TLR Agonists Modify NK Cell Activation and Increase Its Cytotoxicity in Acute Lymphoblastic Leukemia
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Patients
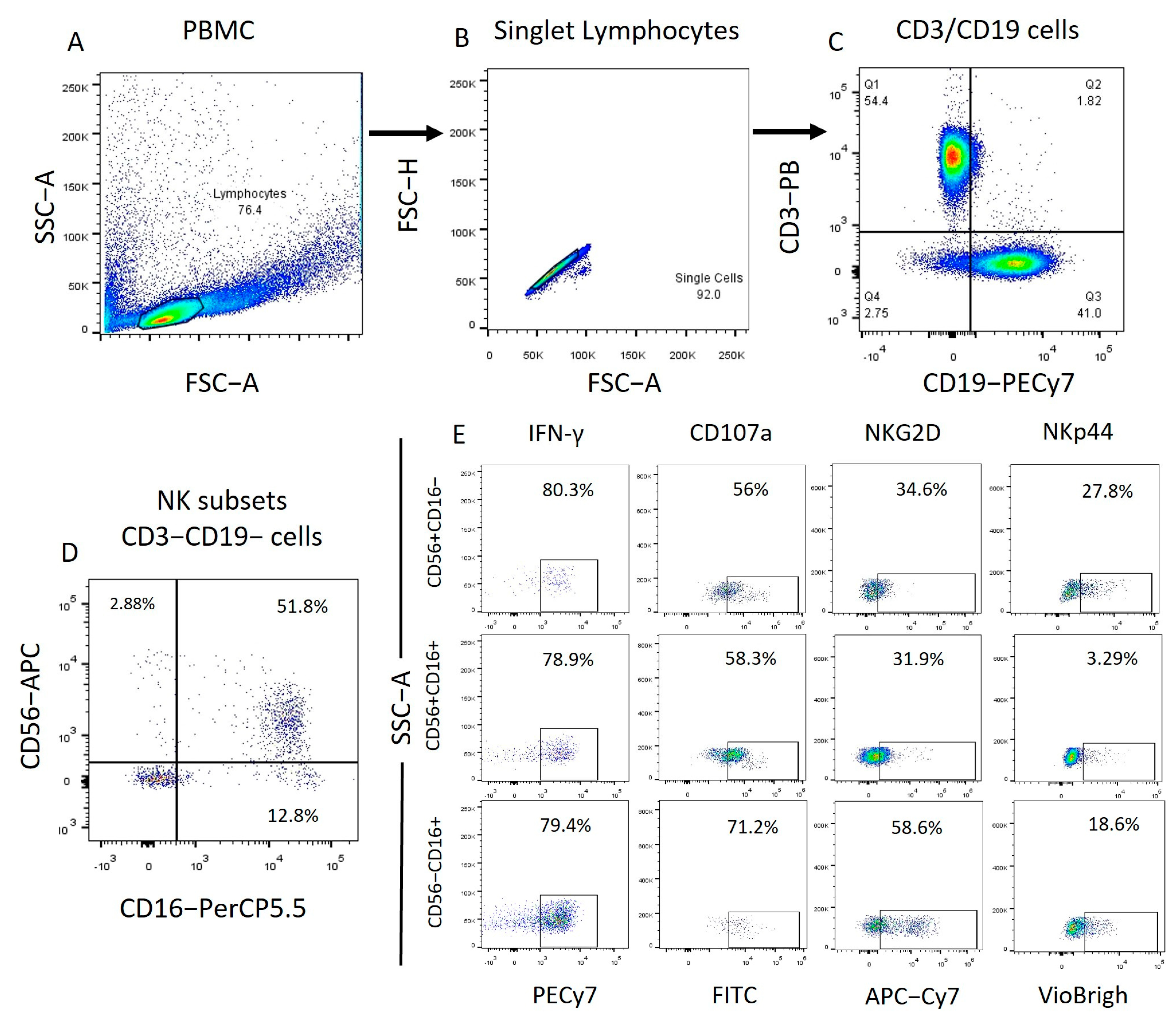
2.2. Identification of the Different NK Cell Subpopulations in Patients with ALL and IFN-γ, CD107a, NKp44, and NKG2D Expression
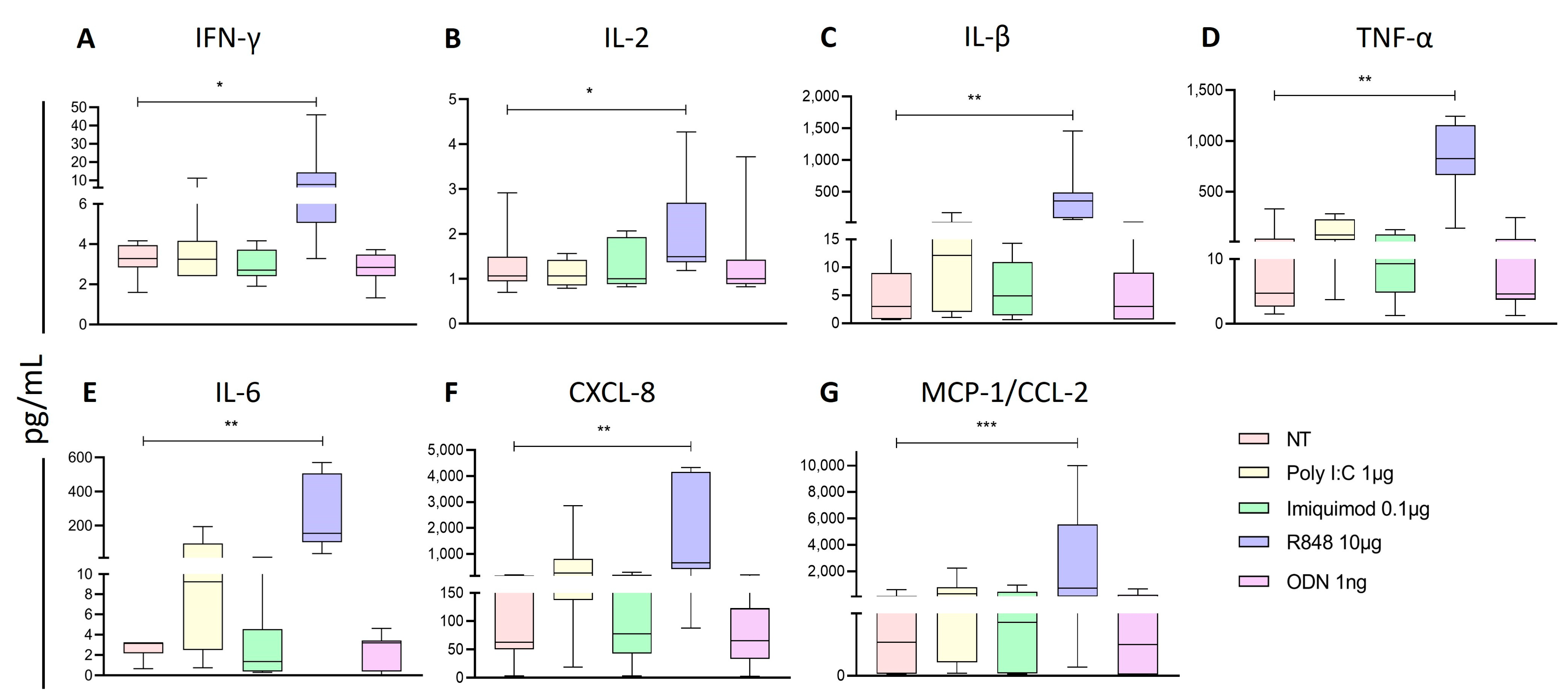
2.3. Poly I:C and R848 Increase the Activity of NK Subpopulations within the PBMC
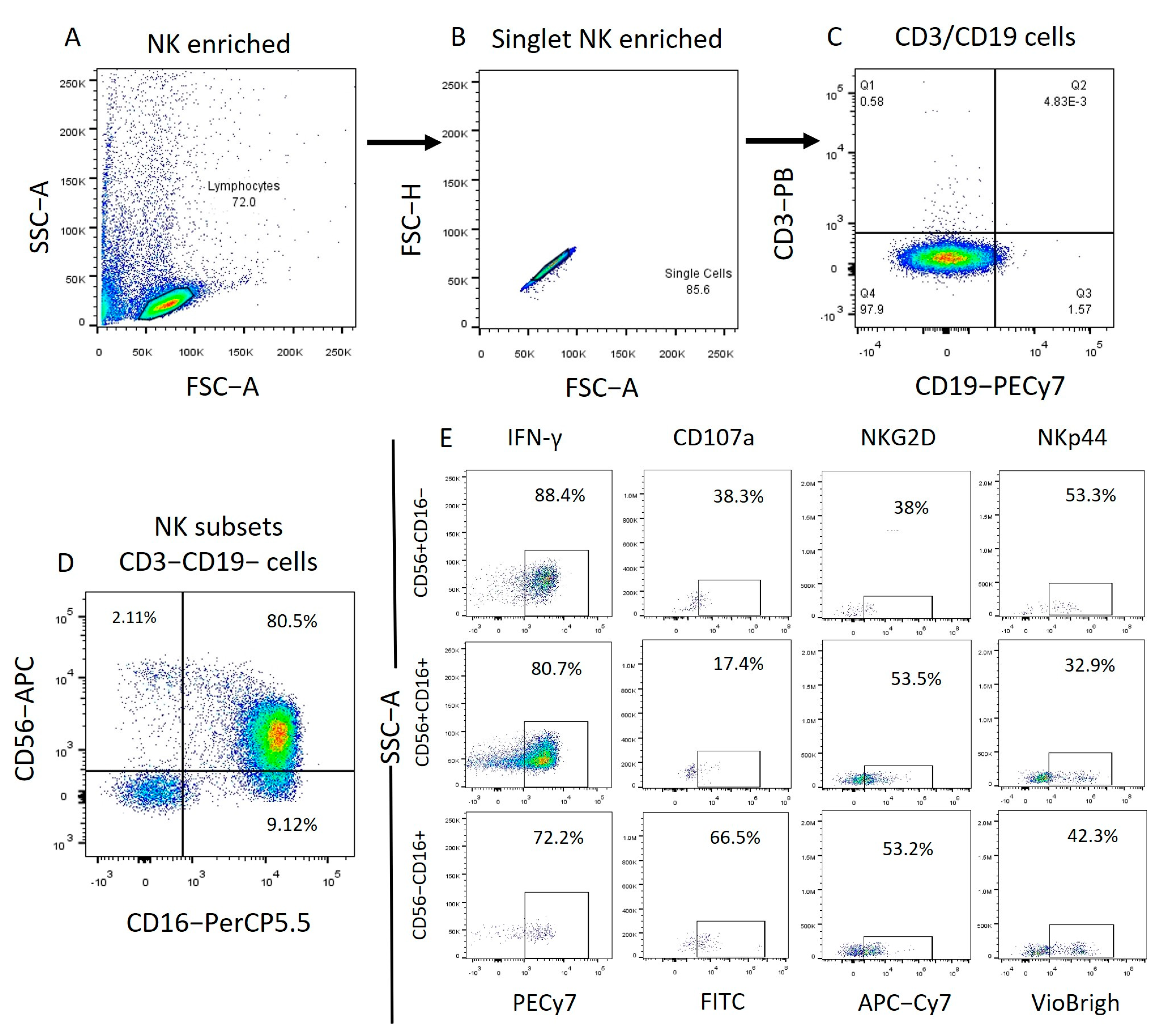
2.4. Imiquimod and ODN2006 Increase the Function of Enriched NK Cells Subpopulations
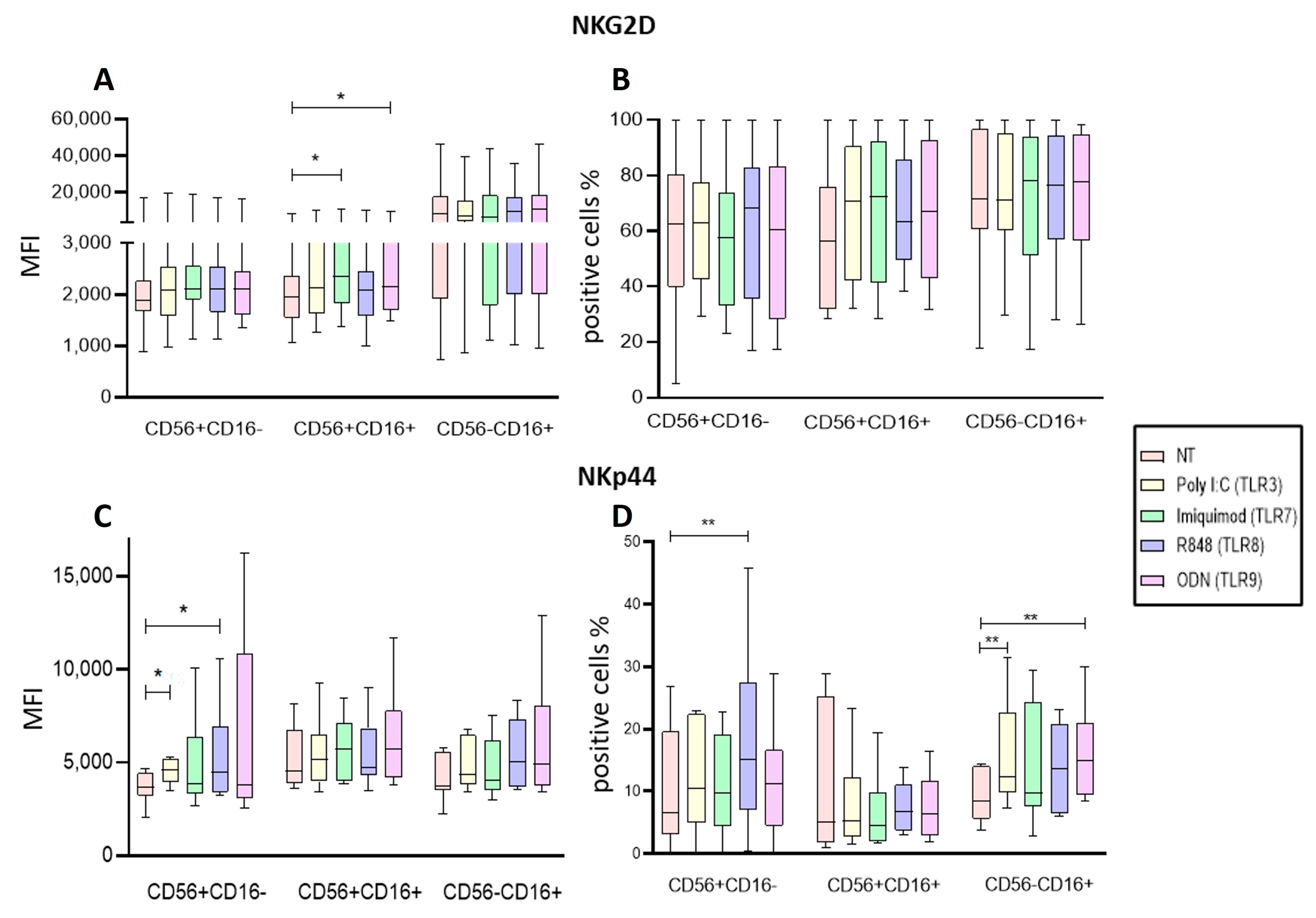
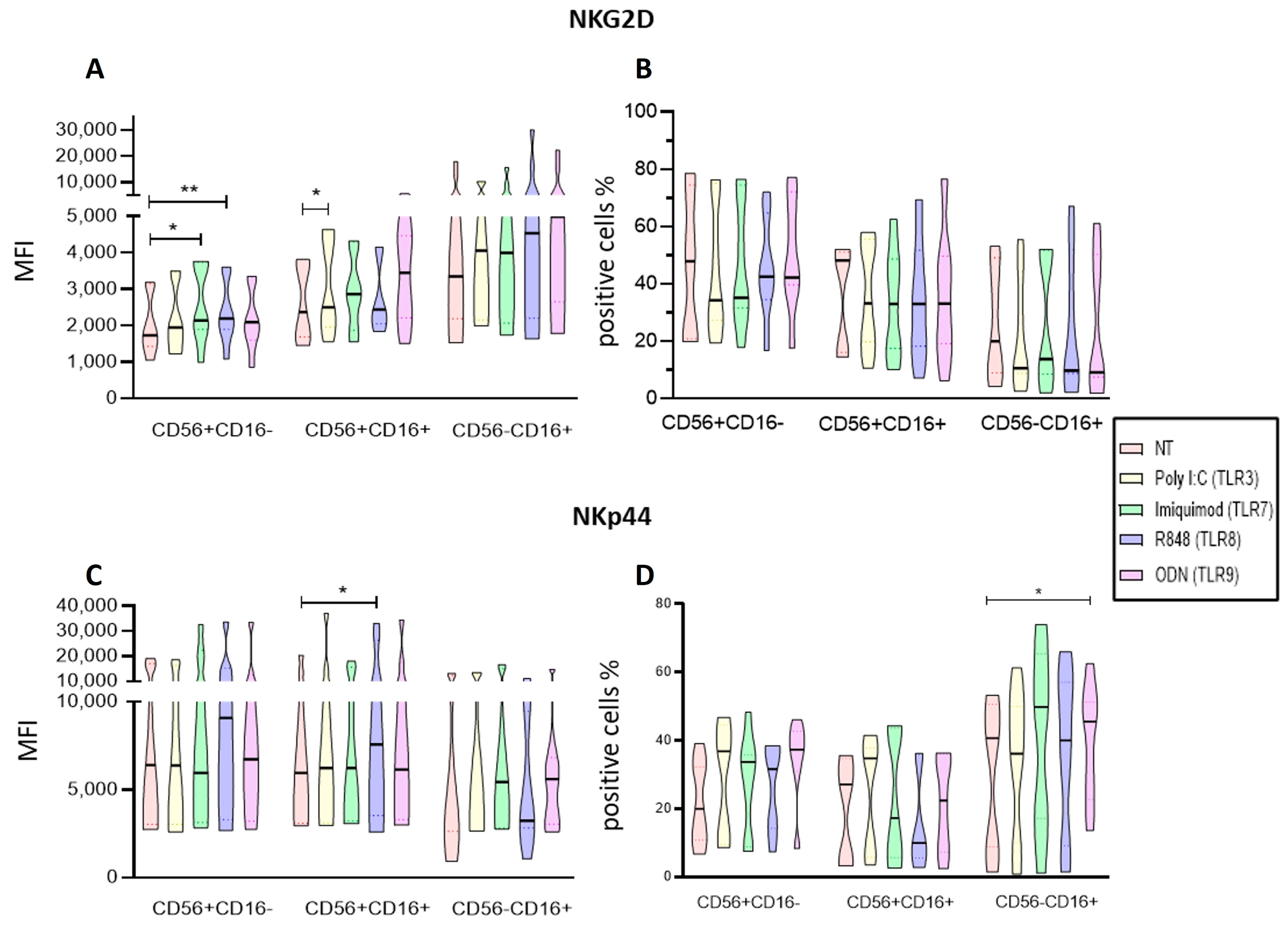
2.5. Endosomal TLRs Increase the Activation of NK Subpopulations within the PBMC
2.6. Endosomal TLR Ligands Increase the Activation of Enriched NK Cell Subpopulations
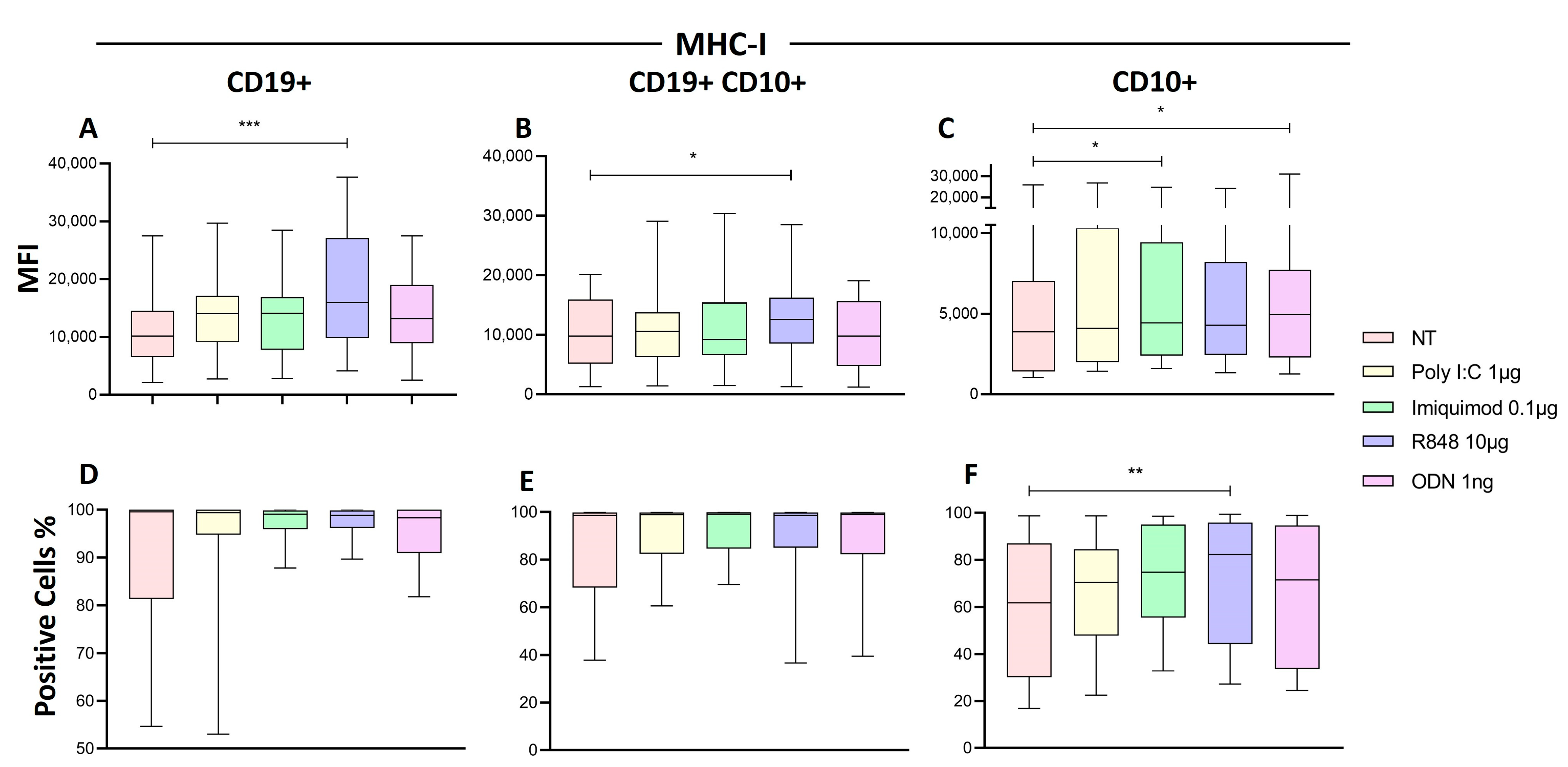
2.7. R848 Increase HLA-I Expression in Immature B Cells of Patients with ALL
2.8. TLR8 and TLR9 Ligands Increase the Cytotoxic Activity of NK Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Obtaining Mononuclear Cells, Isolating NK Cells, Culture and Activation with TLR Ligands
4.3. Characterization of NK Cells and Determination of Activation
4.4. Granzyme B Detection
4.5. Cytokines Determination
4.6. Interferon Gamma Determination
4.7. Cytotoxicity Assays
4.7.1. Zombie NIR Assay
