Comparative Transcriptome Analysis of Henosepilachna vigintioctomaculata Reveals Critical Pathways during Development
Abstract
:1. Introduction
2. Results
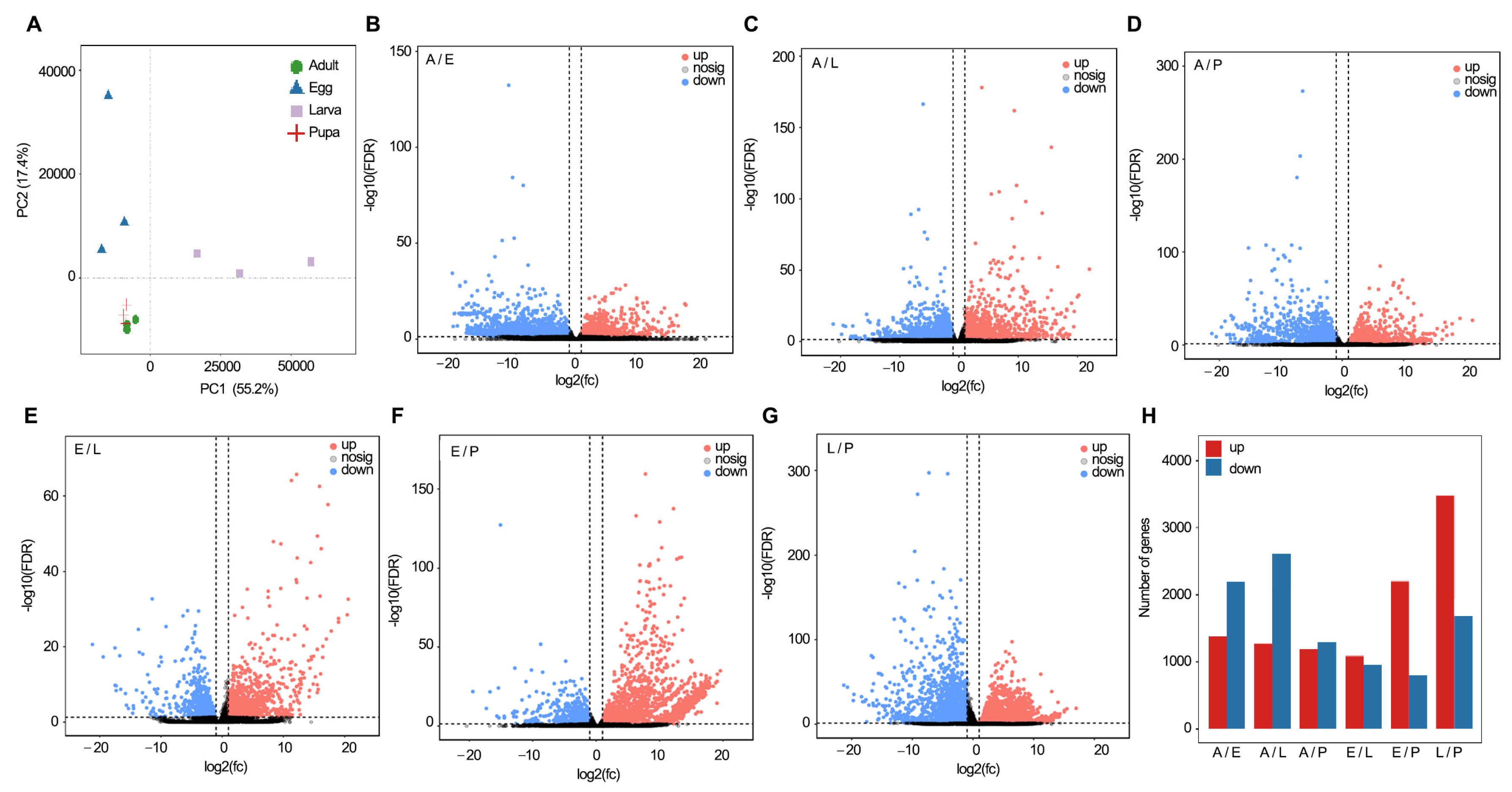
2.1. Data Description and Processing
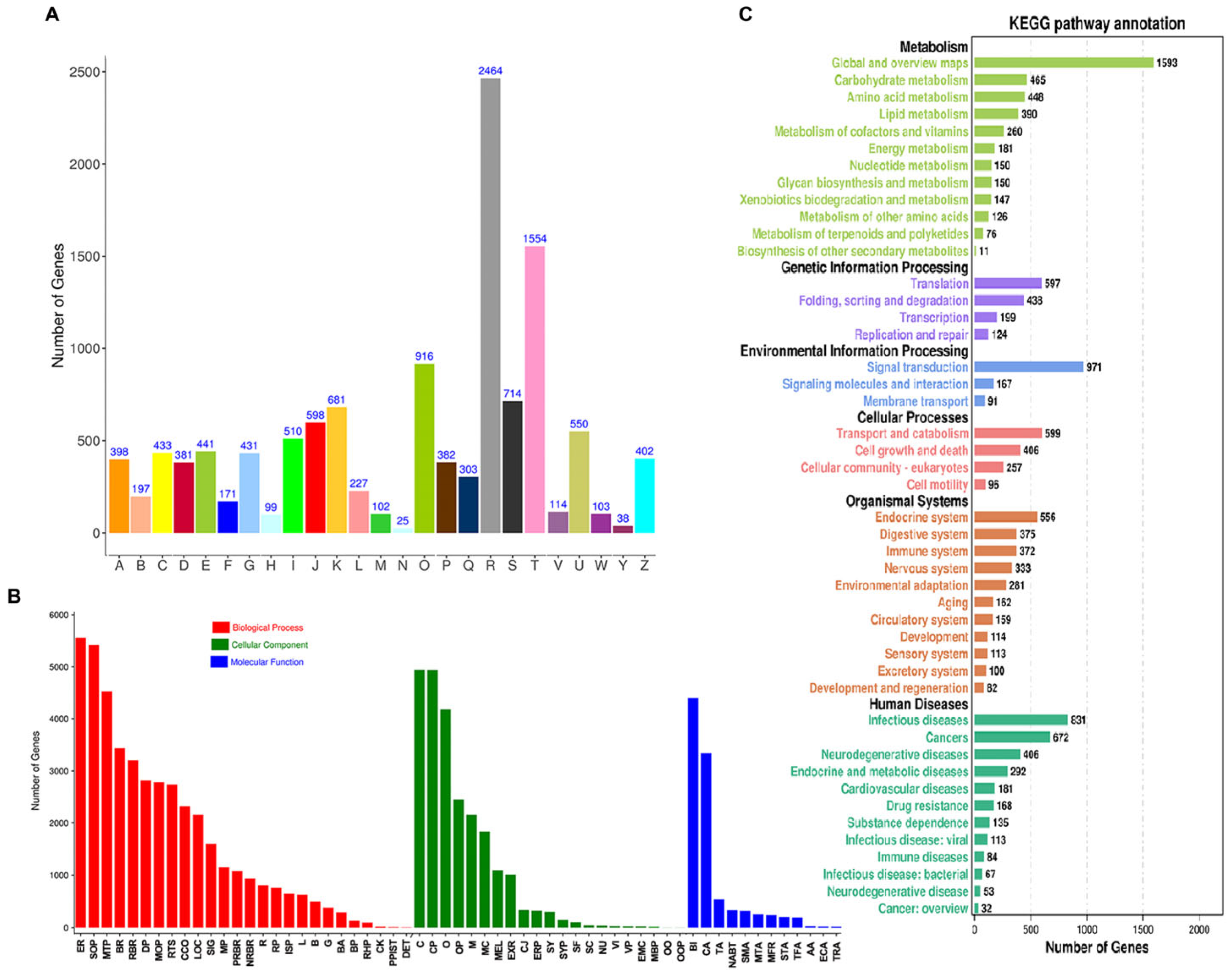
2.2. Function Annotation
2.3. Differential Expression Genes
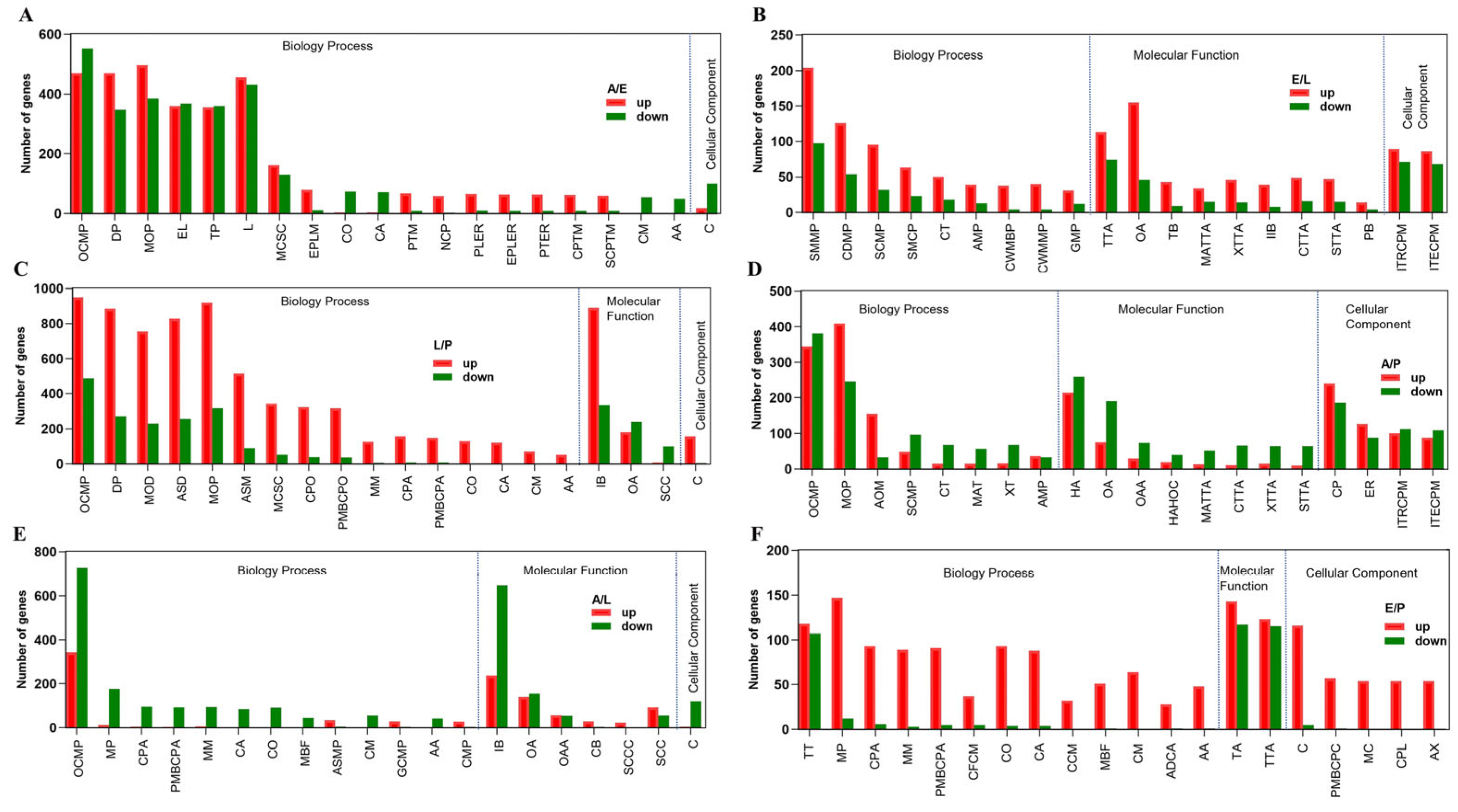
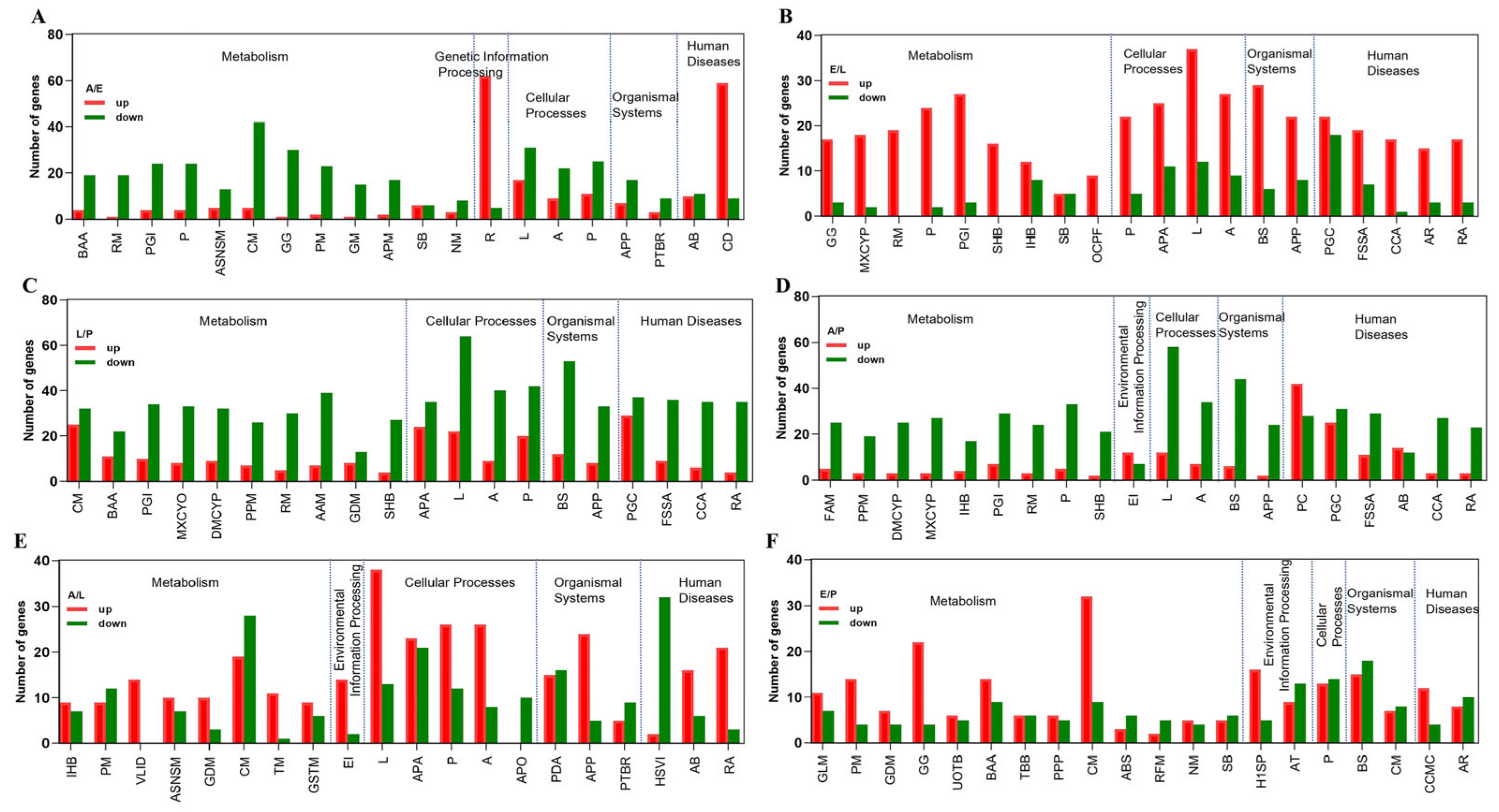
2.4. Adult versus Egg
2.5. Egg versus Larvae
2.6. Larva versus Pupa
2.7. Adult versus Pupa
2.8. Adult versus Larva
2.9. Egg versus Pupa
3. Discussion
3.1. Potential Targets for Control throughout the Whole Lifespan
3.2. Targeting Energy Generation Genes in Embryonic and Pupal Stages
3.3. Antifeedants for Larvae and Adults
4. Materials and Methods
4.1. Insect Rearing
4.2. Sample Collection
4.3. Total RNA Isolation
4.4. Library Construction and Transcriptomic Sequencing
4.5. Sequencing Processing and Assembly
4.6. Identification of Differentially Expressed Genes
4.7. Transcript Data Annotation and Gene Ontology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; Chi, S.; Wang, Y.; Li, H.; Wang, Z.; Gu, S.; Sun, T.; Xiang, H.; You, P.; Ren, Y. A chromosome-level genome assembly of the Henosepilachna vigintioctomaculata provides insights into the evolution of ladybird beetles. DNA Res. 2023, 30, dsad001. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamura, H.; Fujii, Y.; Suzuki, Y.; Matsuda, K. Chemicals affecting the feeding preference of the Solanaceae feeding lady beetle Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2011, 135, 121–131. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tan, Q.; Shen, C.H.; Wu, J.J.; Wu, Y.K.; Li, W.Z.; Jin, L.; Li, G.Q. Reference gene selection for transcriptional profiling by RT-qPCR in the 28-spotted larger potato ladybird. J. Asia-Pac. Entomol. 2022, 25, 101900. [Google Scholar] [CrossRef]
