Molecular Lesions in BRI1 and Its Orthologs in the Plant Kingdom
Abstract
:1. Introduction
2. bri1 Alleles in Arabidopsis
2.1. A BR-Insensitive Mutant Shows Multiple Defects
2.2. Brassinosteroids Are Essential in Plant Development
2.3. An LRR Receptor-like Kinase Is Involved in BR Signaling Pathway
2.4. BRI1 Is Involved in BR Homeostasis
2.5. BRI1 Is Ubiquitously Expressed
2.6. Interaction between BR and GA in Plant Development
2.7. BRs Are Involved in Flowering Time in Arabidopsis
2.8. Is the Kinase Activity of BRI1 Essential for Its Function?
2.9. T-DNA Insertion bri1 Alleles
2.10. Intragenic Suppressor of bri1-5
2.11. LRR Domain of BRI1 Is Necessary
2.12. Suppressors of PMEIox
2.13. Additional bri1 Alleles via TILLING Analysis
3. Orthologs of bri1 Allele
3.1. lka Mutant of Garden Pea
3.2. cu3 Alleles of Tomato
3.3. d61 Alleles of Rice
3.4. uzu Alleles of Barley
3.5. Bd21 Allele of Brachypodium
3.6. mtbri1 Alleles of M. truncatula
3.7. E29 Allele of Pepper
4. Biological Uses of bri1 Alleles and Its Orthologs
4.1. bri1 Alleles as a Genetic Tool
4.2. Dwarfism: An Important Trait for Agriculture
4.3. Pathogen Resistance
4.4. Stress Tolerance
4.4.1. Drought
4.4.2. Cold
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Zullo, M.A.T.; Adam, G. Brassinosteroid Phytohormones—Structure, Bioactivity and Applications. Braz. J. Plant Physiol. 2002, 14, 143–181. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential Regulators of Plant Growth and Development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; González-García, M.; Caño-Delgado, A.I.; et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol. 2012, 197, 490–502. [Google Scholar] [CrossRef]
- Yang, D.; Baldwin, I.T.; Wu, J. Silencing Brassinosteroid Receptor BRI1 Impairs Herbivory-elicited Accumulation of Jasmonic Acid-isoleucine and Diterpene Glycosides, but not Jasmonic Acid and Trypsin Proteinase Inhibitors in Nicotiana attenuata. J. Integr. Plant Biol. 2013, 55, 514–526. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid perception in the epidermis controls root meristem size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Schomburg, F.M.; Amasino, R.M.; Vierstra, R.D.; Nagy, F.; Davis, S.J. Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering. Development 2007, 134, 2841–2850. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Schneider-Pizoń, J.; Betti, C.; Mayerhofer, J.; Vanhoutte, I.; van Dongen, W.; Boeren, S.; Zhiponova, M.; de Vries, S.; Jonak, C.; et al. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat. Cell Biol. 2012, 14, 548–554. [Google Scholar] [CrossRef]
- Kim, T.-W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z.-Y. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef]
- Khan, M.; Rozhon, W.; Bigeard, J.; Pflieger, D.; Husar, S.; Pitzschke, A.; Teige, M.; Jonak, C.; Hirt, H.; Poppenberger, B. Brassinosteroid-regulated GSK3/Shaggy-like Kinases Phosphorylate Mitogen-activated Protein (MAP) Kinase Kinases, Which Control Stomata Development in Arabidopsis thaliana. J. Biol. Chem. 2013, 288, 7519–7527. [Google Scholar] [CrossRef]
- Krishna, P. Brassinosteroid-Mediated Stress Responses. J. Plant Growth Regul. 2003, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Boutrot, F.; Segonzac, C.; Schwessinger, B.; Gimenez-Ibanez, S.; Chinchilla, D.; Rathjen, J.P.; de Vries, S.C.; Zipfel, C. Brassinosteroids inhibit pathogen-associated molecular pattern–triggered immune signaling independent of the receptor kinase BAK1. Proc. Natl. Acad. Sci. USA 2011, 109, 303–308. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y.; Epple, P.; Balsemão-Pires, E.; Dangl, J.L.; Chory, J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2011, 109, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Savaldi-Goldstein, S. Growth control: Brassinosteroid activity gets context. J. Exp. Bot. 2015, 66, 1123–1132. [Google Scholar] [CrossRef]