4.7.2. LDH Release Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maldonado-Bernal, C.; Sánchez-Herrera, D. Toll-like Receptors and Natural Killer Cells. In Toll-like Receptors; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Huntington, N.D.; Vosshenrich, C.A.J.; Di Santo, J.P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 2007, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, S.; Hidalgo-Miranda, A.; Ramírez-Bello, J. Acute lymphoblastic leukemia: A genomic perspective. Bol. Med. Hosp. Infant. Mex. 2017, 74, 13–26. [Google Scholar] [CrossRef]
- Ceppi, F.; Cazzaniga, G.; Colombini, A.; Biondi, A.; Conter, V. Risk factors for relapse in childhood acute lymphoblastic leukemia: Prediction and prevention. Expert. Rev. Hematol. 2015, 8, 57–70. [Google Scholar] [CrossRef]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Vazquez, L.; Núñez-Enríquez, J.C.; Sánchez-Herrera, J.; Jiménez-Hernández, E.; Martín-Trejo, J.A.; Espinoza-Hernández, L.E.; Medina-Sanson, A.; Flores-Villegas, L.V.; Peñaloza-González, J.G.; Torres-Nava, J.R.; et al. Functional characterization of NK cells in Mexican pediatric patients with acute lymphoblastic leukemia: Report from the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia. PLoS ONE 2020, 15, e0227314. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Rouce, R.H.; Shaim, H.; Sekine, T.; Weber, G.; Ballard, B.; Ku, S.; Barese, C.; Murali, V.; Wu, M.-F.; Liu, H.; et al. The TGF-β/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 2016, 30, 800–811. [Google Scholar] [CrossRef]