- Li, H.; Xia, X.; He, X.; Li, S.; Dai, L.; Ye, J.; Hao, D. Comparative transcriptome analysis reveals molecular insights in overwintering Monochamus alternatus (Coleoptera: Cerambycidae). J. Insect Sci. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Lu, X.Y.; Ma, J. Characterization and expression analysis of attacins, antimicrobial peptide-encoding genes, from the desert beetle Microdera punctipennis in response to low temperatures. Cryo Lett. 2017, 38, 65–74. [Google Scholar]
- Tian, C.B.; Li, Y.Y.; Huang, J.; Chu, W.Q.; Wang, Z.Y.; Liu, H. Comparative transcriptome and proteome analysis of heat acclimation in predatory mite Neoseiulus barkeri. Front. Physiol. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Park, Y. Differential Transcriptome Analysis Reveals Genes Related to Low- and High-Temperature Stress in the Fall Armyworm, Spodoptera frugiperda. Front. Physiol. 2022, 12, 827077. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Perkin, L.C.; Lorenzen, M.; Dossey, A.T. Transcriptome analysis of life stages of the house cricket, Acheta domesticus, to improve insect crop production. Sci. Rep. 2020, 10, 3471. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Mo, S.H.; Liu, Y.; Wei, D. Transcriptomic profiling of various developmental stages of Aphis aurantii to provide a genetic resource for gene expression and SSR analysis. Front. Physiol. 2020, 11, 578939. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Wang, F.; Guo, J.; Deng, X.Y.; Chen, J.Y.; Lin, L.B. Characterization of ladybird Henosepilachna vigintioctopunctata transcriptomes across various life stages. Sci. Data 2018, 5, 180093. [Google Scholar] [CrossRef]
- Du, H.; Ge, R.; Zhang, L.; Zhang, J.; Chen, K.; Li, C. Transcriptome-wide identification of development related genes and pathways in Tribolium castaneum. Genomics 2023, 115, 110551. