Abstract
Brassinosteroids (BRs) are an important group of polyhydroxylated naturally occurring steroidal phytohormones found in the plant kingdom in extremely low amounts. Due to the low concentrations in which these compounds are found, much effort has been dedicated to synthesizing these compounds or their structural analogs using natural and abundant sterols. In this work, we report the synthesis of new brassinosteroid analogs obtained from hyodeoxycholic acid, with a 3,6 dioxo function, 24-Nor-22(S)-hydroxy side chain and p-substituted benzoate function at C-23. The plant growth activities of these compounds were evaluated by two different bioassays: rice lamina inclination test (RLIT) and BSI. The results show that BRs’ analog with p-Br (compound 41f) in the aromatic ring was the most active at 1 × 10−8 M in the RLIT and BSI assays. These results are discussed in terms of the chemical structure and nature of benzoate substituents at the para position. Electron-withdrawing and size effects seems to be the most important factor in determining activities in the RLIT assay. These results could be useful to propose a new structural requirement for bioactivity in brassinosteroid analogs.
1. Introduction
Brassinosteroids (BRs) are natural polyhydroxysteroid compounds that have been recognized as a different class of plant hormones [1,2]. BRs were first described in the 1970s when Mitchell et al. reported the promotion of stem elongation and cell division by treatment with organic extracts of rapeseed (Brassica napus) pollen [3]. In 1979, brassinolide (1) (Figure 1) was isolated from Brassica napus pollen [3,4], and it has been recognized as the most active BR out of 70 natural occurring BRs isolated so far [5]. In Figure 1, the chemical structures of the most common and active natural BRs are shown, namely, brassinolide, 24-epibrasinolide (2), castasterone (3) and 24-epicastasterone (4). It is currently known that BRs regulate plant growth and development by producing an array of physiological changes and eliciting very important functions, such as plant growth regulation and cell division and differentiation in young tissues of growing plants [6,7,8,9]. BRs also play an important role in molecular and physiological responses in plant growth and abiotic stress such as drought, salinity, high temperature, low temperature, and heavy metal stresses [7,8,9].
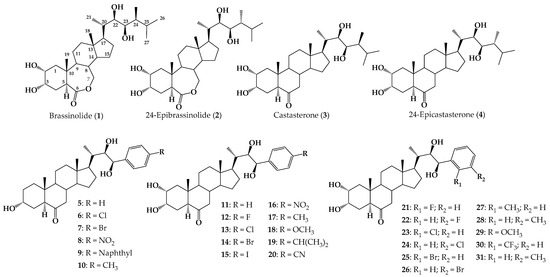
Figure 1.
Structure of natural occurring BRs 1–4, and synthetic arylbrassinosteroid analogs 5–31.
These compounds are found at extremely low concentrations in nature, and therefore much effort has been dedicated to synthesizing these compounds or their structural analogs using natural and accessible sterols [1]. In this task, side chain design remains the main synthetic challenge [10]. In that direction, an efficient method for construction of 23-arylbrassinosteroidal side chains was described via Heck coupling and asymmetric dihydroxylation as key steps, and several (22R,23R)-23-arylbrassinosteroids (compounds 5–10, Figure 1) were synthesized [11]. Biological activity of these compounds was evaluated using the rice lamina inclination test (RLIT) bioassay, and analog 5 was as active as 24-epibrassinolide [12].
A series of 23-p-substituted phenyl BRs analogs (compounds 11–20, Figure 1) was designed by molecular docking and synthesized using the Horner–Wadsworth––Emmons (HWE) reaction [13]. These analogs were tested in several biological assays, in which compounds 11 and 12 were the most active [13]. The series 21–31 comprises phenyl brassinosteroid analogs that were prepared via alkene cross-metathesis, and all of them were docked into the active site of BRI1 using AutoDock Vina. Plant growth-promoting activity was measured using the bean second internode (BSI) and Arabidopsis root sensitivity assays and compared with 2 [14]. The results indicate that phenyl substitution with a F atom at ortho or Cl atom at meta position generates the most active compounds of this series (21 and 24), whereas substitution with methyl or trifluoromethyl groups causes a significant decrease in or complete loss of plant activity [14].
In the same context, our research group reported the synthesis and biological evaluation of 24-norcholane type BR analogs, with 22(S)-hydroxy and benzoate functions at the C-23 position of the side chain (compounds 32–35, Figure 2) [15,16,17]. Analog 32 exhibited similar activity behavior to 1, which was used as a positive control in the RLIT, at all tested concentrations [18]. Additionally, the synthesis and biological activity of BR analogs with benzoate groups at the C-22 position, substituted with a F atom at the “ortho” or “para” positions, (37 and 38, Figure 2) were described [19]. Plant growth-promoting activities were evaluated by using RLIT and BSI biotests and the obtained results indicate that compound 38 (F atom in para position) is the most active BR analog, and in some cases was even more active than 1. Molecular docking results show that analogs with a fluorine-substituted aromatic ring in the side chain adopt an orientation that is similar to that predicted for brassinolide [19].
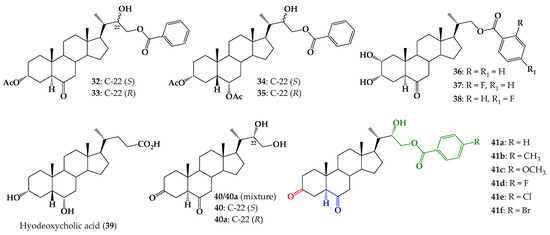
Figure 2.
Structure of 24-norcholane type BR analogs with benzoate function at C-23 (compounds 32–35), 23,24-bisnorcholanic analogs (compounds 36–38), hyodeoxycholic acid, precursor mixture 40/40a and new analogs 3,6-dioxo with benzoate function at C-23 (compounds 41a–f).
Considering the important effect on biological activity produced by incorporating substituted aromatic rings in the side chain, herein the synthesis of 24-norcholane type BR analogs with 3,6-dioxo function and different substituents at the “para” position of the aromatic ring (compounds 41a–f, Figure 2) is described.
The idea of attaching electron-donating and electron-withdrawing substituents to the aromatic ring is to evaluate the effect of electron density and substituents size on biological activity, as reported by other authors [13,14]. All obtained compounds were characterized by IR, HRMS, and 1D and 2D NMR spectroscopic techniques. Additionally, these compounds were evaluated for hormonal activity by plant bioassays RLIT and BSI. The obtained results could be important to propose a new structural requirement for BR analog bioactivity.
2. Results and Discussion
2.1. Chemical Synthesis
Commercially available hyodeoxycholic acid (39, Figure 2) (AK Scientific Inc., Union City, CA, USA) was used as starting material for preparation of new BR analogs, 41a–f. The synthesis route developed is shown in Figure 3.
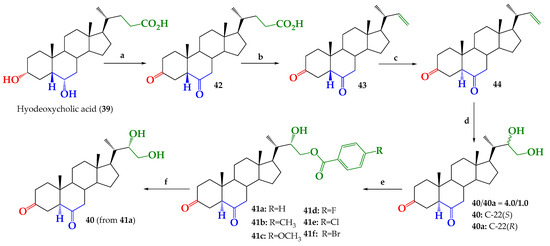
Figure 3.