- Valenzuela-Vázquez, L.; Nuñez-Enriquez, J.C.; Sánchez-Herrera, J.; Medina-Sanson, A.; Pérez-Saldivar, M.L.; Jiménez-Hernández, E.; Martiín-Trejo, J.A.; Campo-Martínez, M.d.L.D.; Flores-Lujano, J.; Amador-Sánchez, R.; et al. NK cells with decreased expression of multiple activating receptors is a dominant phenotype in pediatric patients with acute lymphoblastic leukemia. Front. Oncol. 2022, 12, 1023510. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, C. Critical role of Toll-like receptor signaling in NK cell activation. Chin. Sci. Bull. 2012, 57, 3192–3202. [Google Scholar] [CrossRef]
- Vacchelli, E.; Eggermont, A.; Sautès-Fridman, C.; Galon, J.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch toll-like receptor agonists for cancer therapy. Oncoimmunology 2013, 2, e25238. [Google Scholar] [CrossRef]
- Barbalat, R.; Ewald, S.E.; Mouchess, M.L.; Barton, G.M. Nucleic Acid Recognition by the Innate Immune System. Annu. Rev. Immunol. 2011, 29, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like receptors and cancer. Nat. Rev. Cancer 2009, 9, 57–63. [Google Scholar] [CrossRef]
- Sánchez-Herrera, D.; Contreras-Ramos, A.; Jiménez-Hernández, E.; Medina-Sansón, A.; Giono-Cerezo, S.; Maldonado-Bernal, C. NK Cell Subpopulation Is Altered and the Expression of TLR1 and TLR9 Is Decreased in Patients with Acute Lymphoblastic Leukemia. J. Oncol. 2021, 2021, 5528378. [Google Scholar] [CrossRef]
- Della Chiesa, M.; De Maria, A.; Muccio, L.; Bozzano, F.; Sivori, S.; Moretta, L. Human NK Cells and Herpesviruses: Mechanisms of Recognition, Response and Adaptation. Front. Microbiol. 2019, 10, 2297. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Fuchs, A.; Colonna, M.; Caligiuri, M.A. NK cell and DC interactions. Trends Immunol. 2004, 25, 47–52. [Google Scholar] [CrossRef]
