Spectroscopic Study of a Novel Binaphthyl Amine Fluorescent Probe for Chiral Recognition of D/L-Lysine
Abstract
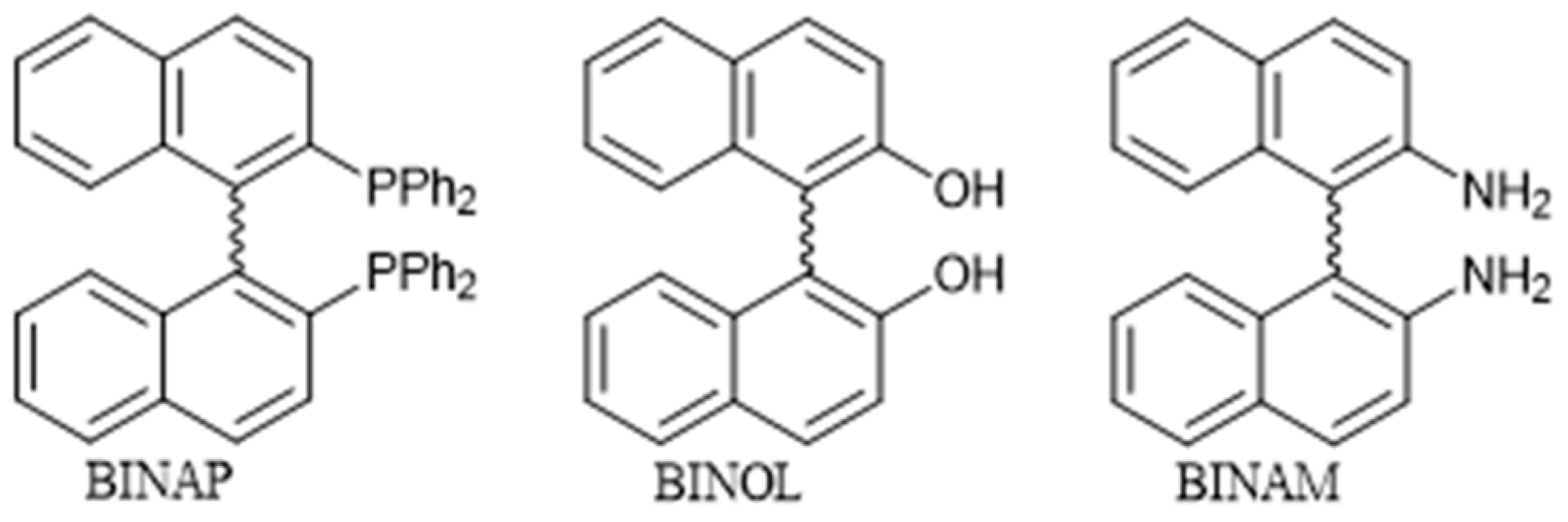
1. Introduction
2. Results and Discussion
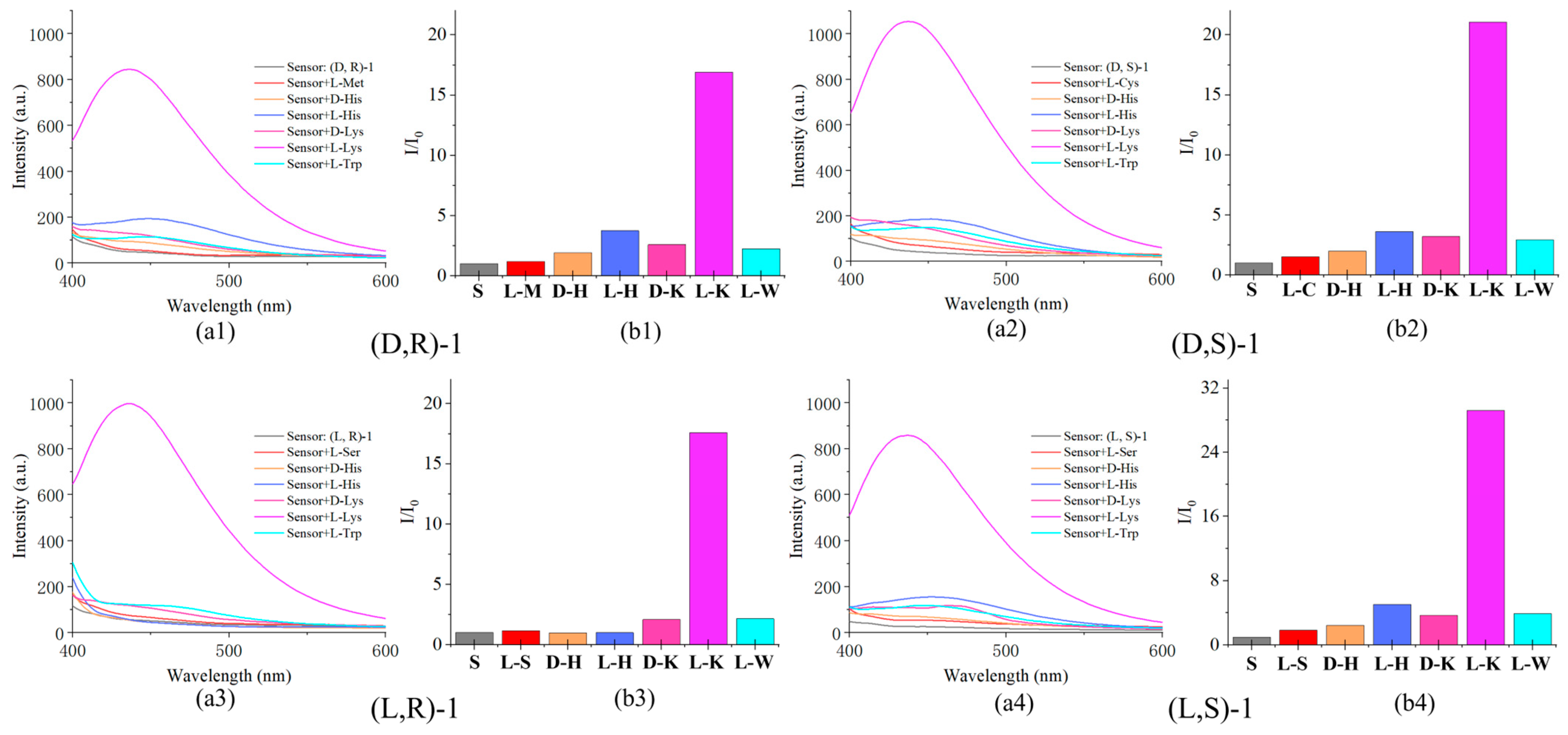
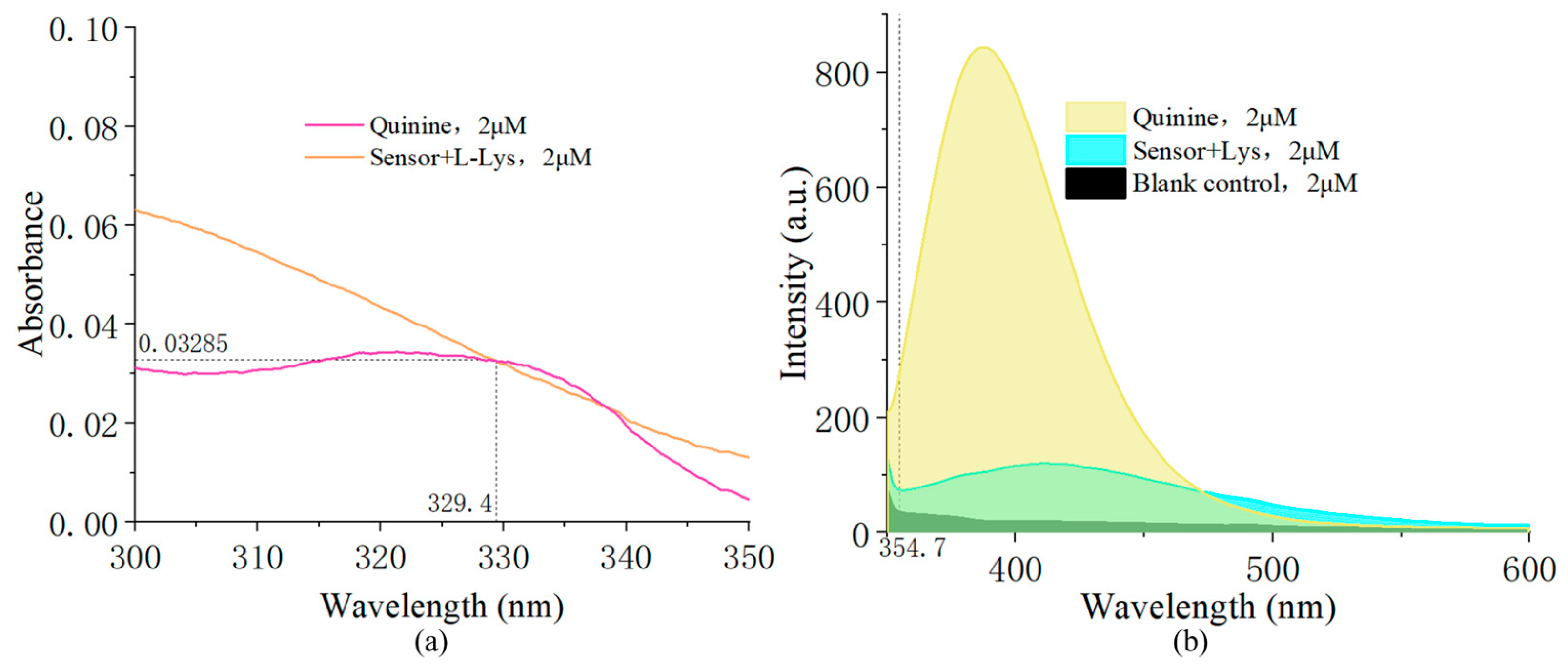
2.1. Chiral Recognition of D/L-Lysine
2.2. Recognition in Different pH
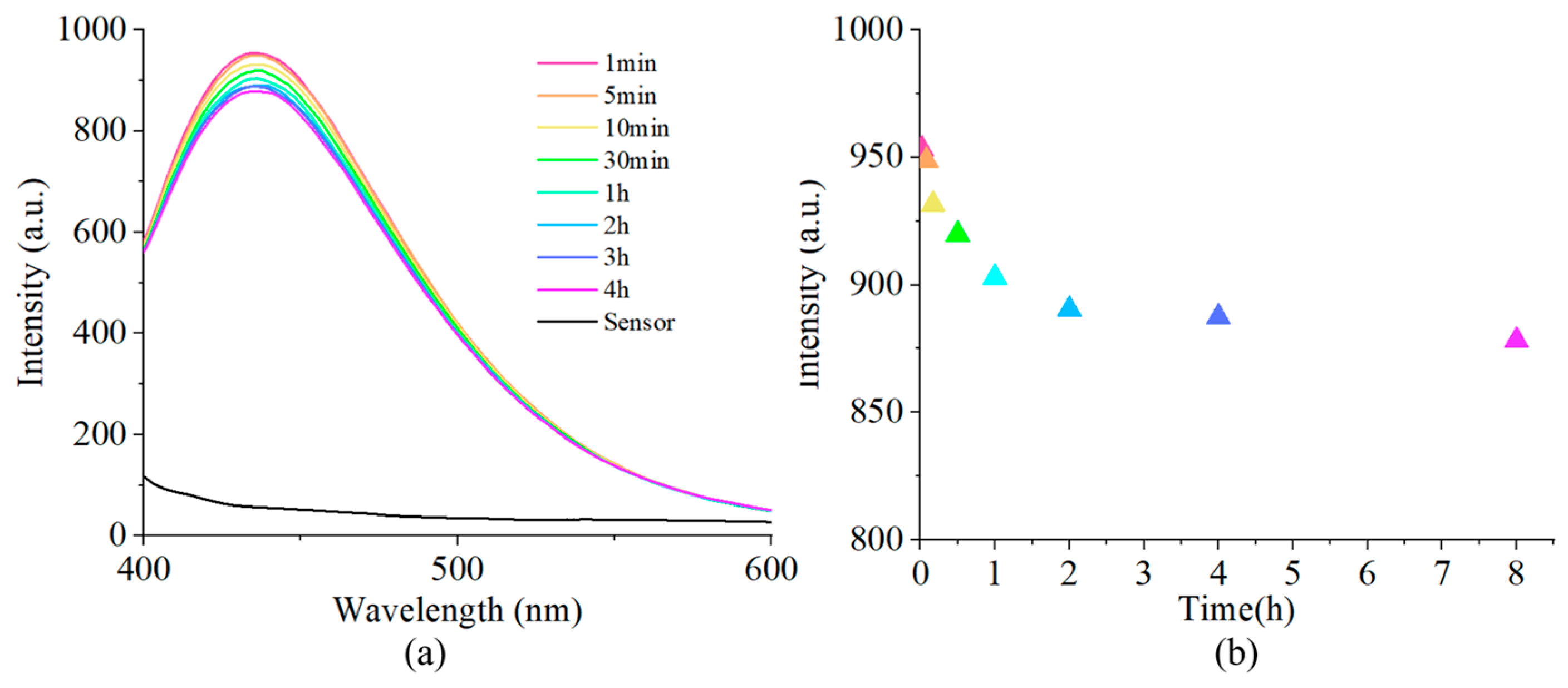
2.3. The Reaction Time of the Probes
2.4. Limit of Detection
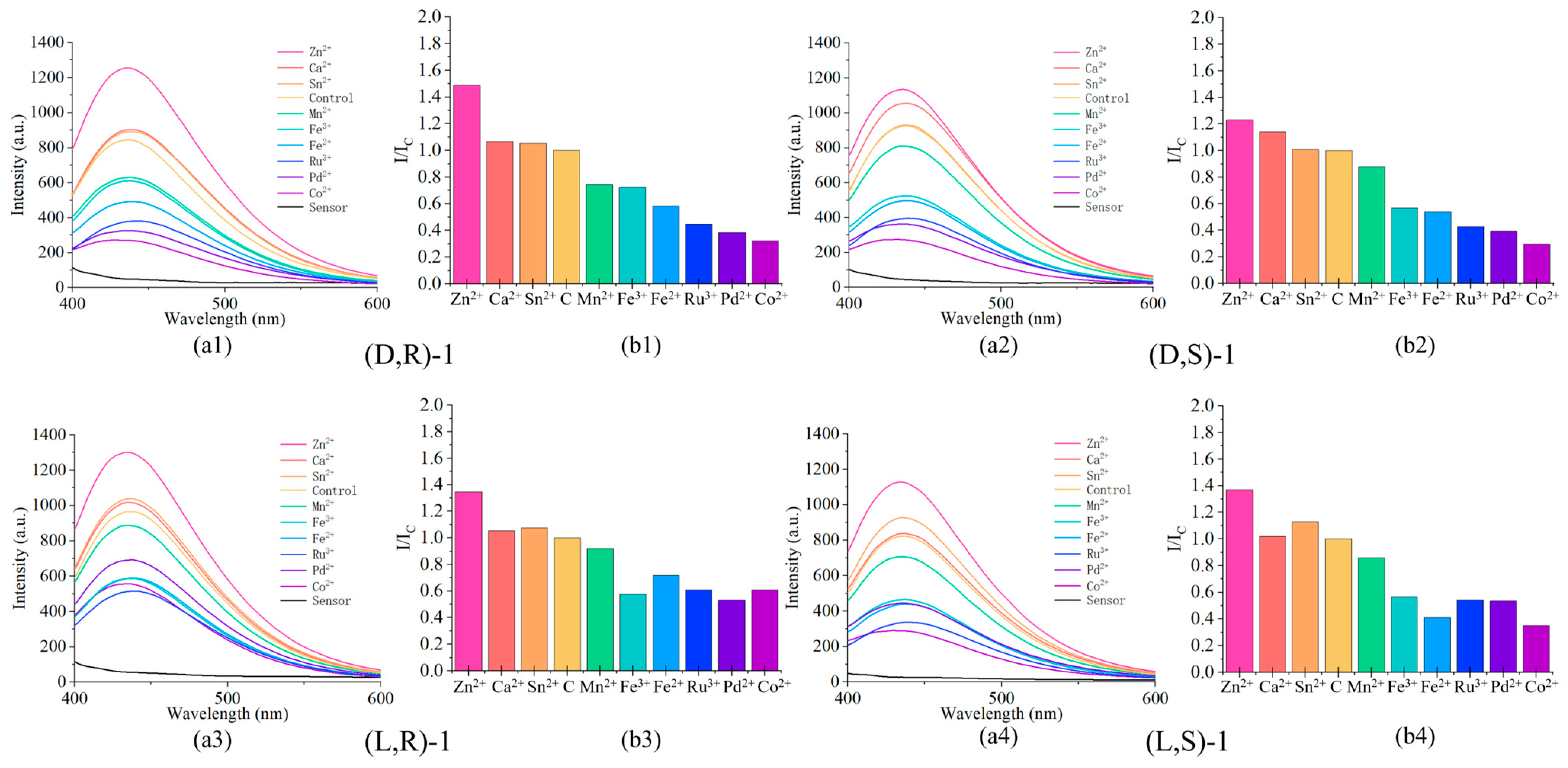
2.5. The Effect of Metal Ions
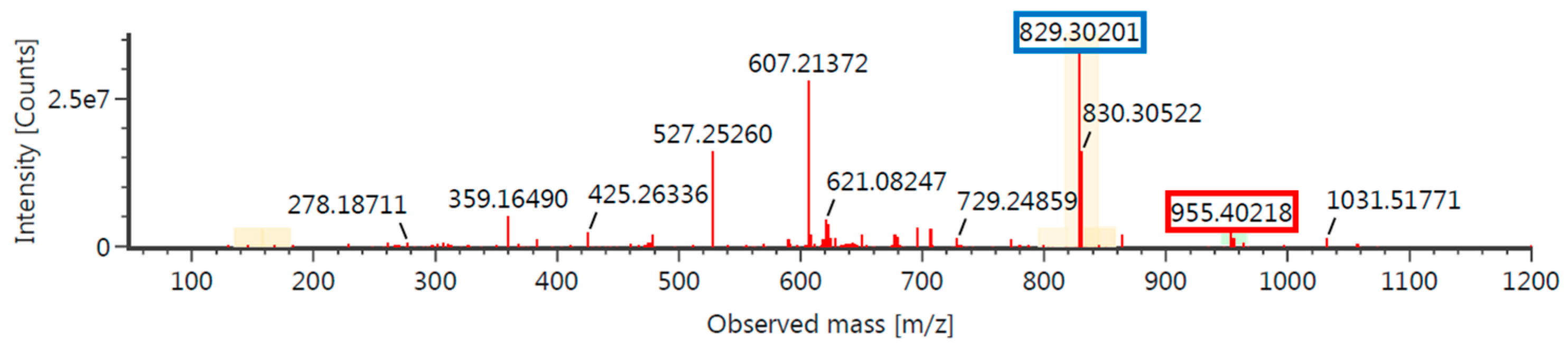
2.6. Confirm the Reaction Site by 19F NMR
2.7. Quantum Yield
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis of L-Lys-Boc2
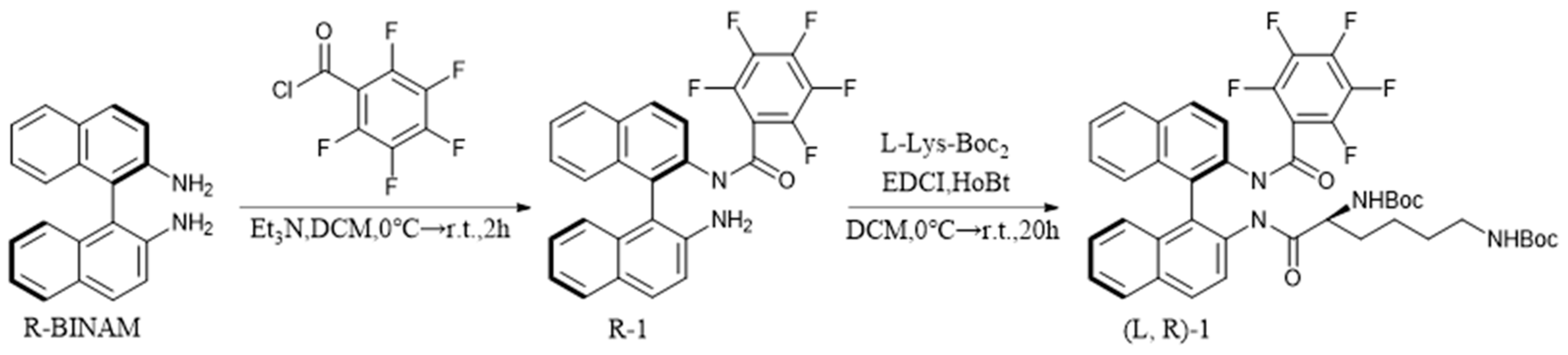
3.3. Synthesis of (L,R)-1
3.4. Preparation of Samples for Fluorescence Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozturk, S.F.; Sasselov, D.D. On the origins of life’s homochirality: Inducing enantiomeric excess with spin-polarized electrons. Proc. Natl. Acad. Sci. USA 2022, 119, e2204765119. [Google Scholar] [CrossRef] [PubMed]
- Beatty-Desana, J.W.; Hoggard, M.J.; Cooledge, J.W. Letters to nature. Nature 2001, 255, 242–243. [Google Scholar] [CrossRef]