- Li, J.; Chory, J. A Putative Leucine-Rich Repeat Receptor Kinase Involved in Brassinosteroid Signal Transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef]
- Ma, X.; Xu, G.; He, P.; Shan, L. SERKing Coreceptors for Receptors. Trends Plant Sci. 2016, 21, 1017–1033. [Google Scholar] [CrossRef]
- Hohmann, U.; Lau, K.; Hothorn, M. The Structural Basis of Ligand Perception and Signal Activation by Receptor Kinases. Annu. Rev. Plant Biol. 2017, 68, 109–137. [Google Scholar] [CrossRef]
- Shiu, S.-H.; Bleecker, A.B. Plant Receptor-Like Kinase Gene Family: Diversity, Function, and Signaling. Sci. STKE 2001, 2001, re22. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; Ray, W.K.; Huber, S.C.; Asara, J.M.; Gage, D.A.; Clouse, S.D. Recombinant Brassinosteroid Insensitive 1 Receptor-Like Kinase Autophosphorylates on Serine and Threonine Residues and Phosphorylates a Conserved Peptide Motif in Vitro. Plant Physiol. 2000, 124, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Meisenhelder, J.; Hunter, T.; Yoshida, S.; Asami, T.; Chory, J. Autoregulation and Homodimerization Are Involved in the Activation of the Plant Steroid Receptor BRI1. Dev. Cell 2005, 8, 855–865. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Han, Z.; Kim, T.-W.; Wang, J.; Cheng, W.; Chang, J.; Shi, S.; Wang, J.; Yang, M.; Wang, Z.-Y.; et al. Structural insight into brassinosteroid perception by BRI1. Nature 2011, 474, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, M.; Belkhadir, Y.; Dreux, M.; Dabi, T.; Noel, J.P.; Wilson, I.A.; Chory, J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 2011, 474, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chory, J. Brassinosteroids Regulate Dissociation of BKI1, a Negative Regulator of BRI1 Signaling, from the Plasma Membrane. Science 2006, 313, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-Garcìa, S.; Cheng, J.-C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wang, H.; Walker, J.C.; Li, J. BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J. 2004, 40, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Canodelgado, A.I.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nat. Cell Biol. 2005, 433, 167–171. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; A Lease, K.; Doke, J.T.; E Tax, F.; Walker, J.C. BAK1, an Arabidopsis LRR Receptor-like Protein Kinase, Interacts with BRI1 and Modulates Brassinosteroid Signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef]
- Nam, K.H.; Li, J. BRI1/BAK1, a Receptor Kinase Pair Mediating Brassinosteroid Signaling. Cell 2002, 110, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mora-García, S.; Vert, G.; Yin, Y.; Caño-Delgado, A.; Cheong, H.; Chory, J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004, 18, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kim, T.-W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. Brassinosteroid-Signaling Kinases (BSKs) Mediate Signal Transduction from the Receptor Kinase BRI1 in Arabidopsis NIH Public Access. Science 2008, 321, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Guan, S.; Burlingame, A.L.; Wang, Z.-Y. The CDG1 Kinase Mediates Brassinosteroid Signal Transduction from BRI1 Receptor Kinase to BSU1 Phosphatase and GSK3-like Kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nam, K.H.; Vafeados, D.; Chory, J. BIN2, a New Brassinosteroid-Insensitive Locus in Arabidopsis. Plant Physiol. 2001, 127, 14–22. [Google Scholar] [CrossRef] [PubMed]
- He, J.-X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.-Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. In Proceedings of the National Academy of Sciences, La Jolla, CA, USA, 23 July 2002; pp. 10185–10190. [Google Scholar]