Synthesis of compounds 40, 42–44, epimeric mixture 40/40a and new BR analogs 41a–f. Conditions: a. Jones ((CrO3/H2SO4/(CH3)2CO), r.t. 4 h, 97.0%. b. Pb(OAc)4/Cu(OAc)2/C5H5N/C6H6, reflux, 4 h, 42.7%. c. HCl 2.5%, CH3OH, reflux, 2 h, 94.0%. d. Upjohn dihydroxylation (OsO4/NMMO/(CH3)2CO), r.t, 36 h, 64.3%. e. 4-R-PhCOCl/CH2Cl2/C5H5N/DMAP, 0–5 °C, 6 h, C.C separation and subsequent crystallization 41a: 25.9%; 41b: 8.4%; 41c: 30.6%; 41d: 23.3%; 41e: 19.9%; 41f: 14.9%. f. K2CO3/MeOH/reflux, 3 h, 36.2%.
In a first step, full Jones oxidation of hyodeoxycholic acid gives dioxidized compound 42 with 97.0% yield, as reported for other bile acids [20]. The presence of both ketone groups is evidenced by 13C NMR spectrum (Figure S2, Supplementary Material), where the signals observed at δC = 210.92 and 208.76 ppm are assigned to carbons C-6 and C-3, respectively. Both signals were correlated and identified by combined 1D and 2D NMR experiments (Figures S1–S5, Supplementary Material). Oxidative decarboxylation of the side chain in compound 42, with the Pb(OAc)4/Cu(OAc)2 system and following a reported procedure [15,21,22,23], leads to olefin 43 with 42.7% yield. The formation of a terminal double bond at the C-22 position on the side chain was confirmed by 1H-NMR and 13C-NMR spectra. Namely, signals appearing at δH = 5.65 ppm (1H, ddd, J = 17.2, 10.1 and 8.5 Hz); 4.91 ppm (1H, dd, J = 17.2 and 1.9 Hz) and 4.83 ppm (1H, dd, J = 10.1 and 1.9 Hz) were assigned to H-22, Htrans-23 and Hcis-23, respectively. In the 13C NMR spectrum, signals observed at δC = 144.69 and 111.96 ppm were assigned to C-22 and C-23, respectively. The presence of a terminal double bond was confirmed by 13C DEPT-135, 2D HSQC and 2D HMBC NMR experiments (Figures S6–S10, Supplementary Material). All NMR spectroscopic data registered for compound 43 were consistent with that reported [24].
Isomerization from 5β to 5α on compounds 43 and 44 was performed under acidic conditions (HCl 2.5%, CH3OH, reflux) [25,26,27]). Isomerization was verified by analyzing changes in the chemical shift and shape of the H-5 signal, and the chemical shift of the C-19 signal, in the 1H and 13C NMR spectra, respectively. These data are summarized in Table 1.

Table 1.
Main spectroscopic differences observed in 1H and 13C NMR spectra of compounds 43 and 44 indicating isomerization from 5β to 5α.
Additionally, registered IR and NMR spectroscopic data (Figures S11–S15, Supplementary Material) were consistent with those reported [26].
Upjohn dihydroxylation (OsO4/NMMO/(CH3)2CO) of alkene 44 produces an epimeric mixture of 40 and 40a (Scheme 1) with 64.3% yield. The relative amount of epimers in this mixture was estimated as 40:40a = 4:1 ratio, by using relative integration areas of CH3-21 group signals for both compounds in the 1H NMR spectrum. The signal observed at δH = 0.962 ppm (d, J = 6.6 Hz, 3H) was assigned to CH3-21 in epimer 40 (C-22S), while the signal observed at δH = 0.923 ppm (d, J = 6.5 Hz, 3H) was assigned to CH3-21 in epimer 40a (C-22R). The corresponding integral values were 6.00 and 0.74, respectively, which give the relative integration ratio 6.00 (40):0.74 (40a) = 4:1 (Figure S17, Supplementary Material). The observed stereoselectivity of this reaction is in line with that reported for the dihydroxylation of terminal alkenes in steroids with similar structures [15,16,17,23,24,27,28]. Additionally, the presence of glycol function in C22–C23 and full structure was confirmed by 13C, 13C DEPT-135, 2D HSQC and 2D HMBC NMR experiments (Figures S18–S21, Supplementary Material).
Selective benzoylation of primary alcohol at C-23 of epimeric mixture 40/40a with the corresponding benzoyl chlorides, according to a reported protocol [15,16,17], followed by CC of reaction products, and subsequent crystallization purification, gives compounds 41a–f with 25.9%; 8.4%; 30.6%; 23.3%; 19.9% and 14.9% yields, respectively (Scheme 1). Since the mixture was benzoylated, in none of these cases was it possible to isolate the benzoylated epimers 22(R). Presumably, this is due to epimer 40a, which is a very minor product, being lost in the purification process. However, pure glycol 40 was obtained with 36.2% yield from saponification reaction of benzoate 41a with the K2CO3/CH3OH system in reflux, according to the described procedure [16]. Finally, compounds 40 and 41a-f, were fully characterized by IR, 1D, 2D NMR and HRMS spectroscopic techniques (Figures S22–S63, Supplementary Material).
2.2. Biological Activity
2.2.1. Bioactivity in the Rice Lamina Inclination Test (RLIT)
Bioactivity in all compounds was evaluated by using RLIT, a highly sensitive and specific assay for BRs, and one of the most widely used methods worldwide. Briefly, the bending angles between the leaf and sheath of rice-growing plants are measured in the presence of exogenously applied BR analogs. The results obtained at different concentrations of BR analogs and brassinolide, used as positive control, are given in Table 2.

Table 2.
Effect of different concentrations of brassinolide (1) and 24-norcholane BR type analogs on lamina inclination of rice seedlings: 3,6-dioxo-23-dihydroxy (40); 2,6-dioxo-22(S)-hydroxy-23-(4-substituted)-benzoate (41a–f).
The data in Table 2 show that benzoylation of primary alcohol at C-23 brings about significant changes in activity. These changes depend on the nature of para position substituent and the tested concentration. For example, at the lowest tested concentration, analogs with non-substituted benzoate (41a) or p-CH3-substituted (41b) show activities that are slightly lower than that observed for 40, whereas the analog with p-OCH3 is completely inactive (41c). On the other hand, the analogs carrying a benzoate group substituted with halogen atoms exhibit enhanced activities as compared to 40. From all these analogs, 41f (p-Br) is the most active, and its activities, at all tested concentrations, are higher than that observed for 40. Moreover, at 1 × 10−8 M, the activity of 41f is comparable to that observed for brassinolide (see also Figure S64, Supplementary Material). Thus, considering the nature of the substituent at the para position, the order of activities is 41f (p-Br) > 41d (p-F) = 41e (p-Cl) > 41a (p-H) = 41b (p-CH3) > 41c (p-OCH3).
2.2.2. Bioactivity in Bean Second Internode Bioassay
All compounds were tested using the BSI bioassay as well, which has also been used to detect biological activity in phytohormones [29,30]. All BR analogs were applied at 1 × 10−8 M concentration according to a previously described procedure [19]. The values obtained from the bean second internode elongation test are summarized in Table 3, and Figure S65 (Supplementary Material).

Table 3.
Activity in bean second internode bioassay of brassinolide, 40, and BR analogs 41a–f, at 1 × 10−8 M.