- Copur, F.; Bekar, N.; Zor, E.; Alpaydin, S.; Bingol, H. Nanopaper-based photoluminescent enantioselective sensing of L-Lysine by L-Cysteine modified carbon quantum dots. Sens. Actuators B Chem. 2019, 279, 305–312. [Google Scholar] [CrossRef]
- Lu, W.; Gao, Y.; Jiao, Y.; Shuang, S.; Li, C.; Dong, C. Carbon nano-dots as a fluorescent and colorimetric dual-readout probe for the detection of arginine and Cu2+ and its logic gate operation. Nanoscale 2017, 9, 11545–11552. [Google Scholar] [CrossRef] [PubMed]
- Bonner, W.A. Parity violation and the evolution of biomolecular homochirality. Chirality 2000, 12, 114–126. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Zheng, M. Chiral carbon dots-based nanosensors for Sn(II) detection and lysine enantiomers recognition. Sens. Actuators B Chem. 2020, 319, 128265. [Google Scholar] [CrossRef]
- Du, S.; Wey, M.; Armstrong, D.W. d-Amino acids in biological systems. Chirality 2023, 35, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.E. Review of Lysine Metabolism with a Focus on Humans. J. Nutr. 2020, 150, 2548S–2555S. [Google Scholar] [CrossRef]
- Chang, D.; Zhao, Z.; Shi, H.; Feng, J.; Yang, Y.; Shi, L. Ratiometric fluorescent carbon dots for enantioselective sensing of L-lysine and pH discrimination in vivo and in vitro. Sens. Actuators B Chem. 2022, 362, 131792. [Google Scholar] [CrossRef]