- Vert, G.; Chory, J. Downstream nuclear events in brassinosteroid signalling. Nature 2006, 441, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, L.; Guo, H.; Yin, Y. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 20142–20147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-Localized BZR1 Mediates Brassinosteroid-Induced Growth and Feedback Suppression of Brassinosteroid Biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.-Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 Accumulates in the Nucleus in Response to Brassinosteroids to Regulate Gene Expression and Promote Stem Elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Chen, W.; Lv, M.; Wang, Y.; Wang, P.-A.; Cui, Y.; Li, M.; Wang, R.; Gou, X.; Li, J. BES1 is activated by EMS1-TPD1-SERK1/2-mediated signaling to control tapetum development in Arabidopsis thaliana. Nat. Commun. 2019, 10, 4164. [Google Scholar] [CrossRef]
- Lundqvist, U. Scandinavian mutation research in barley—A historical review. Hereditas 2014, 151, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Nemhauser, J.L.; Geldner, N.; Hong, F.; Chory, J. Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 2005, 21, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, C.; Wang, X. Ligand Perception, Activation, and Early Signaling of Plant Steroid Receptor Brassinosteroid Insensitive 1. J. Integr. Plant Biol. 2013, 55, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Z.-Y.; Li, J.; Zhu, Q.; Lamb, C.; Ronald, P.; Chory, J. Perception of Brassinosteroids by the Extracellular Domain of the Receptor Kinase BRI1. Science 2000, 288, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 2001, 410, 380–383, Erratum in 2001, 411, 219. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Henzler, C.; Hothorn, M. Molecular Mechanism for Plant Steroid Receptor Activation by Somatic Embryogenesis Co-Receptor Kinases. Science 2013, 341, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Martinez, J.; Santiago, J.; Rybin, V.; Bayliss, R.; Hothorn, M. Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J. 2014, 78, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, D.M.; Joazeiro, C.A.P.; Li, J.; Hunter, T.; Chory, J. Brassinosteroid-Insensitive-1 is a Ubiquitously Expressed Leucine-Rich Repeat Receptor Serine/Threonine Kinase. Plant Physiol. 2000, 123, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Wang, H.; Nam, K.H.; Li, J.; Li, J. Activation-Tagged Suppressors of a Weak Brassinosteroid Receptor Mutant. Mol. Plant 2010, 3, 260–268. [Google Scholar] [CrossRef]
- Sun, C.; Yan, K.; Han, J.-T.; Tao, L.; Lv, M.-H.; Shi, T.; He, Y.-X.; Wierzba, M.; Tax, F.E.; Li, J. Scanning for New BRI1 Mutations via TILLING Analysis. Plant Physiol. 2017, 174, 1881–1896. [Google Scholar] [CrossRef]
- Jin, H.; Yan, Z.; Nam, K.H.; Li, J. Allele-Specific Suppression of a Defective Brassinosteroid Receptor Reveals a Physiological Role of UGGT in ER Quality Control. Mol. Cell 2007, 26, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Jin, H.; Tzfira, T.; Li, J. Multiple Mechanism–Mediated Retention of a Defective Brassinosteroid Receptor in the Endoplasmic Reticulum of Arabidopsis. Plant Cell 2008, 20, 3418–3429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gou, X.; Yin, H.; Mysore, K.S.; Li, J.; Wen, J. Functional characterisation of brassinosteroid receptor MtBRI1 in Medicago truncatula. Sci. Rep. 2017, 7, 9327. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of Function of a Rice brassinosteroid insensitive1 Homolog Prevents Internode Elongation and Bending of the Lamina Joint. Plant Cell 2000, 12, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Goddard, R.; Peraldi, A.; Ridout, C.; Nicholson, P. Enhanced Disease Resistance Caused by BRI1 Mutation Is Conserved Between Brachypodium distachyon and Barley (Hordeum vulgare). Mol. Plant Microbe Interact. 