From the data shown in Table 3, it can be observed that the analog with no benzoate function, 40, is more active than all the analogs with a benzoate group, except 41f. The activity of analogs in this bioassay can be arranged in descending order of biological activity: 41f (p-Br) > 41c (p-OCH3) ≈ 41e (p-Cl) > 41d (p-F) > 41a (p-H) > 41b (p-CH3).
Considering that the only structural difference between these analogs is in the nature of substituents at the para position, these results should be explained in terms of induction and/or steric effects that are not present in the unsubstituted analog 41a (p-H). In this context, it is known that halogen atoms exhibit similar electron-withdrawing inductive effects, whereas methyl and methoxy groups are electron-donating groups. On one hand, the results of the RLIT (Table 2) suggest that the activity of these molecules is enhanced by decreasing electron density on the aromatic ring. However, at the lowest tested concentration, the bending angle measured in the presence of 41f (p-Br) is almost double those measured for 41e (p-Cl) and 41d (p-F). Therefore, the p-Br atom effect on this activity may be attributed to both inductive and size effects of this atom. On the other hand, measuring the activity of BR analogs using the BSI bioassay indicate that substitution in the para position leads to completely different results. For example, analogs 41c (p-OCH3) and 41e (p-Cl), which are substituted with electron-donating and electron-withdrawing groups, respectively, exhibit almost the same activity. This result suggests that inductive effects play no role in the bioactive property determined by this assay.
Interestingly, the reported activities of analogs 37 and 38, which have a F atom-substituted benzoate group, are similar to those measured for 1 and at least twice those found for 41d [19]. The main structural difference between these analogs and 41d is in the presence of hydroxyl groups at C-2 and C-3 instead of the ketone group at C-3. Thus, these results indicate that the effect of the electronic density and size of the alkyl chain on biological activities depends on the entire BR structure, and that hydroxyl groups attached to ring A are essentials for BR activities. To verify this result, a series of analogs with a hydroxyl group at C-3 and similar substitutions in the benzoate group are currently under study. We hope that analyzing both sets of data will allow for us to establish a relationship between chemical structure and biological activity for these BR analogs.
It is worth emphasizing that the RLIT is the most widely used test and most of the proposed SARs are based on these data. However, it is also known that for the same BR, different activities can be obtained by using different bioassays. This occurs because BRs affect a variety of plant processes and consequently many plant responses can be obtained. Therefore, an SAR obtained by RLIT will probably not apply for BR activities in a BSI. For this reason, we used two different tests to evaluate the bioactivity in the synthesized analogs.
Finally, analog 41f was the most active analog in both assays, even though these bioassays measure completely different effects on plant growth. Therefore, an exploratory docking study in the binding site of crystallized protein BRI1-BAK1 was carried out. The results show that the binding energy and orientation adopted by docked analog 41f is like that found for brassinolide (Figures S66–S68, Supplementary Material). Similar results have been observed in previous reports for other analogs with aromatic ring substitutions [13,14,19].
3. Materials and Methods
3.1. General Chemicals and Methods
All reagents were purchased from commercial suppliers and used without further purification. Melting points were measured on an SMP3 apparatus (Stuart-Scientific, now Merck KGaA, Darmstadt, Germany) and are uncorrected. 1H-, 13C-, 13C DEPT-135, gs 2D HSQC and gs 2D HMBC NMR spectra were recorded in CDCl3 solutions, and are referenced to the residual peaks of CHCl3 at δ = 7.26 ppm and δ = 77.00 ppm for 1H and 13C, respectively, on an Avance Neo 400 Digital NMR spectrometer (Bruker, Rheinstetten, Germany) operating at 400.1 MHz for 1H and 100.6 MHz for 13C. Chemical shifts are reported in δ ppm and coupling constants (J) are given in Hz; multiplicities are reported as follows: singlet (s), doublet (d), broad doublet (bd), doublet of doublets (dd), doublet of triplets (dt), triplet (t), broad triplet (bt), quartet (q), doublet of quartet (dq), doublet of double doublets (ddd), triplet of triplets (tt) and multiplet (m). IR spectra were recorded as KBr disks in a FT-IR 6700 spectrometer (Nicolet, Thermo Scientific, San Jose, CA, USA) and frequencies are reported in cm−1. High-resolution mass spectra (HRMS-ESI) were recorded in a Bruker Daltonik. The analysis for the reaction products was performed with the following relevant parameters: dry temperature, 180 °C; nebulizer 0.4 Bar; dry gas, 4 L/min; and spray voltage, 4.5 kV in positive mode. The accurate mass measurements were performed at a resolving power of 140,000 FWHM at a range m/z 50–1300. For analytical TLC, silica gel 60 in a 0.25 mm layer was used and TLC spots were detected by heating after spraying with 25% H2SO4 in H2O. Chromatographic separations were carried out by conventional column on silica gel 60 (230–400 mesh) using hexane–EtOAc mixtures of increasing polarity. All organic extracts were dried over anhydrous magnesium sulfate and evaporated under reduced pressure, below 40 °C.
3.2. Synthesis
3.2.1. 3,6-Dioxo-5β-Cholan-24-Oic Acid (42)
A solution of hyodeoxycholic acid (39) (10.0 g, 25.47 mmol) in 800 mL of acetone was cooled in an ice-water bath at 10 °C with constant stirring. Then, 40 mL of Jones reagent solution (6.5 g CrO3, 6 mL H2SO4 and 18 mL H2O) was added. The reaction mixture was kept under constant stirring at 10 °C for 4 h. (TLC). Then, 80 [mL] of CH3OH was added to destroy the excess oxidizing agent. The resulting mixture was evaporated and diluted with water (400 mL), extracted with CH2Cl2 (300 mL) and washed with water (3 × 100 mL). The organic layer was dried over anhydrous MgSO4 and filtered, and the solvent was removed by distillation in a rotary evaporator. Compound 42 (9.7 g, 97% yield) was a colorless solid (m.p. = 164.4–164.7 °C). IRνmax (KBr, cm−1): 3432–2650 (OH); 2964 and 287 (C-H); 1717 (C=O); 1466 (CH2-); 1387 (CH3-); 1245 and 1167 (C-O). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 2.64 (1H, dd, J = 14.3 and 13.7 Hz, H-4α); 2.48 (1H, dd, J = 12.9 and 4.7 Hz, H-5); 0.955 (3H, s, H-19); 0.945 (3H, d, J = 6.7 Hz, H-21); 0.697 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 210.92 (C-6); 208.76 (C-3); 179.44 (C-24); 59.69 (C-5); 56.76 (C-14); 55.77 (C-17); 43.08 (C-13); 42.13 (C-7); 40.89 (C-9); 39.87 (C-4); 39.47 (C-12); 38.28 (C-10); 36.67 (C-20); 36.47 (C-1); 35.74 (C-2); 35.19 (C-8); 30.84 (C-23); 30.60 (C-22); 27.93 (C-16); 23.89 (C-15); 22.45 (C-19); 21.28 (C-21); 11.98 (C-18) (Figures S1–S5, Supplementary Material).