- Song, L.; Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef]
- Payne, A.; Wang, X.; Ivy, M.T.; Stewart, A.; Nelson, K.; Darris, C.; Nahashon, S.N. Lysine mediation of neuroendocrine food regulation in guinea fowl. Poult. Sci. 2016, 95, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Estaras, M.; Ameur, F.Z.; Estévez, M.; Díaz-Velasco, S.; Gonzalez, A. The lysine derivative aminoadipic acid, a biomarker of protein oxidation and diabetes-risk, induces production of reactive oxygen species and impairs trypsin secretion in mouse pancreatic acinar cells. Food Chem. Toxicol. 2020, 145, 111594. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-L.; Zhang, Z.-M.; Song, Z.-W.; Gao, C.-Q.; Yan, H.-C.; Wang, X.-Q. mTORC1-Mediated Satellite Cell Differentiation Is Required for Lysine-Induced Skeletal Muscle Growth. J. Agric. Food Chem. 2020, 68, 4884–4892. [Google Scholar] [CrossRef]
- Tang, S.; Wei, Z.; Guo, J.; Sun, X.; Hu, Y. Enantioselective Recognition of L-Lysine by ICT Effect with a Novel Binaphthyl-Based Complex. Micromachines 2023, 14, 500. [Google Scholar] [CrossRef]
- Loaëc, G.; Niquet-Léridon, C.; Henry, N.; Jacolot, P.; Jouquand, C.; Janssens, M.; Hance, P.; Cadalen, T.; Hilbert, J.-L.; Desprez, B.; et al. Impact of Variety and Agronomic Factors on Crude Protein and Total Lysine in Chicory; Nε-Carboxymethyl-lysine-Forming Potential during Drying and Roasting. J. Agric. Food Chem. 2015, 63, 10295–10302. [Google Scholar] [CrossRef]