2014, 27, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Estelle, M.A.; Somerville, C.; Kende, H. Insensitivity to Ethylene Conferred by a Dominant Mutation in Arabidopsis thaliana. Science 1988, 241, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis Ethylene-Response Gene ETR1: Similarity of Product to Two-Component Regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chory, J.; Nagpal, P.; Peto, C.A. Phenotypic and Genetic Analysis of det2, a New Mutant That Affects Light-Regulated Seedling Development in Arabidopsis. Plant Cell 1991, 3, 445. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Németh, K.; Koncz-Kálmán, Z.; Mathur, J.; Kauschmann, A.; Altmann, T.; Rédei, G.P.; Nagy, F.; Schell, J.; Koncz, C. Brassinosteroids Rescue the Deficiency of CYP90, a Cytochrome P450, Controlling Cell Elongation and De-etiolation in Arabidopsis. Cell 1996, 85, 171–182. [Google Scholar] [CrossRef]
- Clouse, S.D.; Langford, M.; Mcmorris, T.C. A Brassinosteroid-Lnsensitive Mutant in Arabidopsis thaliana Exhibits Multiple Defects in Growth and Development’. Plant Physiol. 1996, 111, 671–678. [Google Scholar] [CrossRef]
- Kwon, M.; Choe, S. Brassinosteroid Biosynthesis and Dwarf Mutants. J. Plant Biol. 2005, 48, 1–15. [Google Scholar] [CrossRef]
- Kauschmann, A.; Jessop, A.; Koncz, C.; Szekeres, M.; Willmitzer, L.; Altmann, T. Genetic Evidence for an Essential Role of Brassinosteroids in Plant Development. Plant J. 1996, 9, 701–713. [Google Scholar] [CrossRef]
- Noguchi, T.; Fujioka, S.; Choe, S.; Takatsuto, S.; Yoshida, S.; Yuan, H.; Feldmann, K.A.; Tax, F.E. Brassinosteroid-Insensitive Dwarf Mutants of Arabidopsis Accumulate Brassinosteroids. Plant Physiol. 1999, 121, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Belkhadir, Y.; Durbak, A.; Wierzba, M.; Schmitz, R.J.; Aguirre, A.; Michel, R.; Rowe, S.; Fujioka, S.; Tax, F.E. Intragenic Suppression of a Trafficking-Defective Brassinosteroid Receptor Mutant in Arabidopsis. Genetics 2010, 185, 1283–1296. [Google Scholar] [CrossRef]
- Shang, Y.; Lee, M.M.; Li, J.; Nam, K.H. Characterization of Cp3 Reveals a New Bri1 Allele, Bri1-120, and the Importance of the LRR Domain of BRI1 Mediating BR Signaling. BMC Plant Biol. 2011, 11, 8. [Google Scholar] [CrossRef]
- Bouquin, T.; Meier, C.; Foster, R.; Nielsen, M.E.; Mundy, J. Control of Specific Gene Expression by Gibberellin and Brassinosteroid. Plant Physiol. 2001, 127, 450–458. [Google Scholar] [CrossRef]
- Hou, Q.; Saima, S.; Ren, H.; Ali, K.; Bai, C.; Wu, G.; Li, G. Less Conserved LRRS Is Important for BRI1 Folding. Front. Plant Sci. 2019, 10, 634. [Google Scholar] [CrossRef]
- Lv, M.; Li, M.; Chen, W.; Wang, Y.; Sun, C.; Yin, H.; He, K.; Li, J. Thermal-Enhanced Bri1-301 Instability Reveals a Plasma Membrane Protein Quality Control System in Plants. Front. Plant Sci. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Xu, W.; Huang, J.; Li, B.; Li, J.; Wang, Y. Is Kinase Activity Essential for Biological Functions of BRI1? Cell Res. 2008, 18, 472–478. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Qin, Y.; Chen, Y.; Liu, X.; Wang, M.; Mao, J.; Zhang, J.; He, Z.; Liu, L.; et al. A Temperature-Sensitive Misfolded Bri1-301 Receptor Requires Its Kinase Activity to Promote Growth 1[Open]. Plant Physiol. 2018, 178, 1704–1719. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, Y.; Erhardt, M.; Ruan, Y.; Shen, W.H. A Non-Canonical Transferred DNA Insertion at the BRI1 Locus in Arabidopsis thaliana. J. Integr. Plant Biol. 2009, 51, 367–373. [Google Scholar] [CrossRef]
- Gou, X.; Yin, H.; He, K.; Du, J.; Yi, J.; Xu, S.; Lin, H.; Clouse, S.D.; Li, J. Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling. PLoS Genet. 2012, 8, e1002452. [Google Scholar] [CrossRef]
