Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy
Abstract
:1. Introduction
2. Tailoring Exercise Programs to Address Breast Cancer-Related Body Wasting
Exercise Counteracts Breast Cancer- and Chemotherapy-Induced Body Wasting
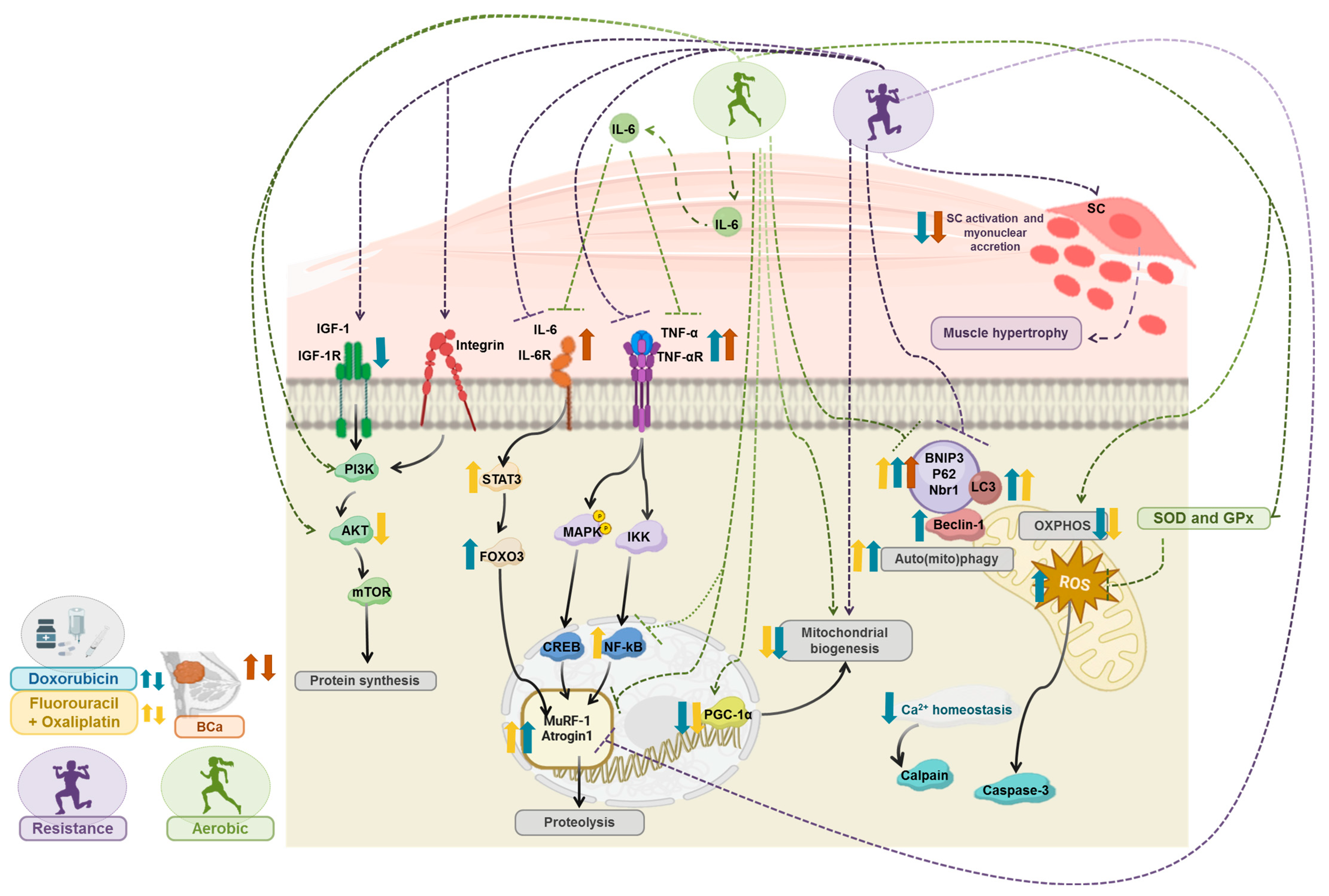
3. Exercise Training Counteracts Cancer- and/or Chemotherapy-Induced Muscle Wasting: The Molecular Targets
3.1. Exercise-Induced Remodeling of Muscle Fiber Phenotype in Cancer-Induced Muscle Wasting
3.2. Exercise Counteracts Oxidative Stress and Inflammation on Cancer and Chemotherapy-Induced Muscle Wasting
3.3. Metabolic Remodeling and Hormonal Influence on Skeletal Muscle Response to Exercise in Cancer Settings
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key, T.J.; Verkasalo, P.K.; Banks, E. Epidemiology of Breast Cancer. Lancet Oncol. 2001, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Tolaney, S.M. HER2-Positive Breast Cancer: New Therapeutic Frontiers and Overcoming Resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment Strategies for Breast Cancer Brain Metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in Adjuvant Chemotherapy for Breast Cancer: An Overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef]
- Torti, F.M.; Bristow, M.M.; Lum, B.L.; Carter, S.K.; Howes, A.E.; Aston, D.A.; Brown, B.W.; Hannigan, J.F.; Meyers, F.J.; Mitchell, E.P. Cardiotoxicity of Epirubicin and Doxorubicin: Assessment by Endomyocardial Biopsy. Cancer Res. 1986, 46, 3722–3727. [Google Scholar]
- Wilson, H.E.; Stanton, D.A.; Rellick, S.; Geldenhuys, W.; Pistilli, E.E. Breast Cancer-Associated Skeletal Muscle Mitochondrial Dysfunction and Lipid Accumulation Is Reversed by PPARG. Am. J. Physiol. Cell Physiol. 2021, 320, C577–C590. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Luca, F.D.; Locatelli, C.A.; Ratto, D.; Di Iorio, C.; Savino, E.; Bottone, M.G.; Rossi, P. From a Medicinal Mushroom Blend a Direct Anticancer Effect on Triple-Negative Breast Cancer: A Preclinical Study on Lung Metastases. Molecules 2020, 25, 5400. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.D.; Roda, E.; Ratto, D.; Desiderio, A.; Venuti, M.T.; Ramieri, M.; Bottone, M.G.; Savino, E.; Rossi, P. Fighting Secondary Triple-Negative Breast Cancer in Cerebellum: A Powerful Aid from a Medicinal Mushrooms Blend. Biomed. Pharmacother. 2023, 159, 114262. [Google Scholar] [CrossRef]
- Rajarajeswaran, P.; Vishnupriya, R. Exercise in Cancer. Indian J. Med. Paediatr. Oncol. 2009, 30, 61–70. [Google Scholar] [CrossRef]
- Frisch, R.E.; Wyshak, G.; Albright, N.L.; Albright, T.E.; Schiff, I.; Jones, K.P.; Witschi, J.; Shiang, E.; Koff, E.; Marguglio, M. Lower Prevalence of Breast Cancer and Cancers of the Reproductive System among Former College Athletes Compared to Non-Athletes. Br. J. Cancer 1985, 52, 885–891. [Google Scholar] [CrossRef]
- Orlandella, F.M.; De Stefano, A.E.; Iervolino, P.L.C.; Buono, P.; Soricelli, A.; Salvatore, G. Dissecting the Molecular Pathways Involved in the Effects of Physical Activity on Breast Cancers Cells: A Narrative Review. Life Sci. 2021, 265, 118790. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Schumann, M.; Freitag, N.; Bloch, W. Advanced Exercise Prescription for Cancer Patients and Its Application in Germany. J. Sci. Sport Exerc. 2020, 2, 201–214. [Google Scholar] [CrossRef]
- Thomas, G.A. Using a Network Physiology Approach to Prescribe Exercise for Exercise Oncology. Front. Netw. Physiol. 2022, 2, 877676. [Google Scholar] [CrossRef]
- Roeland, E.J.; Ma, J.D.; Nelson, S.H.; Seibert, T.; Heavey, S.; Revta, C.; Gallivan, A.; Baracos, V.E. Weight Loss versus Muscle Loss: Re-Evaluating Inclusion Criteria for Future Cancer Cachexia Interventional Trials. Support. Care Cancer 2017, 25, 365–369. [Google Scholar] [CrossRef]
- McMillin, S.L.; Minchew, E.C.; Lowe, D.A.; Spangenburg, E.E. Skeletal Muscle Wasting: The Estrogen Side of Sexual Dimorphism. Am. J. Physiol. Cell Physiol. 2022, 322, C24–C37. [Google Scholar] [CrossRef]
- Montero, D.; Madsen, K.; Meinild-Lundby, A.-K.; Edin, F.; Lundby, C. Sexual Dimorphism of Substrate Utilization: Differences in Skeletal Muscle Mitochondrial Volume Density and Function. Exp. Physiol. 2018, 103, 851–859. [Google Scholar] [CrossRef]