- Jin, C.; Bao, J. Lysine Production by Dry Biorefining of Wheat Straw and Cofermentation of Corynebacterium glutamicum. J. Agric. Food Chem. 2021, 69, 1900–1906. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Nutritional and medicinal aspects of d-amino acids. Amino Acids 2011, 42, 1553–1582. [Google Scholar] [CrossRef]
- Abbasov, M.E.; Kavanagh, M.E.; Ichu, T.-A.; Lazear, M.R.; Tao, Y.; Crowley, V.M.; am Ende, C.W.; Hacker, S.M.; Ho, J.; Dix, M.M.; et al. A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem. 2021, 13, 1081–1092. [Google Scholar] [CrossRef]
- Kugimiya, A.; Fukada, R.; Funamoto, D. A luminol chemiluminescence method for sensing histidine and lysine using enzyme reactions. Anal. Biochem. 2013, 443, 22–26. [Google Scholar] [CrossRef]
- Namera, A.; Yashiki, M.; Nishida, M.; Kojima, T. Direct extract derivatization for determination of amino acids in human urine by gas chromatography and mass spectrometry. J. Chromatogr. B 2002, 776, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Huang, B.; Day, S.; Tang, C.C.; Tsang, S.C.E.; Wong, K.y.; Lo, T.W.B. Differential Adsorption of l- and