- Holzwart, E.; Wanke, F.; Glöckner, N.; Höfte, H.; Harter, K.; Wolf, S. A Mutant Allele Uncouples the Brassinosteroid-Dependent and Independent Functions of Brassinosteroid Insensitive 1. Plant Physiol. 2019, 182, 669–678. [Google Scholar] [CrossRef]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant Cell Wall Homeostasis Is Mediated by Brassinosteroid Feedback Signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Chory, J. Out of Darkness: Mutants Reveal Pathways Controlling Light-Regulated Development in Plants. Trends Genet. 1993, 9, 167–172. [Google Scholar] [CrossRef]
- Deng, X.W. Fresh View of Light Signal Transduction in Plants. Cell 1994, 76, 423–426. [Google Scholar] [CrossRef]
- Altmann, T.; Felix, G.; Jessop, A.; Kauschmann, A.; Uwer, U.; Peña-Cortés, H.; Willmitzer, L. Ac/Ds Transposon Mutagenesis in Arabidopsis thaliana: Mutant Spectrum and Frequency of Ds Insertion Mutants. MGG Mol. Gen. Genet. 1995, 247, 646–652. [Google Scholar] [CrossRef]
- Wei, N.; Kwok, S.F.; Von Arnim, A.G.; Lee, A.; McNellis, T.W.; Piekos, B.; Deng, X.W. Arabidopsis COP8, COP10, and COP11 Genes Are Involved in Repression of Photomorphogenic Development in Darkness. Plant Cell 1994, 6, 629. [Google Scholar] [CrossRef]
- Walker, J.C. Receptor-like Protein Kinase Genes of Arabidopsis thaliana. Plant J. 1993, 3, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, R.; Wu, Y.; LoCascio, J.C.; Feldmann, K.A. An Arabidopsis Brassinosteroid-Dependent Mutant Is Blocked in Cell Elongation. Plant Cell 1998, 10, 219–230. [Google Scholar] [CrossRef]
- Turk, E.M.; Fujioka, S.; Seto, H.; Shimada, Y.; Takatsuto, S.; Yoshida, S.; Wang, H.; Torres, Q.I.; Ward, J.M.; Murthy, G.; et al. BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J. 2005, 42, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Nakayama, M.; Reid, J.B.; Takeuchi, Y.; Yokota, T. Blockage of Brassinosteroid Biosynthesis and Sensitivity Causes Dwarfism in Garden Pea. Plant Physiol. 1997, 113, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kitasaka, Y.; Takatsuto, S.; Reid, J.B.; Fukami, M.; Yokota, T. Brassinosteroid/Sterol Synthesis and Plant Growth as Affected by lka and lkb Mutations of Pea1. Plant Physiol. 1999, 119, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Bishop, G.J.; Kaneta, T.; Reid, J.B.; Chory, J.; Yokota, T. The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J. 2003, 36, 291–300. [Google Scholar] [CrossRef]
- Koka, C.V.; Cerny, R.E.; Gardner, R.G.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Clouse, S.D. A Putative Role for the Tomato Genes DUMPY and CURL-3 in Brassinosteroid Biosynthesis and Response. Plant Physiol. 2000, 122, 85–98. [Google Scholar] [CrossRef]
- Montoya, T.; Nomura, T.; Farrar, K.; Kaneta, T.; Yokota, T.; Bishop, G.J. Cloning the Tomato Curl3 Gene Highlights the Putative Dual Role of the Leucine-Rich Repeat Receptor Kinase tBRI1/SR160 in Plant Steroid Hormone and Peptide Hormone Signaling. Plant Cell 2002, 14, 3163–3176. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological Alteration Caused by Brassinosteroid Insensitivity Increases the Biomass and Grain Production of Rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef]
- Nakamura, A.; Fujioka, S.; Sunohara, H.; Kamiya, N.; Hong, Z.; Inukai, Y.; Miura, K.; Takatsuto, S.; Yoshida, S.; Ueguchi-Tanaka, M.; et al. The Role of OsBRI1 and Its Homologous Genes, OsBRL1 and OsBRL3, in Rice. Plant Physiol. 2006, 140, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, C.; Yuan, S.; Yin, L.; Sun, W.; Zhao, Q.; Zhao, B.; Li, X. Kinase activity of OsBRI1 is essential for brassinosteroids to regulate rice growth and development. Plant Sci. 2013, 199–200, 113–120. [Google Scholar] [CrossRef]
- Takeda, K. Internode Elongation and Dwarfism in Some Gramineous Plants. Gamma Field Symp. 1979, 16, 1–18. [Google Scholar]