- Antoun, S.; Borget, I.; Lanoy, E. Impact of Sarcopenia on the Prognosis and Treatment Toxicities in Patients Diagnosed with Cancer. Curr. Opin. Support. Palliat. Care 2013, 7, 383–389. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength. Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.; Bennett, J.A.; Leo, M.C.; Naik, A.; Schwartz, A. Strength Training Stops Bone Loss and Builds Muscle in Postmenopausal Breast Cancer Survivors: A Randomized, Controlled Trial. Breast Cancer Res. Treat. 2011, 127, 447–456. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A Systematic Review of Rehabilitation and Exercise Recommendations in Oncology Guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Bonsignore, A.; Bland, K.A.; Mckenzie, D.C.; Gelmon, K.A.; Van Patten, C.L.; Campbell, K.L. Exercise Prescription and Adherence for Breast Cancer: One Size Does Not FITT All. Med. Sci. Sports Exerc. 2018, 50, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Mallard, J.; Hucteau, E.; Hureau, T.J.; Pagano, A.F. Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights from Other Cancers. Front. Cell Dev. Biol. 2021, 9, 719643. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Guigni, B.A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; Toth, M.J. Skeletal Muscle Atrophy and Dysfunction in Breast Cancer Patients: Role for Chemotherapy-Derived Oxidant Stress. Am. J. Physiol. Cell Physiol. 2018, 315, C744–C756. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.H.; Lane, K.; et al. Effects of Aerobic and Resistance Exercise in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Weisser, B.; Dürkop, J.; Jonat, W.; Van Mackelenbergh, M.; Röcken, C.; Mundhenke, C. Comparing Endurance and Resistance Training with Standard Care during Chemotherapy for Patients with Primary Breast Cancer. Anticancer Res. 2015, 35, 5623–5629. [Google Scholar] [PubMed]
- Møller, A.B.; Lønbro, S.; Farup, J.; Voss, T.S.; Rittig, N.; Wang, J.; Højris, I.; Mikkelsen, U.R.; Jessen, N. Molecular and Cellular Adaptations to Exercise Training in Skeletal Muscle from Cancer Patients Treated with Chemotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1449–1460. [Google Scholar] [CrossRef]
- Wengström, Y.; Bolam, K.A.; Mijwel, S.; Sundberg, C.J.; Backman, M.; Browall, M.; Norrbom, J.; Rundqvist, H. Optitrain: A Randomised Controlled Exercise Trial for Women with Breast Cancer Undergoing Chemotherapy. BMC Cancer 2017, 17, 100. [Google Scholar] [CrossRef]
- Padrão, A.I.; Figueira, A.C.C.; Faustino-Rocha, A.I.; Gama, A.; Loureiro, M.M.; Neuparth, M.J.; Moreira-Gonçalves, D.; Vitorino, R.; Amado, F.; Santos, L.L.; et al. Long-Term Exercise Training Prevents Mammary Tumorigenesis-Induced Muscle Wasting in Rats through the Regulation of TWEAK Signalling. Acta Physiol. 2017, 219, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Alves de Lima, E.; de Teixeira, A.A.S.; Biondo, L.A.; Diniz, T.A.; Silveira, L.S.; Coletti, D.; Busquets Rius, S.; Rosa Neto, J.C. Exercise Reduces the Resumption of Tumor Growth and Proteolytic Pathways in the Skeletal Muscle of Mice Following Chemotherapy. Cancers 2020, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Ballarò, R.; Beltrà, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate Exercise in Mice Improves Cancer Plus Chemotherapy-Induced Muscle Wasting and Mitochondrial Alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef] [PubMed]
- Smuder, A.J.; Kavazis, A.N.; Min, K.; Powers, S.K. Exercise Protects against Doxorubicin-Induced Markers of Autophagy Signaling in Skeletal Muscle. J. Appl. Physiol. 2011, 111, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Bredahl, E.C.; Pfannenstiel, K.B.; Quinn, C.J.; Hayward, R.; Hydock, D.S. Effects of Exercise on Doxorubicin-Induced Skeletal Muscle Dysfunction. Med. Sci. Sports Exerc. 2016, 48, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.J.; Hydock, D.S. Effects of Endurance Exercise and Doxorubicin on Skeletal Muscle Myogenic Regulatory Factor Expression. Muscles Ligaments Tendons J. 2017, 7, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Klassen, O.; Schmidt, M.E.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Muscle Strength in Breast Cancer Patients Receiving Different Treatment Regimes: Muscle Strength in Breast Cancer Patients. J. Cachexia Sarcopenia Muscle 2017, 8, 305–316. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Torgrimson-Ojerio, B.; Dieckmann, N.F.; Stoyles, S.; Mitri, Z.; Luoh, S.-W. A Randomized-Controlled Trial Comparing Supervised Aerobic Training to Resistance Training Followed by Unsupervised Exercise on Physical Functioning in Older Breast Cancer Survivors. J. Geriatr. Oncol. 2022, 13, 152–160. [Google Scholar] [CrossRef]