- Guillerey, C.; Chow, M.T.; Miles, K.; Olver, S.; Sceneay, J.; Takeda, K.; Möller, A.; Smyth, M.J. Toll-like receptor 3 regulates NK cell responses to cytokines and controls experimental metastasis. Oncoimmunology 2015, 4, e1027468. [Google Scholar] [CrossRef]
- Gruijs, M.; Ganzevles, S.H.; Stigter-van Walsum, M.; van der Mast, R.; van Ostaijen-ten Dam, M.M.; Tuk, C.W.; Schilham, M.W.; Leemans, C.R.; Brakenhoff, R.H.; van Egmond, M.; et al. NK Cell-Dependent Antibody-Mediated Immunotherapy Is Improved In Vitro and In Vivo When Combined with Agonists for Toll-like Receptor 2 in Head and Neck Cancer Models. Int. J. Mol. Sci. 2021, 22, 11057. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Song, H.; Li, H.; Cui, A.; Liu, Y.; Su, L.; Crispe, I.N.; Tu, Z. Activated NK cells kill hepatic stellate cells via p38/PI3K signaling in a TRAIL-involved degranulation manner. J. Leukoc. Biol. 2019, 105, 695–704. [Google Scholar] [CrossRef]
- Samudio, I.; Rezvani, K.; Shaim, H.; Hofs, E.; Ngom, M.; Bu, L.; Liu, G.; Lee, J.T.C.; Imren, S.; Lam, V.; et al. UV-inactivated HSV-1 potently activates NK cell killing of leukemic cells. Blood J. Am. Soc. Hematol. 2016, 127, 2575–2586. [Google Scholar] [CrossRef]