3.2.2. 24-Nor-5β-Chol-22-Ene-3,6-Dione (43)
To a solution of 42 (4.00 g, 10.30 mmol) in dry benzene (120 mL), Cu(OAc)2*H2O (400 mg, 2.0 mmol) and pyridine (2.5 mL) were added. Then, under reflux, Pb(OAc)4 (11.2 g, 12.63 mmol) was added in four portions at hourly intervals. After the addition was completed, the reaction was continued for 4 h. The end of the reaction was verified by TLC; then, the mixture was filtered, and the solvent was evaporated under reduced pressure. The crude was re-dissolved in AcOEt (60 mL) washed with saturated aqueous NaCl solution (3 × 60 mL) and chromatographed on silica gel with hexane–EtOAc mixtures of increasing polarity (19.8:0.2→15.8:4.2). Compound 43 (1.71 g, 42.7% yield) was obtained as a colorless solid, m.p. = 180.5–182.4 °C (AcOEt/hex.), (177.5–178.9 °C [25] and 197–200 °C [29]). IRνmax (KBr, cm−1): 3073 (CH=CH2); 2964, 2947, 2873 and 2855 (C-H); 1716 (C=O); 1693 (C=O); 1632 (C=C); 1466 (CH2-); 1382 (CH3-); 1245 and 1216 (C-O); 908 (CH=CH2). 1H NMR (400.1 MHz, CDCl3), δ (ppm) = 5.65 (1H, ddd, J = 17.2, 10.1 and 8.5 Hz, H-22); 4.91 (1H, dd, J = 17.2 and 1.9 Hz, Htrans-23); 4.83 (1H, dd, J = 10.1 and 1.9 Hz, Hcis-23); 2.64 (1H, dd, J = 14.7 and 13.2 Hz, H-4α); 2.47 (1H, dd, J = 12.5 and 4.9 Hz, H-5); 2.39 (1H, dd, J = 14.0 and 5.0 Hz, H-1α); 1.11 (1H, ddd, J = 12.1, 11.9 and 6.0 Hz, H-15); 1.04 (3H, d, J = 6.7 Hz, H-21); 0.954 (3H, s, H-19); 0.715 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3), δ (ppm) = 210.81 (C-6); 208.63 (C-3); 144.69 (C-22); 111.96 (C-23); 59.71 (C-5); 56.81 (C-14); 55.36 (C-17); 42.99 (C-13); 42.13 (C-7); 41.06 (C-20); 40.93 (C-9); 39.87 (C-4); 39.37 (C-12); 38.27 (C-10); 36.66 (C-8); 36.46 (C-1); 35.74 (C-2); 28.18 (C-16); 23.90 (C-15); 22.45 (C-19); 21.27 (C-11); 20.06 (C-21); 12.13 (C-18). IR, 1H and 13C NMR spectroscopic data were consistent with those reported [24] (Figures S6–S10, Supplementary Material).
3.2.3. 24-Nor-5α-Chol-22-Ene-3,6-Dione (44)
Compound 43 (2.00 g, 5.84 mmol) was dissolved in 195 mL of 2.5% v/v HCl-MeOH at reflux and constant agitation for 2 h. The end of the reaction was verified by TLC. The solvent was evaporated under reduced pressure, and the crude was re-dissolved in 60 mL of EtOAc. The organic layer was washed with a saturated solution of NaHCO3 (3 × 60 mL) and water (2 × 30 mL), dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure. The crude was re-dissolved in CH2Cl2 (5 mL) and chromatographed on silica gel with hexane–EtOAc mixtures of increasing polarity (9.8:0.2→4.0:6.0). Compound 44 (1.88 g, 94.0% yield) was obtained as a colorless solid, m.p. = 148.9–149.7 °C (AcOEt/hex.). IRνmax (KBr, cm−1): 3073 (CH=CH2); 2946, 2870 and 2866 (C-H); 1712 (C=O); 1638 (C=C); 1460 (CH2-); 1389 (CH3-); 1248 and 1216 (C-O); 906 (CH=CH2). 1H NMR (400.1 MHz, CDCl3), δ (ppm) = 5.65 (1H, ddd, J = 17.2, 10.2 and 8.5 Hz, H-22); 4.91 (1H, dd, J = 17.2 and 1.8 Hz, Htrans-23); 4.83 (1H, dd, J = 10.2 and 1.8 Hz, Hcis-23); 2.62–2.54 (2H, m, H-5 and H-2); 2.00 (1H, t, J = 13.0 Hz, H-7β); 1.43 (1H, ddd, J = 16.7, 12.7 and 3.7 Hz, H-11β); 1.10 (1H, ddd, J = 18.0, 11.7 and 5.9 Hz, H-15); 1.03 (d, J = 6.6 Hz, 3H, H-21); 0.953 (s, 3H, H-19); 0.712 (s, 3H, H-18). 13C NMR (100.6 MHz, CDCl3), δ (ppm) = 211.23 (C-3); 209.03 (C-6); 144.75 (C-22); 111.92 (C-23); 57.47 (C-5); 56.58 (C-14); 55.34 (C-9); 53.44 (C-17); 46.55 (C-7); 42.95 (C-13); 41.22 (C-10); 41.06 (C-20); 39.22 (C-12); 38.06 (C-1); 37.96 (C-8); 37.35 (C-2); 36.96 (C-4); 28.15 (C-16); 23.95 (C-15); 21.62 (C-11); 20.03 (C-21); 12.54 (C-19); 12.17 (C-18) (Figures S11–S15, Supplementary Material).
3.2.4. 22(S), 23-Dihydroxy-24-Nor-5α-Cholan-3,6-Dione (40) and 22(R), 23-Dihydroxy-24-Nor-5α-Cholan-3,6-Dione (40a)
To a solution of alkene 44 (2.50 g, 7.30 mmol) in acetone (150 mL), N-Methylmorpholine N-oxide (NMO) (0.45 g, 3.84 mmol) was added. Then, the mixture was homogenized by magnetic stirring and 2.0 mL of 4% OsO4 (0.210 mmol) was added dropwise with stirring for 36 h at room temperature. The end of the reaction was verified by TLC. Then, the solvent was removed (until a 25 mL approximate volume), and water (25 mL) and Na2S2O3.5H2O (25 mL saturated solution) were added. The organic layer was extracted with EtOAc (2 × 30 mL), washed with saturated NaHCO3 (3 × 60 mL) and water (2 × 20 mL), dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure. The crude was re-dissolved in CH2Cl2 (10 mL) and chromatographed on silica gel with hexane–EtOAc mixtures of increasing polarity (19.8:0.2→9.8:10.2). A mixture of 40/40a = 4.0/1.0 was obtained as a colorless solid (1.61 g, 64.3% yield).
3.2.5. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Substituted)-Benzoate-23-yl (41a–41f)
General procedure: A mixture of 40/40a was dissolved in pyridine (py, 15 mL). Later, 4-dimetilaminopiridina (DMAP) (40 mg) and p-PhCOCl were added with slow stirring at 5–10 °C. The end of the reaction was verified by TLC (3 h). Then, 3 mL of water (60 °C) was added and stirred for 20 min. The mixture was extracted with EtOAc (2 × 25 mL), and the organic layer was washed with 5% KHSO4 (2 × 5 mL) and water (2 × 10 mL), dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure. The crude was redissolved in CH2Cl2 (5 mL) and chromatographed on silica gel with hexane–EtOAc mixtures of increasing polarity.