- American College of Sports; Medicine American College of Sports; Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Kannus, P. Isokinetic Evaluation of Muscular Performance: Implications for Muscle Testing and Rehabilitation. Int. J. Sports Med. 1994, 15 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef]
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; Klassen, T.P.; Mackey, J.R.; Courneya, K.S. Effects of Exercise on Breast Cancer Patients and Survivors: A Systematic Review and Meta-Analysis. Can. Med. Assoc. J. 2006, 175, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Floyd, A.; Moyer, A. Group versus Individual Exercise Interventions for Women with Breast Cancer: A Meta-Analysis. Health Psychol. Rev. 2010, 4, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Dobek, J.; Bennett, J.A.; Nail, L.M.; Leo, M.C.; Schwartz, A. The Effect of Resistance Training on Muscle Strength and Physical Function in Older, Postmenopausal Breast Cancer Survivors: A Randomized Controlled Trial. J. Cancer Surviv. 2012, 6, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Mutrie, N.; White, F.; McGuire, F.; Kearney, N. A Pilot Study of a Supervised Group Exercise Programme as a Rehabilitation Treatment for Women with Breast Cancer Receiving Adjuvant Treatment. Eur. J. Oncol. Nurs. 2005, 9, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, L.C.; McNeely, M.L.; Lau, H.Y.; Reimer, R.A.; Giese-Davis, J.; Fung, T.S.; Culos-Reed, S.N. Patient-Reported Outcomes, Body Composition, and Nutrition Status in Patients with Head and Neck Cancer: Results from an Exploratory Randomized Controlled Exercise Trial. Cancer 2016, 122, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Moley, J.F.; Aamodt, R.; Rumble, W.; Kaye, W.; Norton, J.A. Body Cell Mass in Cancer-Bearing and Anorexic Patients. JPEN J. Parenter. Enteral Nutr. 1987, 11, 219–222. [Google Scholar] [CrossRef]
- Friesen, D.E.; Baracos, V.E.; Tuszynski, J.A. Modeling the Energetic Cost of Cancer as a Result of Altered Energy Metabolism: Implications for Cachexia. Theor. Biol. Med. Model. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Mallard, J.; Hucteau, E.; Schott, R.; Trensz, P.; Pflumio, C.; Kalish-Weindling, M.; Favret, F.; Pivot, X.; Hureau, T.J.; Pagano, A.F. Early Skeletal Muscle Deconditioning and Reduced Exercise Capacity during (Neo)Adjuvant Chemotherapy in Patients with Breast Cancer. Cancer 2023, 129, 215–225. [Google Scholar] [CrossRef]
- Cao, D.; Wu, G.; Zhang, B.; Quan, Y.; Wei, J.; Jin, H.; Jiang, Y.; Yang, Z. Resting Energy Expenditure and Body Composition in Patients with Newly Detected Cancer. Clin. Nutr. 2010, 29, 72–77. [Google Scholar] [CrossRef]
- Soom, T.V.; Tjalma, W.; Daele, U.V.; Gebruers, N.; Breda, E.V. Resting Energy Expenditure, Body Composition, and Metabolic Alterations in Breast Cancer Survivors vs. Healthy Controls: A Cross-Sectional Study. BMC Women’s Health 2024, 24, 117. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased Visceral Fat and Decreased Energy Expenditure during the Menopausal Transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body Composition as an Independent Determinant of 5-Fluorouracil-Based Chemotherapy Toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Lane, K.; Martin, A.D.; Gelmon, K.A.; McKenzie, D.C. Resting Energy Expenditure and Body Mass Changes in Women during Adjuvant Chemotherapy for Breast Cancer. Cancer Nurs. 2007, 30, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Durkin, K.; Heetun, A.; Ewings, S.; Munday, R.; Wootton, S.A.; Turner, L.; Copson, E.R.; Cutress, R.I. Body Composition and Chemotherapy Toxicity in Women with Early Breast Cancer (CANDO-3): Protocol for an Observational Cohort Study. BMJ Open 2022, 12, e054412. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential Effects of Resistance and Endurance Exercise in the Fed State on Signalling Molecule Phosphorylation and Protein Synthesis in Human Muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef]
