The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation
Abstract
1. Introduction
2. Results
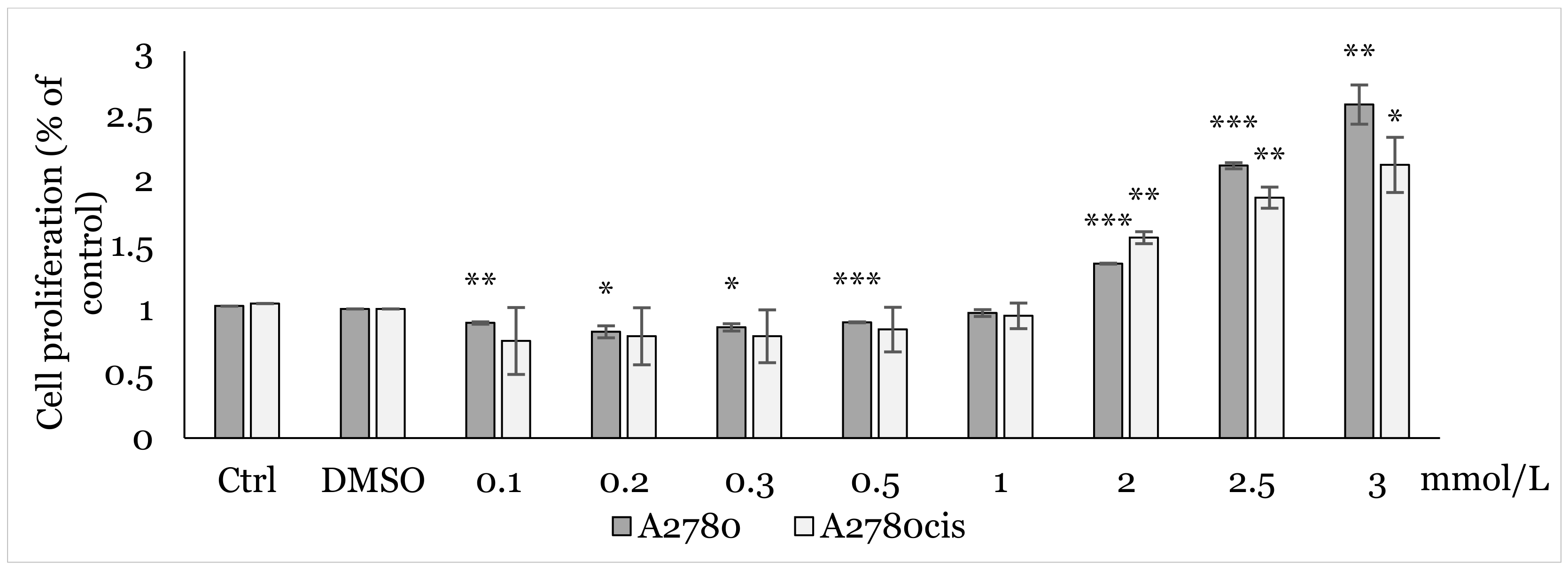