3.2.6. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxobenzoate-23-yl (41a)
Mixture 40/40a (324.0 mg, 0.86 mmol), py (15 mL), DMAP (40 mg) and PhCOCl (392 μL, 2.79 mmol, d = 1.21 g/mL). Compound 41a (107 mg, 25.9% yield) was obtained as a colorless solid, m.p. = 206.4–207.8 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3446 (O-H); 2947 (CH3-); 2871 (CH2-); 1705 (C=O); 1454 (CH2-); 1274 and 1024 (C-O); 718 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 8.05 (2H, dd, J = 7.8 and 1.4 Hz, 2H, H-2’ and H-6’); 7.58 (1H, t, J = 7.4 Hz, H-4’); 7.46 (2H, t, J = 7.8 Hz, H-3’ and H-5’); 4.49 (1H, dd, J = 11.5 and 1.9 Hz, H-23a); 4.20 (1H, dd, J = 11.5 and 9.1 Hz, H-23b); 4.06 (1H, ddd, J = 9.1, 3.6 and 1.9 Hz, H-22); 2.63–2.54 (2H, m, H-5 and H-2); 2.01 (1H, t, J = 12.6 Hz, H-7β); 1.18 (1H, ddd, J = 12.5, 12.0 and 6.0 Hz, H-15); 1.06 (d, J = 6.8 Hz, 3H, H-21); 0.959 (s, 3H, H-19); 0.732 (s, 3H, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.20 (C-3); 208.92 (C-6); 167.00 (Ar-CO2); 133.25 (C-4’); 129.80 (C-1’); 129.62 (C-2’ and C-6’); 128.45 (C-3’ and C-5’); 71.74 (C-22); 66.35 (C-23); 57.44 (C-5); 56.19 (C-14); 53.34 (C-9); 52.83 (C-17); 46.50 (C-7); 43.42 (C-13); 41.17 (C-10); 40.27 (C-20); 39.27 (C-12); 38.01 (C-1); 37.95 (C-8); 37.33 (C-2); 36.94 (C-4); 27.39 (C-16); 24.05 (C-15); 21.61 (C-11); 12.89 (C-21); 12.53 (C-19); 11.80 (C-18) (Figures S27–S31, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C30H40O5: 481.2949 [M + H]+; found 481.2918 [M + H]+ (Figure S58, Supplementary Material).
3.2.7. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Methyl)-Benzoate-23-yl (41b)
Mixture 40/40a (200 mg, 0.53 mmol), py (10 mL), DMAP (70 mg) and p-CH3-PhCOCl (287 μL, 0.68 mmol, d = 1.17 g/mL). Compound 41b (22.1 mg, 8.4% yield) was obtained as a colorless solid, m.p. = 217.9–219.3 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3446 (O-H); 2950 (CH3-); 2905 (CH2-); 2870 (CH-); 1720 (C=O); 1705 (C=O); 1609 (C=C); 1391 (CH3-); 1277 and 1095 (C-O); 757 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 7.95 (2H, d, J = 8.1 Hz, H-2’ and H-6’); 7.27 (2H, d, J = 8.1 Hz, H-3’ and H-5’); 4.49 (1H, dd, J = 11.5 and 1.7 Hz, H-23a); 4.20 (1H, dd, J = 11.5 and 9.1 Hz, H-23b); 4.08–4.05 (1H, m, H-22); 2.65–2.56 (2H, m, H-5 and H-2); 2.44 (3H, s, CH3-Ar); 2.03 (1H, t, J = 12.8 Hz, H-7β); 1.19 (1H, ddd, J = 12.8, 11.7 and 5.7 Hz, H-15); 1.08 (3H, d, J = 6.8 Hz, H-21); 0.978 (3H, s, H-19); 0.750 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.18 (C-3); 208.90 (C-6); 166.06 (Ar-CO2); 143.97 (C-4’); 129.64 (C-3’ and C-5’); 129.14 (C-2’ and C-6’); 127.04 (C-1’); 71.72 (C-22); 66.17 (C-23); 57.42 (C-5); 56.18 (C-14); 53.34 (C-9); 52.81 (C-17); 46.48 (C-7); 43.40 (C-13); 41.15 (C-10); 40.23 (C-20); 39.25 (C-12); 37.99 (C-1); 37.94 (C-8); 37.31 (C-2); 36.92 (C-4); 27.37 (C-16); 24.04 (C-15); 21.65 (CH3-Ar); 21.60 (C-11); 12.88 (C-21); 12.52 (C-19); 11.79 (C-18) (Figures S32–S36, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C31H42O5: 495.3105 [M + H]+; found 495.3119 [M + H]+ (Figure S59, Supplementary Material).
3.2.8. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Methoxy)-Benzoate-23-yl (41c)
Mixture 40/40a (211.3 mg, 0.56 mmol), py (10 mL), DMAP (45 mg) and p-CH3O-PhCOCl (373 mg, 0.73 mmol). Compound 41c (87.7 mg, 30.6% yield) was obtained as a colorless solid, m.p. = 202.3–204.2 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3490 (O-H); 2967 (CH3-); 2890 (CH2-); 1707 (C=O); 1607 (C=C); 1514 (C=C); 1425 (CH2-); 1257 and 1075 (C-O); 771 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 7.99 (2H, d, J = 8.9 Hz, H-2’ and H-6’); 6.91 (2H, d, J = 8.9 Hz, H-3’ and H-5’); 4.45 (1H, dd, J = 11.5 and 1.9 Hz, H-23a); 4.15 (1H, dd, J = 11.5 and 9.1 Hz, H-23b); 4.04–4.00 (1H, m, H-22); 3.85 (3H, s, OCH3); 2.62–2.53 (2H, m, H-5 and H-2); 2.00 (1H, t, J = 13.1 Hz, H-7β); 1.16 (1H, ddd, J = 12.3, 11.5 and 5.7 Hz, H-15); 1.04 (3H, d, J = 6.9 Hz, H-21); 0.943 (3H, s, H-19); 0.715 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.19 (C-3); 208.91 (C-6); 166.73 (Ar-CO2); 163.53 (C-4’); 131.66 (C-2’ and C-6’); 122.14 (C-1’); 113.65 (C-3’ and C-5’); 71.74 (C-22); 66.05 (C-23); 57.39 (C-5); 56.15 (C-14); 55.42 (C-9); 53.31 (C-17); 52.79 (C-17); 46.46 (C-7); 43.38 (C-13); 41.14 (C-10); 40.23 (C-20); 39.23 (C-12); 37.97 (C-1); 37.92 (C-8); 37.29 (C-2); 36.90 (C-4); 27.36 (C-16); 24.03 (C-15); 21.58 (C-11); 12.87 (C-21); 12.50 (C-19); 11.77 (C-18) (Figures S37–S41, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C31H42O6: 511.3054 [M + H]+; found 511.3071 [M + H]+ (Figure S60, Supplementary Material).