- Martin, A.; Freyssenet, D. Phenotypic Features of Cancer Cachexia-related Loss of Skeletal Muscle Mass and Function: Lessons from Human and Animal Studies. J. Cachexia Sarcopenia Muscle 2021, 12, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Dunlap, K.R.; Rosa-Caldwell, M.E.; Haynie, W.S.; Jansen, L.T.; Washington, T.A.; Greene, N.P. Comparative Plasma Proteomics in Muscle Atrophy during Cancer-Cachexia and Disuse: The Search for Atrokines. Physiol. Rep. 2020, 8, e14608. [Google Scholar] [CrossRef]
- Hain, B.A.; Xu, H.; Wilcox, J.R.; Mutua, D.; Waning, D.L. Chemotherapy-Induced Loss of Bone and Muscle Mass in a Mouse Model of Breast Cancer Bone Metastases and Cachexia. JCSM Rapid Commun. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Min, K.; Kwon, O.-S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.-K.; Hwang, M.-H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased Mitochondrial Emission of Reactive Oxygen Species and Calpain Activation Are Required for Doxorubicin-Induced Cardiac and Skeletal Muscle Myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 Carcinoma Tumor-Bearing Mouse as a Model for the Study of Cancer Cachexia. JoVE J. Vis. Exp. 2016, 117, e54893. [Google Scholar] [CrossRef]
- Kwon, I. Protective Effects of Endurance Exercise on Skeletal Muscle Remodeling against Doxorubicin-Induced Myotoxicity in Mice. Phys. Act. Nutr. 2020, 24, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, M.D.; Amorese, A.J.; Balestrieri, N.P.; Fisher-Wellman, K.H.; Spangenburg, E.E. Doxorubicin Causes Lesions in the Electron Transport System of Skeletal Muscle Mitochondria That Are Associated with a Loss of Contractile Function. J. Biol. Chem. 2019, 294, 19709–19722. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Andersen, J.L.; Adamsen, L.; Lindegaard, B.; Mackey, A.L.; Nielsen, R.H.; Rørth, M.; Daugaard, G. Progressive Resistance Training and Cancer Testis (PROTRACT)—Efficacy of Resistance Training on Muscle Function, Morphology and Inflammatory Profile in Testicular Cancer Patients Undergoing Chemotherapy: Design of a Randomized Controlled Trial. BMC Cancer 2011, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Collao, N.; Sanders, O.; Caminiti, T.; Messeiller, L.; De Lisio, M. Resistance and Endurance Exercise Training Improves Muscle Mass and the Inflammatory/Fibrotic Transcriptome in a Rhabdomyosarcoma Model. J. Cachexia Sarcopenia Muscle 2023, 14, 781–793. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Sato, S.; Gao, S.; Price, R.L.; Baynes, J.W.; Kostek, M.C.; Matesic, L.E.; Carson, J.A. IL-6 Regulation on Skeletal Muscle Mitochondrial Remodeling during Cancer Cachexia in the ApcMin/+ Mouse. Skelet. Muscle 2012, 2, 14. [Google Scholar] [CrossRef]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Busquets, S.; Toledo, M.; Camperi, A.; Lopez-Soriano, F.J.; Costelli, P.; Argilés, J.M.; Penna, F. Combination of Exercise Training and Erythropoietin Prevents Cancer-Induced Muscle Alterations. Oncotarget 2015, 6, 43202–43215. [Google Scholar] [CrossRef]
- LIU, M.-H.; ZHANG, Y.; HE, J.; TAN, T.-P.; WU, S.-J.; FU, H.-Y.; CHEN, Y.-D.; LIU, J.; LE, Q.-F.; HU, H.-J.; et al. Upregulation of Peroxiredoxin III in Doxorubicin-Induced Cytotoxicity and the FoxO3a-Dependent Expression in H9c2 Cardiac Cells. Exp. Ther. Med. 2015, 10, 1515–1520. [Google Scholar] [CrossRef]
- Constantinou, C.; Fontes de Oliveira, C.C.; Mintzopoulos, D.; Busquets, S.; He, J.; Kesarwani, M.; Mindrinos, M.; Rahme, L.G.; Argilés, J.M.; Tzika, A.A. Nuclear Magnetic Resonance in Conjunction with Functional Genomics Suggests Mitochondrial Dysfunction in a Murine Model of Cancer Cachexia. Int. J. Mol. Med. 2011, 27, 15–24. [Google Scholar] [CrossRef]
- McLean, J.B.; Moylan, J.S.; Andrade, F.H. Mitochondria Dysfunction in Lung Cancer-Induced Muscle Wasting in C2C12 Myotubes. Front. Physiol. 2014, 5, 503. [Google Scholar] [CrossRef]