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, R.; Li, X.; Gao, B.; Chen, S. Transcriptome and Gene Expression Analysis of Cylas formicarius (Coleoptera: Brentidae) During Different Development Stages. J. Insect Sci. 2016, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Graveley, B.R.; Brooks, A.N.; Carlson, J.W.; Duff, M.O.; Landolin, J.M.; Yang, L.; Artieri, C.G.; van Baren, M.J.; Boley, N.; Booth, B.W.; et al. The developmental transcriptome of Drosophila melanogaster. Nature 2011, 471, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xu, H.Q.; Chen, D.; Zhang, S.Y.; Li, W.J.; Smagghe, G.; Wang, J.J. Genome-wide gene expression profiling of the melon fly, Zeugodacus cucurbitae, during thirteen life stages. Sci. Data 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Xia, Y.D.; Zhang, Q.; Li, W.; Li, R.Y.; Liu, Y.; Chen, E.H.; Dou, W.; Stelinski, L.L.; Wang, J.J. Potential targets for controlling Bactrocera dorsalis using cuticle- and hormone-related genes revealed by a developmental transcriptome analysis. Pest Manag. Sci. 2020, 76, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, C.; Geib, S.M.; Zheng, J.; Wu, S.; Zhang, F.; Liang, G. Characterization of Dendrolimus houi Lajonquiere (Lepidoptera: Lasiocampidae) transcriptome across all life stages. Insects 2019, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Morandin, C.; Pulliainen, U.; Bos, N.; Schultner, E. De novo transcriptome assembly and its annotation for the black ant Formica fusca at the larval stage. Sci. Data 2018, 5, 180282. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Patnaik, B.B.; Baliarsingh, S.; Patnaik, H.H.; Sang, M.K.; Park, J.E.; Cho, H.C.; Song, D.K.; Jeong, J.Y.; Hong, C.E.; et al. Transcriptome analysis of the endangered dung beetle Copris tripartitus (Coleoptera: Scarabaeidae) and characterization of genes associated to immunity, growth, and reproduction. BMC Genom. 2023, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, H.M.; Yang, W.J.; Wei, D.D.; Dou, W.; Huang, Y.; Wang, J.J. Transcriptome profiling of the testis reveals genes involved in spermatogenesis and marker discovery in the oriental fruit fly, Bactrocera dorsalis. Insect Mol. Biol. 2015, 24, 41–57. [Google Scholar] [CrossRef]