- Gorski, K.S.; Waller, E.L.; Bjornton-Severson, J.; Hanten, J.A.; Riter, C.L.; Kieper, W.C.; Gorden, K.B.; Miller, J.S.; Vasilakos, J.P.; Tomai, M.A.; et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int. Immunol. 2006, 18, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, N.M.; Mian, F.; MacKenzie, R.; Ashkar, A.A. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006, 241, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ronsley, R.; Kariminia, A.; Ng, B.; Mostafavi, S.; Reid, G.; Subrt, P.; Hijiya, N.; Schultz, K.R. The TLR9 agonist (GNKG168) induces a unique immune activation pattern in vivo in children with minimal residual disease positive acute leukemia: Results of the TACL T2009-008 phase I study. Pediatr. Hematol. Oncol. 2019, 36, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Falco, M.; Della Chiesa, M.; Carlomagno, S.; Vitale, M.; Moretta, L.; Moretta, A. CpG and double-stranded RNA trigger human NK cells by toll-like receptors: Induction of cytokine release and cytotoxicity against tumors dendritic cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10116–10121. [Google Scholar] [CrossRef]
- Khanna, V.; Kim, H.; Zhang, W.; Larson, P.; Shah, M.; Griffith, T.S.; Ferguson, D.; Panyam, J. Novel TLR 7/8 agonists for improving NK cell mediated antibody-dependent cellular cytotoxicity (ADCC). Sci. Rep. 2021, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Portale, F.; Di Mitri, D. NK Cells in Cancer: Mechanisms of Dysfunction and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 9521. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, I.; Alicata, C.; Moretta, L.; Maggi, E. The Latest Approach of Immunotherapy with Endosomal TLR Agonists Improving NK Cell Function: An Overview. Biomedicines 2022, 11, 64. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Veneziani, I.; Alicata, C.; Pelosi, A.; Landolina, N.; Ricci, B.; D’Oria, V.; Fagotti, A.; Scambia, G.; Moretta, L.; Maggi, E. Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56brightCD16- subset. J. Immunother. Cancer 2022, 10, e003385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012, 91, 299. [Google Scholar] [CrossRef]
- Montaldo, E.; Vitale, C.; Cottalasso, F.; Conte, R.; Glatzer, T.; Ambrosini, P.; Moretta, L.; Mingari, M.C. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood 2012, 119, 3987–3996. [Google Scholar] [CrossRef]
- Morvan, M.; Lanier, L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Girart, M.V.; Fuertes, M.B.; Domaica, C.I.; Rossi, L.E.; Zwirner, N.W. Engagement of TLR3, TLR7, and NKG2D regulate IFN-γ secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 2007, 179, 3472–3479. [Google Scholar] [CrossRef]
- Kasahara, Y.; Shin, C.; Kubo, N.; Mihara, K.; Iwabuchi, H.; Takachi, T.; Imamura, M.; Saitoh, A.; Imai, C. Development and characterisation of NKp44-based chimeric antigen receptors that confer T cells with NK cell-like specificity. Clin. Transl. Immunol. 2020, 9, e1147. [Google Scholar] [CrossRef]
- Müller-Durovic, B.; Grählert, J.; Devine, O.P.; Akbar, A.N.; Hess, C. CD56-negative NK cells with impaired effector function expand in CMV and EBV co-infected healthy donors with age. Aging 2019, 11, 724–740. [Google Scholar] [CrossRef]