3.2.9. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Fluoro)-Benzoate-23-yl (41d)
Mixture 40/40a (160.6 mg, 0.43 mmol), py (10 mL), DMAP (50 mg) and p-F-PhCOCl (160 μL, 1.35 mmol, d = 1.34 g/mL). Compound 41d (49.6 mg, 23.3% yield) was obtained as a colorless solid, m.p. = 189.3–190.1 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3505 (O-H); 2967 (CH3-); 2867 (CH2-); 1716 (C=O); 1600 (C=C); 1508 (C=C); 1455 (CH2-); 1380 (CH3-); 1287 and 1071 (C-O); 768 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 8.06 (2H, dd, J = 8.7 and 5.4 Hz, H-2’ and H-6’); 7.12 (2H, t, J = 8.7 Hz, H-3’ and H-5’); 4.47 (1H, dd, J = 11.4 and 1.9 Hz, H-23a); 4.18 (1H, dd, J = 11.3 and 9.1 Hz, H-23b); 4.05–4.02 (1H, m, H-22); 2.62–2.53 (2H, m, H-5 and H-2); 2.00 (1H, t, J = 12.7 Hz, H-7β); 1.17 (1H, ddd, J = 12.1, 11.5 and 5.8 Hz, H-15); 1.05 (3H, d, J = 7.0 Hz, H-21); 0.951 (3H, s, H-19); 0.723 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.18 (C-3); 208.88 (C-6); 166.01 (Ar-CO2); 165.89 (d, 1JCF = 255.0 Hz, C-4’); 132.20 (d, 3JCF = 9.3 Hz, C-2’ and C-6’); 126.05 (d, 4JCF = 2.7 Hz, C-1’); 115.61 (d, 2JCF = 22.0 Hz, C-3’ and C-5’); 71.69 (C-22); 66.45 (C-23); 57.2 (C-5); 56.17 (C-14); 53.33 (C-9); 52.82 (C-17); 46.47 (C-7); 43.41 (C-13); 41.15 (C-10); 40.35 (C-20); 39.25 (C-12); 37.99 (C-1); 37.93 (C-8); 37.31 (C-2); 36.91 (C-4); 27.37 (C-16); 24.04 (C-15); 21.60 (C-11); 12.87 (C-21); 12.52 (C-19); 11.79 (C-18) (Figures S42–S46, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C30H39FO5: 499.2854 [M + H]+; found 499.2839 [M + H]+ (Figure S61, Supplementary Material).
3.2.10. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Chloro)-Benzoate-23-yl (41e)
Mixture 40/40a (120.0 mg, 0.32 mmol), py (10 mL), DMAP (35 mg) and p-Cl-PhCOCl (485 μL, 1.28 mmol, d = 1.36 g/mL). Compound 41e (32.7 mg, 19.9% yield) was obtained as a colorless solid, m.p. = 196.9–198.8 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3466 (O-H); 2970 (CH3-); 2869 (CH2-); 1709 (C=O); 1596 (C=C); 1487 (CH2-); 1281 and 1016 (C-O); 763 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 8.00 (2H, d, J = 8.8 Hz, H-2’ and H-6’); 7.45 (2H, d, J = 8.8 Hz, H-3’ and H-5’); 4.50 (1H, dd, J = 11.6 and 2.1 Hz, H-23a); 4.20 (1H, dd, J = 11.6 and 9.2 Hz, H-23b); 4.07 (1H, ddd, J = 9.1, 3.8 and 2.1 Hz, H-22); 2.68–2.57 (2H, m, H-5 and H-2); 2.04 (1H, t, J = 12.8 Hz, H-7β); 1.20 (1H, ddd, J = 12.5, 11.7 and 5.8 Hz, H-15); 1.08 (3H, d, J = 7.0 Hz, H-21); 0.985 (3H, s, H-19); 0.756 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.19 (C-3); 208.88 (C-6); 166.12 (Ar-CO2); 139.70 (C-4’); 131.01 (C-2’ and C-6’); 128.25 (C-3’ and C-5’); 128.25 (C-1’); 71.66 (C-22); 66.55 (C-23); 57.43 (C-5); 56.18 (C-14); 53.34 (C-9); 52.83 (C-17); 46.48 (C-7); 43.41 (C-13); 41.16 (C-10); 40.38 (C-20); 39.26 (C-12); 38.00 (C-1); 37.93 (C-8); 37.31 (C-2); 36.92 (C-4); 27.38 (C-16); 24.04 (C-15); 21.60 (C-11); 12.87 (C-21); 12.52 (C-19); 11.80 (C-18) (Figures S47–S51, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C30H39ClO5: 515.2559 [M + H]+; found 515.2538 [M + H]+ (Figure S62, Supplementary Material).
3.2.11. 22(S)-Hydroxy-24-Nor-5α-Cholan-3,6-Dioxo-(4-Bromo)-Benzoate-23-yl (41f)
Mixture 40/40a (206.0 mg, 0.55 mmol), py (10 mL), DMAP (62.8 mg) and p-Br-PhCOCl (921 mg, 1.65 mmol). Compound 41f (45.5 mg, 14.9% yield) was obtained as a colorless solid, m.p. = 206.1–208.0 °C (EtOAc/hex). IRνmax (KBr, cm−1): 3482 (O-H); 2957 (CH3-); 2890 (CH2-); 1722 (C=O); 1690 (C=O); 1591 (C=C); 1462 (CH2-); 1398 (CH3-); 1272 and 1071 (C-O); 760 (Ar-H). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 7.91 (2H, d, J = 8.5 Hz, H-2’ and H-6’); 7.59 (2H, d, J = 8.5 Hz, H-3’ and H-5’); 4.48 (1H, dd, J = 11.7 and 1.9 Hz, H-23a); 4.19 (1H, dd, J = 11.7 and 9.1 Hz, H-23b); 4.04 (1H, ddd, J = 8.9, 3.4 and 2.5 Hz, H-22); 2.63–2.55 (2H, m, H-5 and H-2); 2.01 (1H, t, J = 12.5 Hz, H-7β); 1.18 (1H, ddd, J = 12.1, 11.6 and 5.8 Hz, H-15); 1.05 (3H, d, J = 6.9 Hz, H-21); 0.959 (3H, s, H-19); 0.730 (s, 3H, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.19 (C-3); 208.88 (C-6); 166.28 (Ar-CO2); 131.81 (C-2’ and C-6’); 131.14 (C-3’ and C-5’); 128.71 (C-1’); 128.41 (C-4’); 71.68 (C-22); 66.58 (C-23); 57.44 (C-5); 56.19 (C-14); 53.36 (C-9); 52.84 (C-17); 46.49 (C-7); 43.43 (C-13); 41.17 (C-10); 40.39 (C-20); 39.27 (C-12); 38.02 (C-1); 37.94 (C-8); 37.32 (C-2); 36.93 (C-4); 27.39 (C-16); 24.04 (C-15); 21.61 (C-11); 12.88 (C-21); 12.54 (C-19); 11.81 (C-18) (Figures S52–S56, Supplementary material). HRMS-ESI (positive mode): m/z calculated for C30H39BrO5: 561.2038 [M + H]+; found 561.1994 [M + H]+ (Figure S63, Supplementary material).