- Yang, H.; Xu, D.; Zhuo, Z.; Hu, J.; Lu, B. Transcriptome and gene expression analysis of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) during developmental stages. PeerJ 2020, 8, e10223. [Google Scholar] [CrossRef]
- Guo, W.; Lü, J.; Guo, M.; Chen, S.; Qiu, B.; Sang, W.; Yang, C.; Zhang, Y.; Pan, H. De novo transcriptome analysis reveals abundant gonad-specific genes in the ovary and testis of Henosepilachna vigintioctopunctata. Int. J. Mol. Sci. 2019, 20, 4084. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Rolff, J.; Johnston, P.R.; Reynolds, S. Complete metamorphosis of insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190063. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W.; Riddiford, L.M. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 2002, 47, 467–500. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W.; Riddiford, L.M.; Konopová, B.; Nouzova, M.; Noriega, F.G.; Herko, M. The embryonic role of juvenile hormone in the firebrat, Thermobia domestica, reveals its function before its involvement in metamorphosis. Elife 2024, 12, RP92643. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Bai, C.; Wang, Z.; Wang, P.; Fan, Q.; Mi, X.; Wang, L.; He, J.; Pang, J.; Luo, X.; et al. Double-Stranded RNAs High-Efficiently Protect Transgenic Potato from Leptinotarsa decemlineata by Disrupting Juvenile Hormone Biosynthesis. J. Agric. Food Chem. 2018, 66, 11990–11999. [Google Scholar] [CrossRef]
- Yin, Y.; Qiu, Y.W.; Huang, J.; Tobe, S.S.; Chen, S.S.; Kai, Z.P. Enzymes in the juvenile hormone biosynthetic pathway can be potential targets for pest control. Pest Manag. Sci. 2020, 76, 1071–1077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wei, L.; Zhang, L.; Liu, J.; Huang, S.; Li, S.; Yang, W.; Li, K. E93 promotes transcription of RHG genes to initiate apoptosis during Drosophila salivary gland metamorphosis. Insect Sci. 2023, 30, 588–598. [Google Scholar] [CrossRef]