- Forconi, C.S.; Cosgrove, C.P.; Saikumar-Lakshmi, P.; Nixon, C.E.; Foley, J.; Ong’echa, J.M.; Otieno, J.A.; Alter, G.; Münz, C.; Moormann, A.M. Poorly cytotoxic terminally differentiated CD56negCD16pos NK cells accumulate in Kenyan children with Burkitt lymphomas. Blood Adv. 2018, 2, 1101–1114. [Google Scholar] [CrossRef]
- Chretien, A.-S.; Devillier, R.; Granjeaud, S.; Cordier, C.; Demerle, C.; Salem, N.; Wlosik, J.; Orlanducci, F.; Gorvel, L.; Fattori, S.; et al. High-dimensional mass cytometry analysis of NK cell alterations in AML identifies a subgroup with adverse clinical outcome. Proc. Natl. Acad. Sci. USA 2021, 118, e2020459118. [Google Scholar] [CrossRef]
- Voigt, J.; Malone, D.F.G.; Dias, J.; Leeansyah, E.; Björkström, N.K.; Ljunggren, H.; Gröbe, L.; Klawonn, F.; Heyner, M.; Sandberg, J.K.; et al. Proteome analysis of human CD56negNK cells reveals a homogeneous phenotype surprisingly similar to CD56dimNK cells. Eur. J. Immunol. 2018, 48, 1456–1469. [Google Scholar] [CrossRef]
- Reiners, K.S.; Topolar, D.; Henke, A.; Simhadri, V.R.; Kessler, J.; Sauer, M.; Bessler, M.; Hansen, H.P.; Tawadros, S.; Herling, M.; et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013, 121, 3658–3665. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Poly-ICLC (Hiltonol) and Anti-PD1 or Anti-PD-L1 (NCT03721679). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2018. Available online: https://clinicaltrials.gov/study/NCT03721679?cond=Cancer&term=Polyinosinic-polycytidylic%20acid&rank=1 (accessed on 15 January 2024).