d-Lysine on Achiral MFI Zeolites as Determined by Synchrotron X-Ray Powder Diffraction and Thermogravimetric Analysis. Angew. Chem. Int. Ed. 2019, 59, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Pu, L. Micelle-Encapsulated Fluorescent Probe: Chemoselective and Enantioselective Recognition of Lysine in Aqueous Solution. Org. Lett. 2019, 21, 4777–4781. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.J.; Yan, L.; Tian, S.K.; Wu, M.Y.; Wang, L.X.; Fan, Z.L.; Zheng, S.C.; Liu, X.Y.; Tan, B. Highly Enantioselective Kinetic Resolution of Axially Chiral BINAM Derivatives Catalyzed by a Brønsted Acid. Angew. Chem. Int. Ed. 2014, 53, 3684–3687. [Google Scholar] [CrossRef]
- Pu, L. Fluorescence of Organic Molecules in Chiral Recognition. Chem. Rev. 2004, 104, 1687–1716. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. Enantioselective Fluorescent Sensors: A Tale of BINOL. Acc. Chem. Res. 2012, 45, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Wu, X.-D.; Gu, S.-X.; Pu, L. Free Amino Acid Recognition: A Bisbinaphthyl-Based Fluorescent Probe with High Enantioselectivity. J. Am. Chem. Soc. 2018, 141, 175–181. [Google Scholar] [CrossRef]