- Ying, W.; Garnier, P.; Swanson, R.A. NAD+ Repletion Prevents PARP-1-Induced Glycolytic Blockade and Cell Death in Cultured Mouse Astrocytes. Biochem. Biophys. Res. Commun. 2003, 308, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.A.; Moylan, J.S.; Patterson, E.W.; Smith, J.D.; Wilson, A.S.; Rabbani, Z.; Reid, M.B. Doxorubicin Acts via Mitochondrial ROS to Stimulate Catabolism in C2C12 Myotubes. Am. J. Physiol. Cell Physiol. 2012, 302, C195–C202. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.C.; Cheregi, B.D.; Timpani, C.A.; Nurgali, K.; Hayes, A.; Rybalka, E. Mitochondria: Inadvertent Targets in Chemotherapy-Induced Skeletal Muscle Toxicity and Wasting? Cancer Chemother. Pharmacol. 2016, 78, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Doerr, V.; Montalvo, R.N.; Kwon, O.S.; Talbert, E.E.; Hain, B.A.; Houston, F.E.; Smuder, A.J. Prevention of Doxorubicin-Induced Autophagy Attenuates Oxidative Stress and Skeletal Muscle Dysfunction. Antioxidants 2020, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, e7582730. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.A.; Ferreira, L.F.; Bruton, J.D.; Moylan, J.S.; Westerblad, H.; St Clair, D.K.; Reid, M.B. Doxorubicin Acts through Tumor Necrosis Factor Receptor Subtype 1 to Cause Dysfunction of Murine Skeletal Muscle. J. Appl. Physiol. 2009, 107, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. PI3 Kinase Regulation of Skeletal Muscle Hypertrophy and Atrophy. In Phosphoinositide 3-kinase in Health and Disease: Volume 1; Rommel, C., Vanhaesebroeck, B., Vogt, P.K., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin, Heidelberg, Germany, 2011; pp. 267–278. ISBN 978-3-642-13663-4. [Google Scholar]
- Merino, H.; Singla, D.K. Secreted Frizzled-Related Protein-2 Inhibits Doxorubicin-Induced Apoptosis Mediated through the Akt-mTOR Pathway in Soleus Muscle. Oxidative Med. Cell. Longev. 2018, 2018, e6043064. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; Holmes, M.; Papoutsi, E.; Panton, L.; Koutakis, P. Resistance Training, Antioxidant Status, and Antioxidant Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Exercise and Hormesis: Activation of Cellular Antioxidant Signaling Pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef]
- Silveira, L.R.; Pilegaard, H.; Kusuhara, K.; Curi, R.; Hellsten, Y. The Contraction Induced Increase in Gene Expression of Peroxisome Proliferator-Activated Receptor (PPAR)-Gamma Coactivator 1alpha (PGC-1alpha), Mitochondrial Uncoupling Protein 3 (UCP3) and Hexokinase II (HKII) in Primary Rat Skeletal Muscle Cells Is Dependent on Reactive Oxygen Species. Biochim. Biophys. Acta 2006, 1763, 969–976. [Google Scholar] [CrossRef]
- Pettersen, K.; Andersen, S.; Degen, S.; Tadini, V.; Grosjean, J.; Hatakeyama, S.; Tesfahun, A.N.; Moestue, S.; Kim, J.; Nonstad, U.; et al. Cancer Cachexia Associates with a Systemic Autophagy-Inducing Activity Mimicked by Cancer Cell-Derived IL-6 Trans-Signaling. Sci. Rep. 2017, 7, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Paranandi, K.S.; Sridharan, S.; Basu, A. Autophagy in Breast Cancer and Its Implications for Therapy. Am. J. Cancer Res. 2013, 3, 251–265. [Google Scholar]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between Autophagy Receptors and Ubiquitin-like Proteins Form the Molecular Basis for Selective Autophagy. Mol. Cell 2014, 53, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Autophagy in Health and Disease. 3. Involvement of Autophagy in Muscle Atrophy. Am. J. Physiol. Cell Physiol. 2010, 298, C1291–C1297. [Google Scholar] [CrossRef]
- Wang, X.; Blagden, C.; Fan, J.; Nowak, S.J.; Taniuchi, I.; Littman, D.R.; Burden, S.J. Runx1 Prevents Wasting, Myofibrillar Disorganization, and Autophagy of Skeletal Muscle. Genes Dev. 2005, 19, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- de Testa, M.T.J.; Cella, P.S.; Marinello, P.C.; Frajacomo, F.T.T.; de S. Padilha, C.; Perandini, P.C.; Moura, F.A.; Duarte, J.A.; Cecchini, R.; Guarnier, F.A.; et al. Resistance Training Attenuates Activation of STAT3 and Muscle Atrophy in Tumor-Bearing Mice. Front. Oncol. 2022, 12, 880787. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, I.R.; Kamen, C.; Cole, C.; Fung, C.; Heckler, C.E.; Guido, J.J.; Culakova, E.; Onitilo, A.A.; Conlin, A.; Kuebler, J.P.; et al. Effects of Exercise on Inflammation in Patients Receiving Chemotherapy: A Nationwide NCORP Randomized Clinical Trial. Support. Care Cancer 2019, 27, 4615–4625. [Google Scholar] [CrossRef]
- Kosmidou, I.; Vassilakopoulos, T.; Xagorari, A.; Zakynthinos, S.; Papapetropoulos, A.; Roussos, C. Production of Interleukin-6 by Skeletal Myotubes. Am. J. Respir. Cell Mol. Biol. 2002, 26, 587–593. [Google Scholar] [CrossRef]