- Liu, Z.; Nanda, S.; Yang, C.; Chen, S.; Guo, M.; Khan, M.M.; Qiu, B.; Zhang, Y.; Zhou, X.; Pan, H. RNAi suppression of the nuclear receptor FTZ-F1 impaired ecdysis, pupation, and reproduction in the 28-spotted potato ladybeetle, Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2022, 182, 105029. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Yang, Y.; Liu, J.; Lu, J.; Ren, M.; Abbas, M.; Zhu, K.Y.; Zhang, J. Identification and RNAi-based functional analysis of chitinase family genes in Agrotis ipsilon. Pest Manag. Sci. 2022, 78, 4278–4287. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, L.; Li, G. RNAi-mediated functional analysis reveals the regulation of oocyte vitellogenesis by ecdysone signaling in two Coleoptera species. Biology 2023, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Tan, Q.; Jin, L.; Li, G.Q. Molecular characterization of the cytochrome P450 enzyme CYP18A1 in Henosepilachna vigintioctopunctata. Arch. Insect Biochem. Physiol. 2024, 115, e22111. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. Silencing uridine diphosphate N-acetylglucosamine pyrophosphorylase gene impairs larval development in Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2022, 78, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. RNAi for chitin synthase 1 rather than 2 causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2021, 178, 104934. [Google Scholar] [CrossRef]
- Shi, J.F.; Fu, J.; Mu, L.L.; Guo, W.C.; Li, G.Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylase genes LdUAP1 and LdUAP2 are specialized for synthesis of chitin in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochem. Mol. 2016, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef]
- Zhu, B.; Shan, J.; Li, R.; Liang, P.; Gao, X. Identification and RNAi-based function analysis of chitinase family genes in diamondback moth, Plutella xylostella. Pest Manag. Sci. 2019, 75, 1951–1961. [Google Scholar] [CrossRef]