3.2.12. 22(S), 23-Dihydroxy-24-Nor-5α-Cholan-3,6-Dione (40) from 41a
To a solution of benzoate 41a (60 mg, 0.13 mmol) in methanol (20 mL) and water (10 mL), K2CO3 (63.2 mg, 0.46 mmol) was added. Then the mixture was homogenized by magnetic stirring and reflux for 3 h (TLC). The reaction mixture was cooled in an ice-water bath (0–5 °C) then, 50 mL HCl (5% w/w) were added until the formation of a white precipitate. The organic layer was extracted with EtOAc (2 × 60 mL), washed with water (2 × 50 mL), dried over MgSO4, and filtered. The solvent was evaporated under reduced pressure. The crude was re-dissolved in CH2Cl2 (10 mL) and chromatographed on silica gel with hexane–EtOAc (3:7 ratio). Compound 40 (17 mg, 36.2% yield) was obtained as a colorless solid, m.p. = 226.3–227.2 °C (ether/MeOH). IRνmax (KBr, cm−1): 3510 (O-H); 2968 (CH3-); 2935 (CH2-); 2865 (CH-); 1705 (C=O); 1428 (CH2-); 1381 (CH3-); 1264 and 1064 (C-O). 1H NMR (400.1 MHz, CDCl3): δ (ppm) = 3.81 (1H, dt, J = 9.6 and 3.1 Hz, H-22); 3.65 (1H, dd, J = 11.3 and 2.3 Hz, H-23a); 3.54 (1H, dd, J = 11.3 and 9.6 H, H-23b); 2.63–2.54 (2H, m, H-5 and H-2); 2.00 (1H, t, J = 12.4 Hz, H-7β); 0.965 (3H, d, J = 6.8 Hz, H-21); 0.956 (3H, s, H-19); 0.706 (3H, s, H-18). 13C NMR (100.6 MHz, CDCl3): δ (ppm) = 211.24 (C-3); 208.92 (C-6); 73.82 (C-22); 66.48 (C-23); 57.55 (C-5); 56.20 (C-14); 53.43 (C-9); 52.81 (C-17); 46.58 (C-7); 43.44 (C-13); 41.22 (C-10); 40.01 (C-20); 39.31 (C-12); 38.10 (C-1); 38.00 (C-8); 37.37 (C-2); 36.99 (C-4); 27.39 (C-16); 24.11 (C-15); 21.67 (C-11); 13.09 (C-21); 12.58 (C-19); 11.78 (C-18) (Figures S22–S26, Supplementary Material). HRMS-ESI (positive mode): m/z calculated for C23H36O4: 377.2686 [M + H]+; found 377.2672 [M + H]+ (Figure S57, Supplementary Material).
3.3. Biological Activity
3.3.1. Bioactivity in the Rice Lamina Inclination Test (RLIT)
The bioactivity of BR analogs (40, and 41a–41f) was evaluated by RLIT [31] and using a modified procedure previously described [19]. Seeds of a local rice cultivar (Oryza sativa L.) of the Zafiro variety (provided by INIA-QUILAMAPU, Chillán, Ñuble, Chile) were sterilized, sown and grown for about 10 days in pots with substrate, maintained at 22 °C, with a 16 h light/8 h dark photoperiod, and 50–60% relative humidity in a plant growth chamber. Once the rice plants presented the second internode of the lamina, an 8 cm segment was cut. These segments were then placed in a Petri dish containing sterile distilled water (60 mL) with the amounts of BR analogs required to reach the treatment concentration (1 × 10−8; 1 × 10−7 and 1 × 10−6 M). Brassinolide (APExBIO) at the same tested concentrations was used as positive control, whereas the negative control was an aqueous solution with the same volume of dimethyl sulfoxide (DMSO, 1%) used to dilute the BR analogs. All treatments were incubated for 72 h at 25 °C in the dark to finally measure the angle of inclination in the unrolled sheet between the leaf and the sheath. Each treatment consisted of 8 independent replicates. The effect produced in the negative control was subtracted from each treatment. Mean values with at least significant difference (p < 0.05; Student’s t-test) were considered using Excel software (Microsoft Corporation, (Microsoft Corporation, WA, USA)). Images were taken with a Leica EZ4HD stereo microscope with camera software LAS EZ (Leica Microsystems, Wetzlar, Alemania).
3.3.2. Bean Second Internode Bioassay
The bean second internode test for compounds 40 and 41a–41f was carried out using a previously reported procedure [32], with some modifications [18]. Bean seeds (Phaseolus vulgaris L., cv. Pinto) were sown and germinated for three days, and subsequently were transplanted into pots containing perlite, vermiculite and substrate. The pots were kept in a plant growth chamber at 22 °C, with 48 W/m2 light at a 16 h/8 h light/dark photoperiod. When the second internode reached 1–2 mm long, the bean plants were treated with tested compounds dissolved in DMSO (1%) and water at 1 × 10−8 M concentration, by adding them into a small scar generated once the bract was removed from the base of the second internode. At the time of application, a 5 µL drop of each solution was mixed with a 2 µL drop of TWEEN® 20 (AMRESCO®) for adhesion. Control plants were treated with water and TWEEN® 20 only. Measurements of second internode length were made after 5 days. The difference between the length of the second internode of treated and control plants was used as a measure of activity. Mean values with at least significant difference (p < 0.05; Student’s t-test) were considered using Excel software 2021 (Microsoft Corporation, WA, USA).
3.3.3. Molecular Docking Study
The docking procedure involved positioning brassinolide (1) and synthetic compound 41f within the active site of the crystallized protein BRI1-BAK1 (PDB: 4m7e) using the AutoDock Vina program. The protocol used to carry out this study was reported previously [19].
4. Conclusions
Herein, a short synthesis route (five steps) was developed, which, starting from hyodeoxycholic acid (39), allowed for obtaining compound 40 and five new brassinosteroid analogs with 3,6 dioxo function, 24-Nor-22(S)-hydroxy side chain and p-substituted benzoate function at C-23 (compounds 41a–f). This effort is part of a more general task in which the final goal is to establish a relationship between the chemical structure of BR analogs and their biological activity. With this aim, synthesized BR analogs were tested for activity using two different assays, namely, the rice lamina inclination test and the bean second-node bioassay, and the results indicate that compounds 40 and 41f are the more active analogs at 1 × 10−8 M concentration. RLIT activities were correlated with inductive and size effects of substituents at the para position in the benzoate group. A comparison of these results with those previously obtained for 37 and 38 seems to indicate that the effect of the electronic density and size of the alkyl chain on biological activities depends on the entire BR structure, and that hydroxyl groups attached to ring A are essentials for BR activities. More data are needed to verify this conclusion before it can be used as a structural requirement for bioactivity in brassinosteroid analogs.
On the other hand, much more work is needed to establish a relationship between data obtained by using different bioassays. It is also necessary to find a way to define which bioassay gives the best indication of BR activities in each application.
Finally, it has become common to explain BR activities by molecular docking studies in which new molecules are docked to the binding site of natural brassinolide on Arabidopsis Thaliana. In light of these results, it is unclear how docking studies can explain BR activities occurring by different mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25147515/s1.
Author Contributions
Conceptualization, L.E.-C., S.J., M.N., M.A.C., M.S. and K.D.; methodology, L.E.-C., S.J. and M.N., synthesis and compounds structural determination.; K.D. development of biological assay methodologies and results discussion; L.E.-C., S.J., K.D. and A.F.O.; investigation, L.E.-C., K.D., S.J., M.S. and A.F.O.; writing—original draft preparation, M.S. docking studies and results discussion.; K.D. and A.F.O.; writing—review and editing, L.E.-C., project administration, L.E.-C.; funding acquisition, L.E.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico) (grant no. 1191330 and no. 1231502) and FONDEQUIP (Fondo de Equipamiento Científico y Tecnológico, grant no. EQM190025 and EQM200241).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is available at Supplementary Material.