- Schauer, T.; Mazzoni, A.-S.; Henriksson, A.; Demmelmaier, I.; Berntsen, S.; Raastad, T.; Nordin, K.; Pedersen, B.K.; Christensen, J.F. Exercise Intensity and Markers of Inflammation during and after (Neo-) Adjuvant Cancer Treatment. Endocr. Relat. Cancer 2021, 28, 191–201. [Google Scholar] [CrossRef]
- Serra, M.C.; Ryan, A.S.; Ortmeyer, H.K.; Addison, O.; Goldberg, A.P. Resistance Training Reduces Inflammation and Fatigue and Improves Physical Function in Older Breast Cancer Survivors. Menopause 2018, 25, 211. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.D.; Patrizi, R.M.; Cheek, D.J.; Wooten, J.S.; Barbee, J.J.; Mitchell, J.B. Resistance Training Reduces Subclinical Inflammation in Obese, Postmenopausal Women. Med. Sci. Sports Exerc. 2012, 44, 2099. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.; Lopes, M.A.; Franco, F.O.; Knobl, P.; Santos, K.B.; Bueno, L.L.; Correa, V.A.; Bedard, A.H.; Guilherme, A.; Birbrair, A.; et al. Toll-Like Receptor-4 Disruption Suppresses Adipose Tissue Remodeling and Increases Survival in Cancer Cachexia Syndrome. Sci. Rep. 2018, 8, 18024. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Noguchi, Y.; Matsumoto, A. Effects of Tumor Removal and Body Weight Loss on Insulin Resistance in Patients with Cancer. Surgery 1994, 116, 62–66. [Google Scholar] [PubMed]
- Noguchi, Y.; Yoshikawa, T.; Marat, D.; Doi, C.; Makino, T.; Fukuzawa, K.; Tsuburaya, A.; Satoh, S.; Ito, T.; Mitsuse, S. Insulin Resistance in Cancer Patients Is Associated with Enhanced Tumor Necrosis Factor-α Expression in Skeletal Muscle. Biochem. Biophys. Res. Commun. 1998, 253, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary Exercise Prevents Cisplatin-Induced Muscle Wasting during Chemotherapy in Mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Johannsen, N.M.; Swift, D.L.; Gillison, F.B.; Mikus, C.R.; Lucia, A.; Kramer, K.; Lavie, C.J.; Church, T.S. Aerobic and Strength Training in Concomitant Metabolic Syndrome and Type 2 Diabetes. Med. Sci. Sports Exerc. 2014, 46, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective Exercise Modality to Reduce Insulin Resistance in Women with Type 2 Diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1α Protects Skeletal Muscle from Atrophy by Suppressing FoxO3 Action and Atrophy-Specific Gene Transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Arany, Z.; Lebrasseur, N.; Morris, C.; Smith, E.; Yang, W.; Ma, Y.; Chin, S.; Spiegelman, B.M. The Transcriptional Coactivator PGC-1β Drives the Formation of Oxidative Type IIX Fibers in Skeletal Muscle. Cell Metab. 2007, 5, 35–46. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Padilha, C.S.; Borges, F.H.; Costa Mendes da Silva, L.E.; Frajacomo, F.T.T.; Jordao, A.A.; Duarte, J.A.; Cecchini, R.; Guarnier, F.A.; Deminice, R. Resistance Exercise Attenuates Skeletal Muscle Oxidative Stress, Systemic Pro-Inflammatory State, and Cachexia in Walker-256 Tumor-Bearing Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 916–923. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Plummer, E.; Thomas, M.; Hennebry, A.; Ashby, M.; Ling, N.; Smith, H.; Sharma, M.; Kambadur, R. Myostatin Induces Cachexia by Activating the Ubiquitin Proteolytic System through an NF-κB-Independent, FoxO1-Dependent Mechanism. J. Cell. Physiol. 2006, 209, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed Muscle Protein Synthesis and Breakdown after Resistance Exercise in Humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Adams, G.R. Role of Insulin-like Growth Factor-I in the Regulation of Skeletal Muscle Adaptation to Increased Loading. Exerc. Sport Sci. Rev. 1998, 26, 31–60. [Google Scholar] [CrossRef]
- Basualto-Alarcón, C.; Jorquera, G.; Altamirano, F.; Jaimovich, E.; Estrada, M. Testosterone Signals through mTOR and Androgen Receptor to Induce Muscle Hypertrophy. Med. Sci. Sports Exerc. 2013, 45, 1712–1720. [Google Scholar] [CrossRef]
- Alexander, S.E.; Abbott, G.; Aisbett, B.; Wadley, G.D.; Hnatiuk, J.A.; Lamon, S. Total Testosterone Is Not Associated with Lean Mass or Handgrip Strength in Pre-Menopausal Females. Sci. Rep. 2021, 11, 10226. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.K.; Rook, K.M.; Siddle, N.C.; Bruce, S.A.; Woledge, R.C. Muscle Weakness in Women Occurs at an Earlier Age than in Men, but Strength Is Preserved by Hormone Replacement Therapy. Clin. Sci. 1993, 84, 95–98. [Google Scholar] [CrossRef]