- Averin, A.D.; Grigorova, O.K.; Malysheva, A.S.; Shaferov, A.V.; Beletskaya, I.P. Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors. Pure Appl. Chem. 2020, 92, 1367–1386. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Cao, Y.; Gong, G.; Zhou, Y.; Gao, X.; Pu, L.; Zhao, G. Spectroscopic studies of a BINAM-based sensor: Highly selective fluorescent recognition of lysine in water solution through a nucleophilic substitution reaction. Tetrahedron Lett. 2019, 60, 1238–1242. [Google Scholar] [CrossRef]
- Jiao, S.-Y.; Li, K.; Wang, X.; Huang, Z.; Pu, L.; Yu, X.-Q. Making pyrophosphate visible: The first precipitable and real-time fluorescent sensor for pyrophosphate in aqueous solution. Analyst 2015, 140, 174–181. [Google Scholar] [CrossRef]
- Kondo, S.-i.; Nakadai, Y.; Unno, M. Recognition of dicarboxylates in aqueous acetonitrile by a dinuclear zinc(II) complex of 2,2’-binaphthalene-based receptor. Supramol. Chem. 2018, 31, 9–18. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kojima, S.; Hamedpour, V.; Kubota, R.; Takizawa, S.-y.; Yoshikawa, I.; Houjou, H.; Kubo, Y.; Minami, T. Accurate chiral pattern recognition for amines from just a single chemosensor. Chem. Sci. 2020, 11, 3790–3796. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, S.; Wen, K.; Yu, X.; Pu, L. Zn(ii) promoted dramatic enhancement in the enantioselective fluorescent recognition of functional chiral amines by a chiral aldehyde. Chem. Sci. 2014, 5, 3457–3462. [Google Scholar] [CrossRef]