- Zhao, X.; Situ, G.; He, K.; Xiao, H.; Su, C.; Li, F. Functional analysis of eight chitinase genes in rice stem borer and their potential application in pest control. Insect Mol. Biol. 2018, 27, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Pan, P.L.; Ye, Y.X.; Yu, B.; Xu, H.J.; Zhang, C.X. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2015, 24, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ze, L.J.; Jin, L.; Li, G.Q. Silencing of Adc and Ebony causes abnormal darkening of cuticle in Henosepilachna vigintioctopunctata. Front. Physiol. 2022, 13, 829675. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Nanda, S.; Guo, M.; Kong, L.; Yang, C.; Liu, Z.; Gao, R.; Qiu, B.; Zhang, Y.; Zhou, X.; et al. Tyrosine hydroxylase involved in cuticle tanning and reproduction in the 28-spotted potato ladybeetle, Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2022, 78, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Ze, L.J.; Jin, L.; Li, G.Q. The compatible effects of RNA interference targeting laccase2 with biocontrol in Henosepilachna vigintioctopunctata. Entomol. Gen. 2023, 43, 117–126. [Google Scholar] [CrossRef]
- Telegina, T.A.; Vechtomova, Y.L.; Borzova, V.A.; Buglak, A.A. Tetrahydrobiopterin as a Trigger for Vitiligo: Phototransformation during UV Irradiation. Int. J. Mol. Sci. 2023, 24, 13586. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Park, J.O.; Tanner, L.; Nagano, Y.; Rabinowitz, J.D.; Shvartsman, S.Y. Energy budget of Drosophila embryogenesis. Curr. Biol. 2019, 29, R566–R567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.K.; Huang, R.X. Targeting of the respiratory chain by toxicants: Beyond the toxicities to mitochondrial morphology. Toxicol. Res. 2018, 7, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; Yuan, L.; Wu, C.; Liu, Y.J.; Jiang, F.L.; Hu, Y.J.; Liu, Y. Mitochondrial toxicity of organic arsenicals: Membrane permeability transition pore opening and respiratory dysfunction. Toxicol. Res. 2017, 7, 191–200. [Google Scholar] [CrossRef]
- Yu, B.; Ma, L.; Jin, J.; Jiang, F.; Zhou, G.; Yan, K.; Liu, Y. Mitochondrial toxicity induced by a thiourea gold(i) complex: Mitochondrial permeability transition and respiratory deficit. Toxicol. Res. 2018, 7, 1081–1090. [Google Scholar] [CrossRef]