- Smith, G.I.; Yoshino, J.; Reeds, D.N.; Bradley, D.; Burrows, R.E.; Heisey, H.D.; Moseley, A.C.; Mittendorfer, B. Testosterone and Progesterone, but Not Estradiol, Stimulate Muscle Protein Synthesis in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 256–265. [Google Scholar] [CrossRef]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.-K.; Laakkonen, E.K.; Sipilä, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381. [Google Scholar] [CrossRef]
- de Jonge, X.A.K.J.; Boot, C.R.L.; Thom, J.M.; Ruell, P.A.; Thompson, M.W. The Influence of Menstrual Cycle Phase on Skeletal Muscle Contractile Characteristics in Humans. J. Physiol. 2001, 530, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.; Han, A.; Hinrichs, T.; Vorgerd, M.; Manchado, C.; Platen, P. Effects of Follicular versus Luteal Phase-Based Strength Training in Young Women. Springerplus 2014, 3, 668. [Google Scholar] [CrossRef] [PubMed]
- Ruzić, L.; Matković, B.R.; Leko, G. Antiandrogens in Hormonal Contraception Limit Muscle Strength Gain in Strength Training: Comparison Study. Croat. Med. J. 2003, 44, 65–68. [Google Scholar] [PubMed]

| Article | Randomization | Exercise | Muscle | Cancer Stage | Chemotherapy | Data Collected | Outcomes |
|---|---|---|---|---|---|---|---|
| Pre-Clinical Studies | |||||||
| Padrão et al. [31] | Female Sprague-Dawley rats injected with N-Methyl-N-nitrosourea (MNU; 50 mg/kg) at 50 days of age and divided into 2 groups: MNU + SED; MNU + EX | Started 2 days after tumor induction and performed for 35 weeks on a treadmill (20 m/min; 60 min/day; 5 days/week). | Gastrocnemius | Mammary lesions (induced by MNU (50 mg/kg)with 100% incidence | No treatment |
|
|
| Alves de Lima et al. [32] | C57 BL/6 mice were subcutaneously implanted with Lewis lung carcinoma cells (8–10 wks old) divided into 3 groups: LLC; LLC + DOX; LLC + DOX + EXER | Started 1 wk after tumor inoculation and performed for 2 wk (21 d) or 3 wk (28 d). Aerobic: 5 times/wk, 40–60 min, on treadmill at 60% oxygen consumption | Gastrocnemius | Syngeneic model but not with BC | Doxorubicin 2 times/wk (cumulative dose 10 mg/kg) |
|
|
| Ballarò et al. [33] | Female and male BALB/c mice (6 wks old) divided in 4 groups: Control (n = 7), C26 (n = 6), C26 OXFU (n = 8) and C26 OXFU ex (n = 8) C26 mice were subcutaneously implanted with Colon 26 carcinoma cells | Combined: 5 times/wk for 28 d in custom motorized wheel. Started 5 d before tumor injection at 5 m/min for 15 min, and ↑ until 11 m/min for 45 min. | Gastrocnemius; Tibialis anterior | Syngeneic model but not with BC | Oxaliplatin (6 mg/kg) followed (2 h later) by 5-fluorouracil (50 mg/kg), weekly (for 28 d) starting 7 d after tumor injection. |
|
|
| Smuder et al. [34] | 23 male adult (6 month old) Sprague-Dawley rats, divided by 4 groups SED (n = 7); EXETR (n = 6); SEDDOX (n = 5); EXDOX (n = 6) | Aerobic: Treadmill running 5 times/wk (10, 20, 30, 40, 50 min/d on days 1–5). After 2 d rest, performed 60 min/day at 30 m/min (70% oxygen consumption) for more 5 d. | Soleus | No cancer | Doxorubicin (20 mg/kg), administrated 24 h before sacrifice |
|
|
| Bredahl et al. [35] | 60 Sprague-Dawley rats, were divided in 4 groups (RT (n = 20); TM (n = 20); SED (n = 20)). After the exercise protocol were divided in 6 groups of 10 rats (RT + SAL;TM + SAL;SED + SAL;RT + DOX; TM + DOX; SED + DOX) | A total of 10 wks. RT: Jump to reach an elevated food and water container, with ↑height during the wk until reaching 8 inches by wk 8. TM: Progressive treadmill training until 60 min, 30 m/min and 18°, representing 65–75% of oxygen consumption. | Soleus; EDL | No cancer | A single doxorubicin administration (15 mg/Kg) after the 10 wk exercise program |
|
|
| Quinn et al. [36] | 47 male Sprague-Dawley rats (10 wk old) divided in 2 groups: SED (n = 20); EXER (n = 27), after 2 wks they were separated into 4 groups: SED + SAL (n = 10); SED + DOX (n = 10); EXER + SAL (n = 13); EXER + DOX (n = 14) | Aerobic: Progressive treadmill protocol, starting at 30 m/min for 10 min by wk 1, and ↑ until 60 min by wk 2. | Soleus; EDL; DIA | No cancer | A single doxorubicin administration (15 mg/Kg) 24 h after the last exercise session |