- Wang, S.; Men, G.; Zhao, L.; Hou, Q.; Jiang, S. Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sens. Actuators B Chem. 2010, 145, 826–831. [Google Scholar] [CrossRef]
- Song, T.; Cao, Y.; Zhao, G.; Pu, L. Fluorescent Recognition of Zn2+ by Two Diastereomeric Salicylaldimines: Dramatically Different Responses and Spectroscopic Investigation. Inorg. Chem. 2017, 56, 4395–4399. [Google Scholar] [CrossRef]
- Wang, X.; Shi, D.; Xu, Y.; Yu, S.; Zhao, F.; Wang, Y.; Hu, L.; Tian, J.; Yu, X.; Pu, L. Reaction of Zn(II) with a BINOL-amino-acid Schiff base: An unusual off-on-off fluorescence response. Tetrahedron Lett. 2018, 59, 2332–2334. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Y.; Zhang, Y.; Zhou, Y.; Zhao, G.; Pu, L. Study of the ZnII Complexes of 1,1′-Binaphthyl-Based Schiff Bases: Fluorescent Detection of Thiocyanate. Eur. J. Inorg. Chem. 2018, 2018, 4153–4157. [Google Scholar] [CrossRef]
- Levitus, M. Tutorial: Measurement of fluorescence spectra and determination of relative fluorescence quantum yields of transparent samples. Methods Appl. Fluoresc. 2020, 8, 033001. [Google Scholar] [CrossRef]