- Hong, Z.; Ueguchi-Tanaka, M.; Shimizu-Sato, S.; Inukai, Y.; Fujioka, S.; Shimada, Y.; Takatsuto, S.; Agetsuma, M.; Yoshida, S.; Watanabe, Y.; et al. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 2002, 32, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Chono, M.; Honda, I.; Zeniya, H.; Yoneyama, K.; Saisho, D.; Takeda, K.; Takatsuto, S.; Hoshino, T.; Watanabe, Y. A Semidwarf Phenotype of Barley uzu Results from a Nucleotide Substitution in the Gene Encoding a Putative Brassinosteroid Receptor. Plant Physiol. 2003, 133, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Dockter, C.; Gruszka, D.; Braumann, I.; Druka, A.; Druka, I.; Franckowiak, J.; Gough, S.P.; Janeczko, A.; Kurowska, M.; Lundqvist, J.; et al. Induced Variations in Brassinosteroid Genes Define Barley Height and Sturdiness, and Expand the Green Revolution Genetic Toolkit. Plant Physiol. 2014, 166, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D.; Szarejko, I.; Maluszynski, M. New allele of HvBRI1 gene encoding brassinosteroid receptor in barley. J. Appl. Genet. 2011, 52, 257–268. [Google Scholar] [CrossRef]
- Bosshard, H.R.; Marti, D.N.; Jelesarov, I. Protein stabilization by salt bridges: Concepts, experimental approaches and clarification of some misunderstandings. J. Mol. Recognit. 2004, 17, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Yan, W. Molecular Characterization of Barley 3H Semi-Dwarf Genes. PLoS ONE 2015, 10, e0120558. [Google Scholar] [CrossRef]
- Jiang, C.; Lei, M.; Guo, Y.; Gao, G.; Shi, L.; Jin, Y.; Cai, Y.; Himmelbach, A.; Zhou, S.; He, Q.; et al. A reference-guided TILLING by amplicon-sequencing platform supports forward and reverse genetics in barley. Plant Commun. 2022, 3, 100317. [Google Scholar] [CrossRef] [PubMed]
- Thole, V.; Peraldi, A.; Worland, B.; Nicholson, P.; Doonan, J.H.; Vain, P. T-DNA mutagenesis in Brachypodium distachyon. J. Exp. Bot. 2011, 63, 567–576. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, S.; Ou, L.; Liu, F.; Yang, L.; Zheng, J.; Chen, W.; Zhang, Z.; Yang, S.; Ma, Y.; et al. A novel single-base mutation in CaBRI1 confers dwarf phenotype and brassinosteroid accumulation in pepper. Mol. Genet. Genom. 2019, 295, 343–356. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential Transphosphorylation of the BRI1/BAK1 Receptor Kinase Complex Impacts Early Events in Brassinosteroid Signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef]
- Li, J.; Lease, K.A.; Tax, F.E.; Walker, J.C. BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Fujioka, S.; Takatsuto, S.; Matsumoto, S.; Gou, X.; He, K.; Russell, S.D.; Li, J. BEN1, a gene encoding a dihydroflavonol 4-reductase (DFR)-like protein, regulates the levels of brassinosteroids in Arabidopsis thaliana. Plant J. 2007, 51, 220–233. [Google Scholar] [CrossRef]
- Guo, Z.; Fujioka, S.; Blancaflor, E.B.; Miao, S.; Gou, X.; Li, J. TCP1 Modulates Brassinosteroid Biosynthesis by Regulating the Expression of the Key Biosynthetic Gene DWARF4 in Arabidopsis thaliana. Plant Cell 2010, 22, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yuan, T.; Tarkowská, D.; Kim, J.; Nam, H.G.; Novák, O.; He, K.; Gou, X.; Li, J. Brassinosteroid Biosynthesis Is Modulated via a Transcription Factor Cascade of COG1, PIF4, and PIF5. Plant Physiol. 2017, 174, 1260–1273. [Google Scholar] [CrossRef]
- Su, W.; Liu, Y.; Xia, Y.; Hong, Z.; Li, J. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 108, 870–875. [Google Scholar] [CrossRef]
- Hong, Z.; Kajiura, H.; Su, W.; Jin, H.; Kimura, A.; Fujiyama, K.; Li, J. Evolutionarily conserved glycan signal to degrade aberrant brassinosteroid receptors in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11437–11442. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Wang, D.; Su, W.; Liu, L.; Wang, M.; Li, J. EBS7 is a plant-specific component of a highly conserved endoplasmic reticulum-associated degradation system in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 12205–12210. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Fujioka, S.; Asami, T.; Li, J.; Li, J. Regulation of Arabidopsis Brassinosteroid Signaling by Atypical Basic Helix-Loop-Helix Proteins. Plant Cell 2009, 21, 3781–3791. [Google Scholar] [CrossRef]