- DiGregorio, P.J.; Ubersax, J.A.; O’Farrell, P.H. Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 2001, 276, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Alyokhin, A. Lethal and sublethal effects of mineral oil on potato pests. J. Econ. Entomol. 2018, 111, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Roczeń-Karczmarz, M.; Demkowska-Kutrzepa, M.; Zdybel, J.; Szczepaniak, K.; Studzińska, M.; Tomczuk, K. Comparison of the effectiveness of selected essential oils with mineral oil and spinosad on Dermanyssus gallinae. Pol. J. Vet. Sci. 2022, 25, 261–268. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.D.B.; Nie, X.; Singh, M. Comparison of mineral oil, insecticidal, and biopesticide spraying regimes for reducing spread of three potato virus Y strains in potato crops. Plant Dis. 2022, 106, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D. J and Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Bu, D.C.; Luo, H.T.; Huo, P.P.; Wang, Z.H.; Zhang, S.; He, Z.H. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-X.; Wu, Y.-K.; Liu, H.-H.; Li, W.-Z.; Jin, L.; Li, G.-Q. Comparative Transcriptome Analysis of Henosepilachna vigintioctomaculata Reveals Critical Pathways during Development. Int. J. Mol. Sci. 2024, 25, 7505. https://doi.org/10.3390/ijms25147505
Zhang Y-X, Wu Y-K, Liu H-H, Li W-Z, Jin L, Li G-Q. Comparative Transcriptome Analysis of Henosepilachna vigintioctomaculata Reveals Critical Pathways during Development. International Journal of Molecular Sciences. 2024; 25(14):7505. https://doi.org/10.3390/ijms25147505
Chicago/Turabian StyleZhang, Yu-Xing, Yi-Kuan Wu, Hai-Hui Liu, Wen-Ze Li, Lin Jin, and Guo-Qing Li. 2024. "Comparative Transcriptome Analysis of Henosepilachna vigintioctomaculata Reveals Critical Pathways during Development" International Journal of Molecular Sciences 25, no. 14: 7505. https://doi.org/10.3390/ijms25147505