- Melhuish, W.H. Quantum Efficiencies of Fluorescence of Organic Substances: Effect of Solvent and Concentration of the Fluorescent Solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]



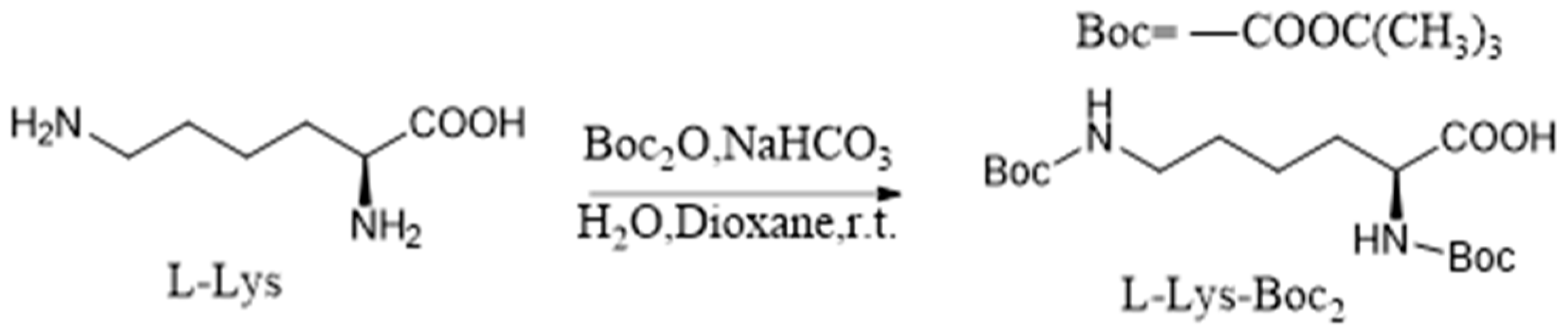
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Lu, X.; Cai, W.; Zou, Y.; Zhang, X.; Yang, J.; Zhao, G. Spectroscopic Study of a Novel Binaphthyl Amine Fluorescent Probe for Chiral Recognition of D/L-Lysine. Int. J. Mol. Sci. 2024, 25, 7504. https://doi.org/10.3390/ijms25147504
Wu L, Lu X, Cai W, Zou Y, Zhang X, Yang J, Zhao G. Spectroscopic Study of a Novel Binaphthyl Amine Fluorescent Probe for Chiral Recognition of D/L-Lysine. International Journal of Molecular Sciences. 2024; 25(14):7504. https://doi.org/10.3390/ijms25147504
Chicago/Turabian StyleWu, Liji, Xiangyu Lu, Wentao Cai, Yajun Zou, Xiaoyu Zhang, Jialiang Yang, and Gang Zhao. 2024. "Spectroscopic Study of a Novel Binaphthyl Amine Fluorescent Probe for Chiral Recognition of D/L-Lysine" International Journal of Molecular Sciences 25, no. 14: 7504. https://doi.org/10.3390/ijms25147504
APA StyleWu, L., Lu, X., Cai, W., Zou, Y., Zhang, X., Yang, J., & Zhao, G. (2024). Spectroscopic Study of a Novel Binaphthyl Amine Fluorescent Probe for Chiral Recognition of D/L-Lysine. International Journal of Molecular Sciences, 25(14), 7504. https://doi.org/10.3390/ijms25147504