- Khush, G.S. Green Revolution: Preparing for the 21st Century. Genome 1999, 42, 646–655. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green Revolution: A Mutant Gibberellin-Synthesis Gene in Rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Vriet, C.; Russinova, E.; Reuzeau, C. Boosting Crop Yields with Plant Steroids. Plant Cell 2012, 24, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Saisho, D.; Tanno, K.-I.; Chono, M.; Honda, I.; Kitano, H.; Takeda, K. Spontaneous Brassinolide-insensitive Barley Mutants ‘uzu’ Adapted to East Asia. Breed. Sci. 2004, 54, 409–416. [Google Scholar] [CrossRef]
- Honda, I.; Zeniya, H.; Yoneyama, K.; Chono, M.; Kaneko, S.; Watanabe, Y. UzuMutation in Barley (Hordeum vulgare L.) Reduces the Leaf Unrolling Response to Brassinolide. Biosci. Biotechnol. Biochem. 2003, 67, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yan, W.; Liu, Y.; Wei, Y.; Zhou, M.; Zheng, Y.-L.; Manners, J.M.; Liu, C. The non-gibberellic acid-responsive semi-dwarfing gene uzu affects Fusarium crown rot resistance in barley. BMC Plant Biol. 2014, 14, 22. [Google Scholar] [CrossRef]
- Ali, S.S.; Gunupuru, L.R.; Kumar, G.B.S.; Khan, M.; Scofield, S.; Nicholson, P.; Doohan, F.M. Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol. 2014, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Oklestkova, J.; Szarejko, I. Barley Brassinosteroid Mutants Provide an Insight into Phytohormonal Homeostasis in Plant Reaction to Drought Stress. Front. Plant Sci. 2016, 7, 1824. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Fodor, J. Non-enzymatic antioxidant accumulations in BR-deficient and BR-insensitive barley mutants under control and drought conditions. Physiol. Plant. 2017, 163, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, B.H.; Lim, C.J.; Lim, C.O.; Nam, K.H. Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold. Physiol. Plant. 2010, 138, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D. Exploring the Brassinosteroid Signaling in Monocots Reveals Novel Components of the Pathway and Implications for Plant Breeding. Int. J. Mol. Sci. 2020, 21, 354. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Gruszka, D. Crosstalk of the Brassinosteroid Signalosome with Phytohormonal and Stress Signaling Components Maintains a Balance between the Processes of Growth and Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 2675. [Google Scholar] [CrossRef]
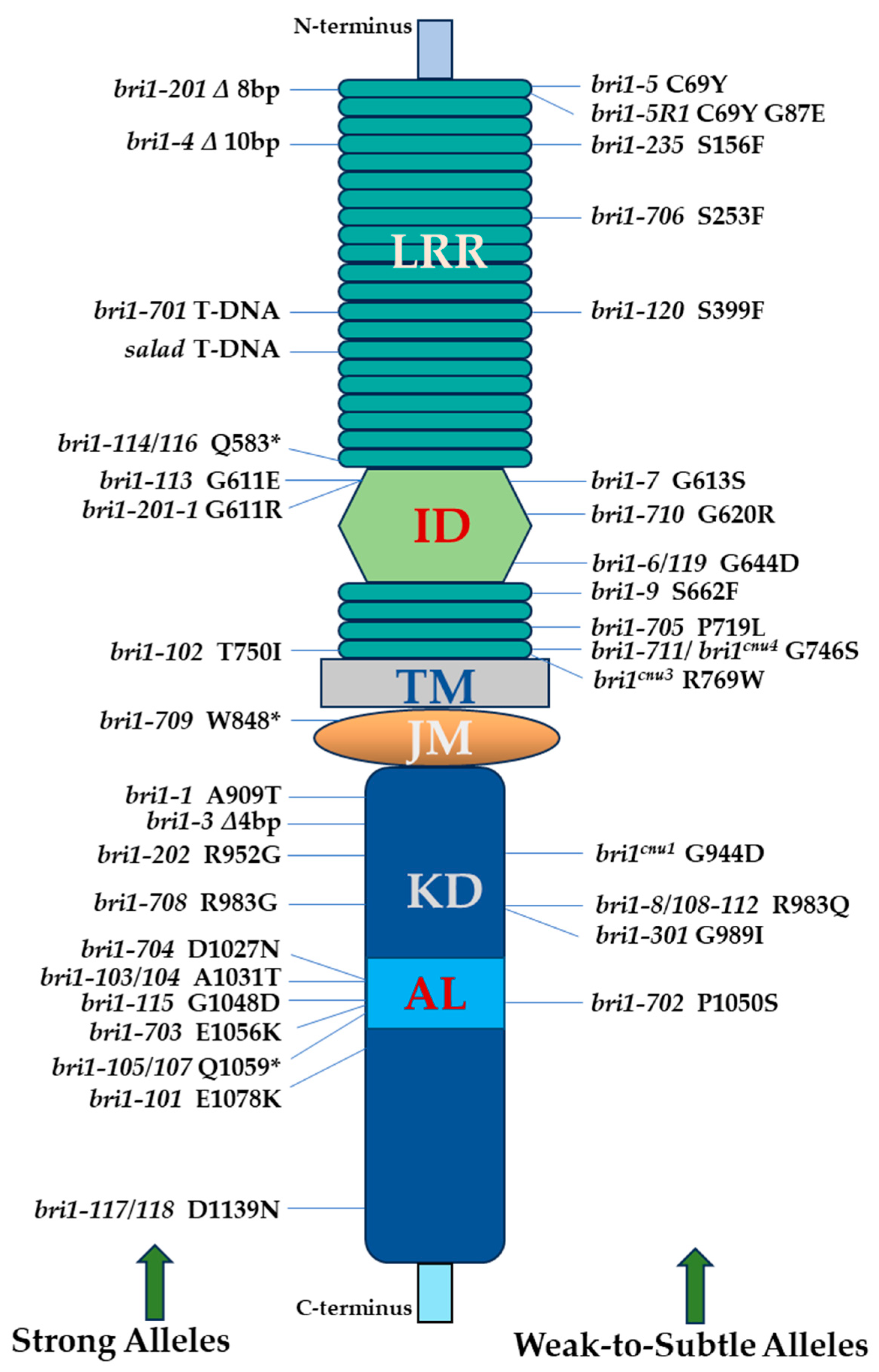




| Alleles | Base Pair Change | Amino Acid Change | Accession | Allelic Strength/ Phenotype | Possible Mechanism | References |
|---|---|---|---|---|---|---|
| bri1-1 | G2725A | Ala-909-Thr | Col-0 | Strong | Extremely weak in vivo BL-stimulated BAK1 phosphorylation | [49,61] |
| bri1-2/cbb2 | Transposon insertion | Nil | Col-0 | Strong | Impaired BRI1 | [63] |
| bri1-3 | 4-bp deletion after 2745 | STOP 44 aa downstream | Wassilewskija-2 (WS2) | Strong | Impaired BRI1 KD | [64] |
| bri1-4 | 10-bp deletion after 459 | STOP 13 aa downstream | WS2 | Strong | No BRI1 | [64] |
| bri1-5 | G206A | Cys-69-Tyr | WS2 | Weak | ER retention | [64] |
| bri1-5R1 | G260A | Gly-87-Glu | bri1-5/WS2 | Weak | Partially restore ER retention of bri1-5 | [65] |
| bri1-6/119 | G1931A | Gly-644-Asp | Enkheim-2 (En-2) | Weak | Unknown | [49,64] |
| bri1-7 | G1838A | Gly-613-Ser | WS2 | Weak | Impaired BL binding | [64] |
| bri1-8/108/112 | G2948A | Arg-983-Gln | WS2/Col-0 | Intermediate | Autophosphorylation cannot be detected in vitro | [49,64] |