- Clinicaltrials.gov. Study of PolyIC and PD-1 mAb in Subjects with Unresectable Hepatocellular Carcinoma (NCT03732547). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2018. Available online: https://clinicaltrials.gov/study/NCT03732547?cond=Cancer&term=poly-IC&intr=Therapy&page=2&rank=11 (accessed on 15 January 2024).
- ClinicalTrials.gov. Combination Therapy of Topical Imiquimod Plus Multipeptide Vaccination for Cutaneous Metastases of Melanoma (MEL53) (NCT01264731). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2011. Available online: https://clinicaltrials.gov/study/NCT01264731?cond=Cancer&term=imiquimod&intr=Therapy&page=3&rank=25 (accessed on 13 January 2024).
- Clinicaltrials.Gov. Predicting Response in Cervical Intraepithelial Neoplasia to Topical Imiquimod Treatment (PRedICT-TOPIC) (NCT05405270). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2022. Available online: https://clinicaltrials.gov/study/NCT05405270?cond=Cancer&term=imiquimod&intr=Therapy&page=1&rank=6 (accessed on 13 January 2024).
- Rook, A.H.; Gelfand, J.M.; Wysocka, M.; Troxel, A.B.; Benoit, B.; Surber, C.; Elenitsas, R.; Buchanan, M.A.; Leahy, D.S.; Watanabe, R.; et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015, 126, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Vaccine Therapy and Resiquimod in Treating Patients with Stage II, Stage III, or Stage IV Melanoma That Has Been Completely Removed by Surgery (NCT00470379). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2006. Available online: https://clinicaltrials.gov/study/NCT00470379?cond=Cancer&term=R848&intr=Therapy&page=1&rank=2 (accessed on 15 January 2024).
- Clinicaltrials.gov. Intratumor CpG-ODN Injection Boosters Immune Killing Against In Situ Tumor Antigen Release for Advanced Solid Tumors (NCT04952272). Bethesda (MD): National Library of Medicine (US) 2000. 29 February 2021. Available online: https://clinicaltrials.gov/study/NCT04952272?cond=Cancer&term=ODN%20&intr=Therapy&rank=1 (accessed on 15 January 2024).



| Characteristics (Peripheral blood) | |
| N° of cases | 24 |
| Sex M:F | 14:10 |
| Age media (range) | 7 (1–17) years |
| Immunophenotype | |
| Pre-B | 22 |
| not defined | 2 |
| Chromosomic alterations | |
| SNP TPMT Heterozygous | 4 |
| Translocation (12:21) | 2 |
| Translocation (2:3) | 1 |
| Translocation (9:22) | 2 |
| Translocation (1:19) | 2 |
| Without translocations | 6 |
| Not defined | 7 |
| % NK Cells (range) | 1.87 (0.4–5.97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Zapata, J.; Pérez-Figueroa, E.; Olivar-López, V.; Medina-Sansón, A.; Jiménez-Hernández, E.; Ortega, E.; Maldonado-Bernal, C. TLR Agonists Modify NK Cell Activation and Increase Its Cytotoxicity in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 7500. https://doi.org/10.3390/ijms25137500
Gallardo-Zapata J, Pérez-Figueroa E, Olivar-López V, Medina-Sansón A, Jiménez-Hernández E, Ortega E, Maldonado-Bernal C. TLR Agonists Modify NK Cell Activation and Increase Its Cytotoxicity in Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2024; 25(13):7500. https://doi.org/10.3390/ijms25137500
Chicago/Turabian StyleGallardo-Zapata, Janet, Erandi Pérez-Figueroa, Víctor Olivar-López, Aurora Medina-Sansón, Elva Jiménez-Hernández, Enrique Ortega, and Carmen Maldonado-Bernal. 2024. "TLR Agonists Modify NK Cell Activation and Increase Its Cytotoxicity in Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 25, no. 13: 7500. https://doi.org/10.3390/ijms25137500






