A.; del Moral-Salmoral, J.; Morato, E.; Marina, A.; Barrocal, B.; Fresno, M.; Gironès, N. Quantitative Proteomic Analysis of Macrophages Infected with Trypanosoma cruzi Reveals Different Responses Dependent on the SLAMF1 Receptor and the Parasite Strain. Int. J. Mol. Sci. 2024, 25, 7493. https://doi.org/10.3390/ijms25137493
Herreros-Cabello A, del Moral-Salmoral J, Morato E, Marina A, Barrocal B, Fresno M, Gironès N. Quantitative Proteomic Analysis of Macrophages Infected with Trypanosoma cruzi Reveals Different Responses Dependent on the SLAMF1 Receptor and the Parasite Strain. International Journal of Molecular Sciences. 2024; 25(13):7493. https://doi.org/10.3390/ijms25137493
Chicago/Turabian StyleHerreros-Cabello, Alfonso, Javier del Moral-Salmoral, Esperanza Morato, Anabel Marina, Beatriz Barrocal, Manuel Fresno, and Núria Gironès. 2024. "Quantitative Proteomic Analysis of Macrophages Infected with Trypanosoma cruzi Reveals Different Responses Dependent on the SLAMF1 Receptor and the Parasite Strain" International Journal of Molecular Sciences 25, no. 13: 7493. https://doi.org/10.3390/ijms25137493