Acknowledgments
Sebastián Jorquera, thanks Dirección de Postgrado y Programas de la Universidad Técnica Federico Santa María and Programa de Iniciación a la Investigación Científica (PIIC) N°056/2021.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khripach, V.A.; Zhabinskii, V.; de Groot, A.E. Brassinosteroids. A New Class of Plant Hormones; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Khripach, V.; Zhabinskii, V.; de Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D.; Steffens, G.L.; Flippenanderson, J.L.; Cook, J.C. Brassinolide, A Plant Growth-Promoting Steroid Isolated from Brassica-Napus Pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Bajguz, A. Metabolism of brassinosteroids in plants. Plant Physiol. Biochem. 2007, 45, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Brassinosteroids—Occurence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, L.; Tian, W.; Zhou, W. Methods for Construction of Sidechain of Brassinosteroids and Application to Syntheses of Brassinosteroids. In Structure and Chemistry (Part E); Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 245–287. [Google Scholar]
- Huang, L.F.; Zhou, W.S. Studies on Steroidal Plant-Growth Regulators. Part 33. Novel Method for Construction of the Side-Chain of 23-Arylbrassinosteroids Via Heck Arylation and Asymmetric Dihydroxylation As Key Steps. J. Chem. Soc. Perkin Trans. 1994, 1, 3579–3585. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Luo, W.-H.; Xu, R.-J.; Zhao, Y.-J.; Zhou, W.-S.; Huang, L.-F.; Shen, J.-M. Biological Activity of Brassinosteroids and Relationship of Structure to Plant Growth Promoting Effects. Chin. Sci. Bull. 1994, 39, 1573–1577. [Google Scholar]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rárová, L.; Korinkova, P.; Mikulík, J.; Budesinsky, M.; Béres, T.; Berka, K.; Lu, Q.; et al. Design, synthesis and biological activities of new brassinosteroid analogues with a phenyl group in the side chain. Org. Biomol. Chem. 2016, 14, 8691–8701. [Google Scholar] [CrossRef]
- Korinkova, P.; Bazgier, V.; Oklestkova, J.; Rarova, L.; Strnad, M.; Kvasnica, M. Synthesis of novel aryl brassinosteroids through alkene cross-metathesis and preliminary biological study. Steroids 2017, 127, 46–55. [Google Scholar] [CrossRef]
- Carvajal, R.; Gonzalez, C.; Olea, A.F.; Fuentealba, M.; Espinoza, L. Synthesis of 2-Deoxybrassinosteroids Analogs with 24-nor, 22(S)-23-Dihydroxy-Type Side Chains from Hyodeoxycholic Acid. Molecules 2018, 23, 1306. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, J.; Aitken, V.; Gonzalez, C.; Ferrer, K.; Olea, A.F.; Parella, T.; Espinoza, L. Synthesis and structural determination of new brassinosteroid 24-nor-5α-cholane type analogs. Molecules 2019, 24, 4612. [Google Scholar] [CrossRef]
- Soto, N.; Ferrer, K.; Díaz, K.; González, C.; Taborga, L.; Olea, A.F.; Carrasco, H.; Espinoza, L. Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain. Int. J. Mol. Sci. 2021, 22, 4808. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.; Espinoza, L.; Carvajal, R.; Conde-Gonzalez, M.; Niebla, V.; Olea, A.F.; Coll, Y. Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. Int. J. Mol. Sci. 2020, 21, 1832. [Google Scholar] [CrossRef] [PubMed]
- Aitken, V.; Diaz, K.; Soto, M.; Olea, A.F.; Cuellar, M.A.; Nuñez, M.; Espinoza-Catalán, L. New Brassinosteroid Analogs with 23,24-Dinorcholan Side Chain, and Benzoate Function at C-22: Synthesis, Assessment of Bioactivity on Plant Growth, and Molecular Docking Study. Int. J. Mol. Sci. 2024, 25, 419. [Google Scholar] [CrossRef]
- Leppik, R.A. Improved synthesis of 3-keto, 4-ene-3-keto, and 4,6-diene-3-keto bile acids. Steroids 1983, 41, 475–484. [Google Scholar] [CrossRef]
- Bacha, J.D.; Kochi, J.K. Alkenes from acids by oxidative decarboxylation. Tetrahedron 1968, 24, 2215–2216. [Google Scholar] [CrossRef]
- Vaydia, A.S.; Dixit, S.M.; Rao, A.S. Degradation of bile acid side chain with lead tetraacetate. Tetrahedron 1968, 50, 5173–5174. [Google Scholar] [CrossRef]
- Soto, N.; González, C.; Mellado, M.; Olea, A.F.; Coll, Y.; Díaz, K.; Espinoza, L. Epimeric Mixtures of Brassinosteroid Analogs: Synthesis, Plant Growth, and Germination Effects in Tomato (Lycopersicum esculentum Mill.). Agronomy 2020, 10, 808. [Google Scholar] [CrossRef]
- Ferrer, K.; Díaz, K.; Kvasnica, M.; Olea, A.F.; Cuellar, M.; Espinoza, L. Synthesis of New Brassinosteroid 24-Norcholane Type Analogs Conjugated in C-3 with Benzoate Groups. Molecules 2021, 26, 1173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Zheng, L.T.; Shi, J.J.; Gao, B.; Chen, Y.K.; Yang, H.C.; Chen, H.L.; Li, Y.C.; Zhen, X.C. Synthesis of 5 alpha-cholestan-6-one derivatives and their inhibitory activities of NO production in activated microglia: Discovery of a novel neuroinflammation inhibitor. Bioorg. Med. Chem. Lett. 2014, 24, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.S.; Zhou, W.; Jiang, B.; Pan, X.F. Studies on steroidal plant-growth regulator IX: The preparation of 22R- and 22S-24, 25, 26, 27, 28-penta-nor-brassinolides. Acta Chim. Sin. 1989, 47, 1017–1021. [Google Scholar]
- Ishiguro, M.; Ikekawa, N. Stereochemistry of Electrophilic Reactions at the Steroidal C-22 Double Bond. Chem. Pharm. Bull. 1975, 23, 2860–2866. [Google Scholar] [CrossRef][Green Version]
- Tian, W.S.; Zhou, W.S. Studies on steroidal plant growth hormone VI. Conversion of methyl 3,6-diketoallocholanate into methyl Δ^2-6-ketoallocholenate. Acta Chim. Sin. 1988, 46, 824–826. [Google Scholar]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rarova, L.; Berka, K.; Strnad, M. Biological activities of new monohydroxylated brassinosteroid analogues with a carboxylic group in the side chain. Steroids 2014, 85, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Abón, A.; de Dios-Bravo, G.; Rodríguez-Sotres, R.; Iglesias-Arteaga, M.A. Synthesis and plant growth promoting activity of dinorcholanic lactones bearing the 5α-hydroxy-6-oxo moiety. J. Steroid Biochem. Mol. Biol. 2013, 134, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, H.; Jang, S. Rice Lamina Joint Inclination Assay. Bio-Protocol 2017, 7, e2409. [Google Scholar] [CrossRef]
- Slavikova, B.; Kohout, L.; Budesinsky, M.; Swaczynova, J.; Kasal, A. Brassinosteroids: Synthesis and Activity of Some Fluoro Analogues. J. Med. Chem. 2008, 51, 3979–3984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