| bri1-9 | C1985T | Ser-662-Phe | WS2/Col-0 | Weak | ER retention | [64] |
| bri1-101 | G3232A | Glu-1078-Lys | Col-0 | Strong | Autophosphorylation cannot be detected in vitro | [18,49] |
| bri1-102 | C2249T | Thr-750-Ile | Col-0 | Strong | Unknown | [49] |
| bri1-103/104 | G3091A | Ala-1031-Thr | Col-0 | Strong | Unknown | [18,49] |
| bri1-105/106/107 | C3175T | Gln-1059-Stop | Col-0 | Strong | Impaired BRI1 KD | [18,49] |
| bri1-113 | G1832A | Gly-611-Glu | Col-0 | Strong | Impaired BL binding | [18] |
| bri1-114/116 | C1747T | Gln-583-Stop | Col-0 | Strong | No BRI1 | [18,49] |
| bri1-115 | G3143A | Gly-1048-Asp | Col-0 | Strong | Unknown | [18] |
| bri1-117/118 | G3415A | Asp-1139-Asn | Col-0 | Strong | Unknown | [18,49] |
| bri1-120/cp3 | T1196C | Ser-399-Phe | Landsberg erecta | Weak | Unknown | [66] |
| bri1-201 | 8bp deletion | STOP 44 aa downstream | Col-0 | Strong | Premature stop | [67] |
| bri1-201-1 | G1831A | Gly-611-Arg | WS2 | Strong, late-flowering ld-3 enhancer | Unknown | [8] |
| bri1-202 | C2854T | Arg-952-Trp | WS2 | Strong, late-flowering ld-3 enhancer | Unknown | [8] |
| bri1-235 | C468T | Ser-156-Phe | Col-0 | Weak | ER retention | [68] |
| bri1-301 | GG2965/6AT | Gly-989-Ile | Col-0 | Weak | Kinase dead, thermally unstable | [69,70,71] |
| salade | Transposon insertion | genome deletion | Col-0 | Strong | No BRI1 | [72] |
| bri1-701 | T-DNA insertion | Nil | Col-0 | Strong | No BRI1 | [73] |
| bri1-702 | C3148T | Pro-1050-Ser | Col-0 | Weak | Reduced autophosphorylation in vitro | [51] |
| bri1-703 | G3166A | Glu-1056-Lys | Col-0 | Strong | Autophosphorylation cannot be detected in vitro | [51] |
| bri1-704 | G3079A | Asp-1027-Asn | Col-0 | Strong | Autophosphorylation cannot be detected in vitro | [51] |
| bri1-705 | C2156T | Pro-719-Leu | Col-0 | Subtle | Disrupt the formation of hydrogen bonds among BRI1, BL, and BAK1 | [51] |
| bri1-706 | C758T | Ser-253-Phe | Col-0 | Subtle | Unknown | [51] |
| bri1-708 | C2947G | Arg-938-Gly | Col-0 | Strong | Autophosphorylation cannot be detected in vitro | [51] |
| bri1-709 | G2543A | Trp-848-Stop | Col-0 | Strong | Premature stop | [51] |
| bri1-710 | G1858A | Gly-620-Arg | Col-0 | Subtle | Unknown | [51] |
| bri1-711/bri1cnu4 | G2236A | Gly-746-Ser | Col-0 | Subtle | Unknown | [51,74] |
| bri1cnu1 | G2831A | Gly-944-Asp | Col-0 | Weak | Unknown | [75] |
| bri1cnu3 | G2307T | Arg-769-Trp | Col-0 | Weak | Unknown | [74] |
| Allele | Base Pair Change | Amino Acid Change | Phenotype | Fertility | Reference |
|---|---|---|---|---|---|
| d61-1 | C to T | Thr-989-Ile | Mild | Fertile | [55] |
| d61-2 | G to A | Val-491-Met | Intermediate | Fertile | [55] |
| d61-3 | A to C | His-420-Pro | Severe | Sterile | [89] |
| d61-4 | G to T | Glu-847-Stop | Severe | Sterile | [89] |
| d61-5 | A to T | Asn-426-Tyr | Severe | Sterile | [89] |
| d61-6 | 2 bp insertion | Asp-759-stop | Severe | Sterile | [89] |
| d61-7 | C to T | Ala-467-Val | Mild | Fertile | [89] |
| d61-8 | G to A | Gly-522-Glu | Mild | Fertile | [89] |
| d61-9 | G to A | Gly-539-Asp | Mild | Fertile | [89] |
| d61-10 | C to A | Thr-854-Ile | Mild | Fertile | [89] |
| Fn189 | A to T | Ile-834-Phe | Mild | Fertile | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zada, A.; Lv, M.; Li, J. Molecular Lesions in BRI1 and Its Orthologs in the Plant Kingdom. Int. J. Mol. Sci. 2024, 25, 8111. https://doi.org/10.3390/ijms25158111
Zada A, Lv M, Li J. Molecular Lesions in BRI1 and Its Orthologs in the Plant Kingdom. International Journal of Molecular Sciences. 2024; 25(15):8111. https://doi.org/10.3390/ijms25158111
Chicago/Turabian StyleZada, Ahmad, Minghui Lv, and Jia Li. 2024. "Molecular Lesions in BRI1 and Its Orthologs in the Plant Kingdom" International Journal of Molecular Sciences 25, no. 15: 8111. https://doi.org/10.3390/ijms25158111