|
|
| Clinical Studies | |||||||
| Courneya et al. [27] | 242 women (49 ± 30 years old), only 219 reported the studied outcomes with 80 patients in each group (AET; RET; UC) | Started 1 wk before chemo. and ended 3 wk after last administration (17 wk) AET: at 60% VO2max* (up 10% every 6 wk until 80%) during 15 min (up 5 min every 3 wk until 45 min) RET: 2 sets of 8–12 reps of 9 exe. (60 or 70% the maximal strength), if 12 reps were performed load ↑ 10% | Not available | Breast Cancer in stage I to IIIA | Taxanes or non-taxanes |
|
|
| Klassen et al. [37] | 281 women with (57.1 ± 6.1 years old), divided into 5 groups (No CT (n = 105); Started CT (n = 91); Post neo-adj. CT (n = 31); Post adj. CT (n = 28); Healthy (n = 26)) | Resistance: A total of 12 wk, 2 times/wk. For 60 min, 8 exe. with 1–3 sets, with weight to 8–12 reps (60–80% RM*). 1 min of rest between sets and if 12 reps were performed, the weigh ↑ 5%. | Not available | Breast cancer in stage 0 to III after lumpectomy or mastectomy | Taxanes and/or Anthracyclines and/or Herceptin |
|
|
| Wengström et al. [30] | 240 women (18–70 years old) divided into 3 groups (AET (n = 80); CT (n = 80); UC (n = 80)) | 60 min, 2 times/wk, 16 wk, with 5 min warm-up in aerobic machine at 10–12 RPE* and 10 min cool-down with scratching exercises. AET: 20 min at 13–15 RPE, followed by 3 × 3 min bouts at 16–18 RPE interspersed with ~1 min of passive or active recovery. CT: 8 exe., 2 sets of 10–12 reps at 70% RM and ↑ to 80% if 12 reps were performed, and 3 × 3 min bouts of AET. | Vastus lateralis | Breast cancer in stage I to IIIa undergoing chemotherapy | Taxanes or Anthracyclines |
|
|
| Schmidt et al. [28] | 67 women (18–70 years old) divided into 3 groups (RT (n = 21); ET (n = 20); SC (n = 26)) | 2 times/wk, 60 min, 12 wk. ET: 10 min warm-up, followed by 25–30 min exercising on at 11–14 RPE, and 10 min cool-down. RT: 10 exe. at 1 RM to complete 20 reps. | Not available | Breast cancer with moderate- or high-risk | Adj. chemotherapy |
|
|
| Møller et al. [29] | 10 patients (28–62 years old). Biopsy only from 6 patients | Combined: 3 times/wk, 90-min, 10 wk. A session of 6 resistance exe. at 1 RM (progressively increasing), 10 min break, and patients consumed a whey protein drink (360 kcal), plus group sessions on ergometer bicycles (progressively increasing intensity). | Vastus lateralis | Breast cancer (n = 7), head and neck cancer (n= 1), rectal cancer (n = 1), sarcoma (n = 1) | Epirubicin and/or cyclophosphamide and/or doxorubicin/adramycin and/or carboplatin and/or vinorelbin/navelbine and/or flouroacil and/or trabectedin and/or gemcitabin |
|
|
| Winters-Stone et al. [38] | 144 women ≥65 years old, divided into 3 groups: AET (n = 37), RET (n = 39) or FLEX (n = 38) | Supervised for 12 months, for 60-min, 3 times/wk: AET: low-impact dance, progressed from 20 to 45 min and increasing from 35% to 65% of HRR*. RET: 10 exercises, 2–3 sets of 10–15 reps at RM, (progressively increasing); FLEX: Stretching and relaxation exercise, 2–3 reps, holding for 15–60 s; Unsupervised for 6 months, with DVD-based version of their program, 3 times/wk. | Not available | Breast cancer stage I-III. | ≥2 years postchemotherapy and/or radiation therapy |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aires, I.; Duarte, J.A.; Vitorino, R.; Moreira-Gonçalves, D.; Oliveira, P.; Ferreira, R. Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7533. https://doi.org/10.3390/ijms25147533
Aires I, Duarte JA, Vitorino R, Moreira-Gonçalves D, Oliveira P, Ferreira R. Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy. International Journal of Molecular Sciences. 2024; 25(14):7533. https://doi.org/10.3390/ijms25147533
Chicago/Turabian StyleAires, Inês, José Alberto Duarte, Rui Vitorino, Daniel Moreira-Gonçalves, Paula Oliveira, and Rita Ferreira. 2024. "Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy" International Journal of Molecular Sciences 25, no. 14: 7533. https://doi.org/10.3390/ijms25147533
APA StyleAires, I., Duarte, J. A., Vitorino, R., Moreira-Gonçalves, D., Oliveira, P., & Ferreira, R. (2024). Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy. International Journal of Molecular Sciences, 25(14), 7533. https://doi.org/10.3390/ijms25147533