2.1. MTT Screening Assay
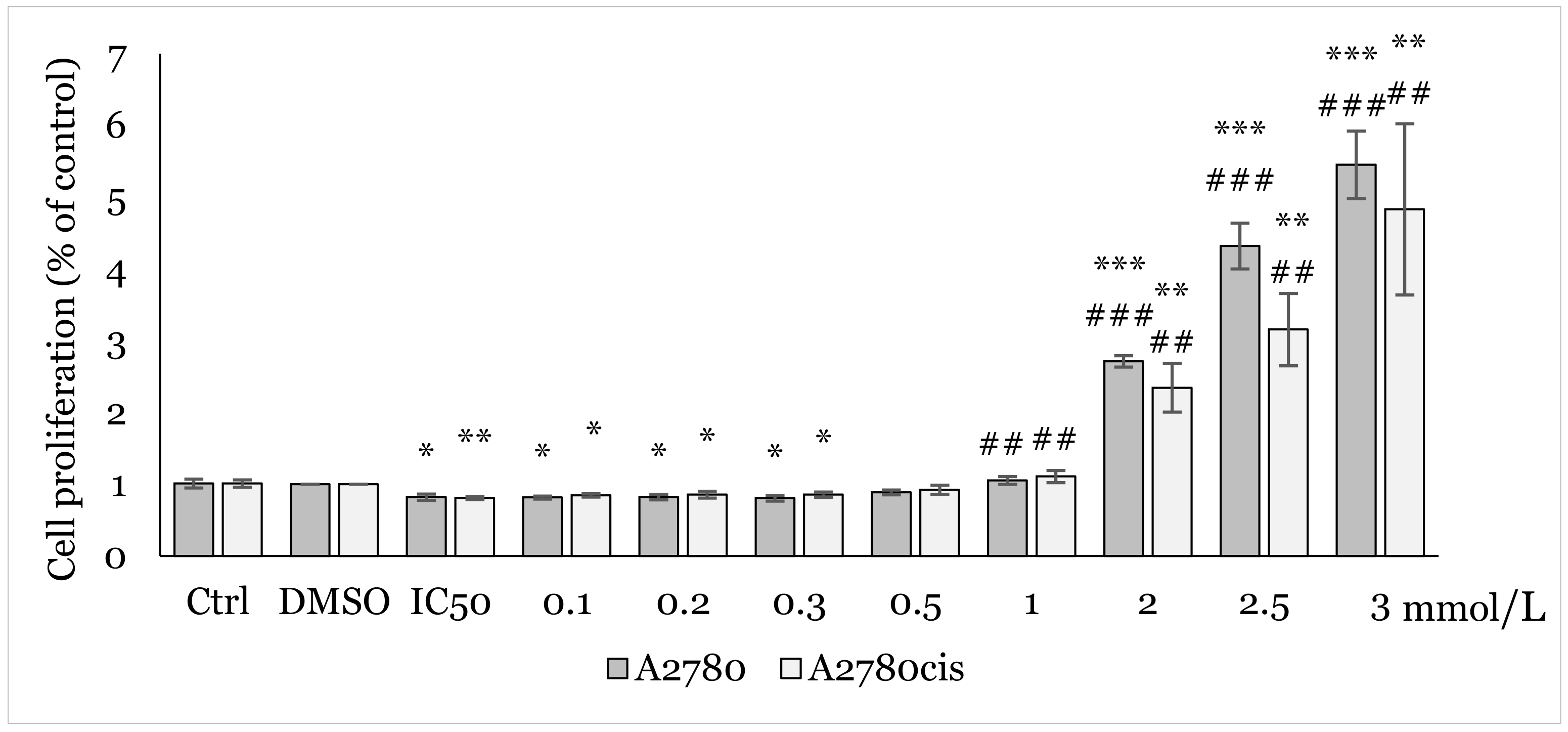
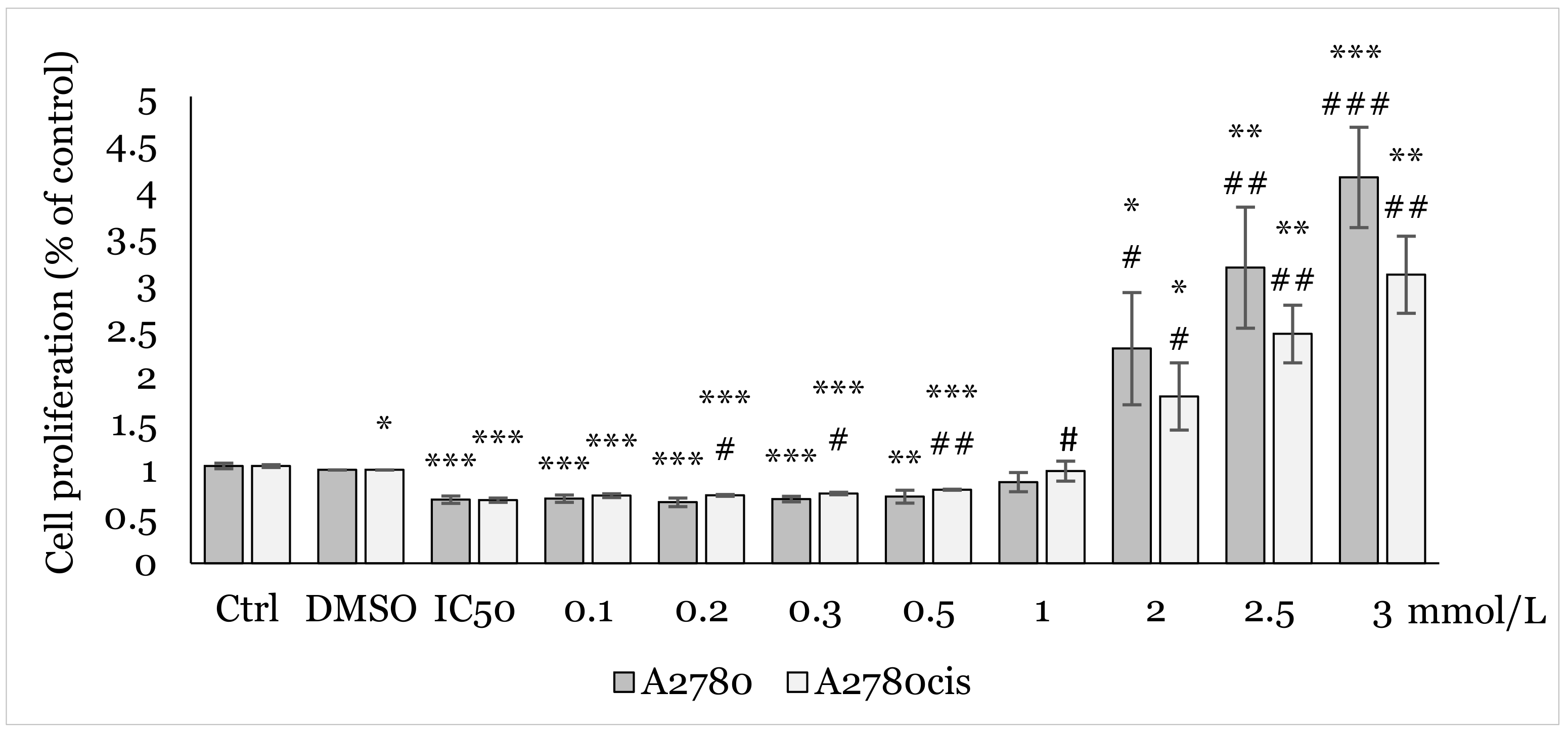
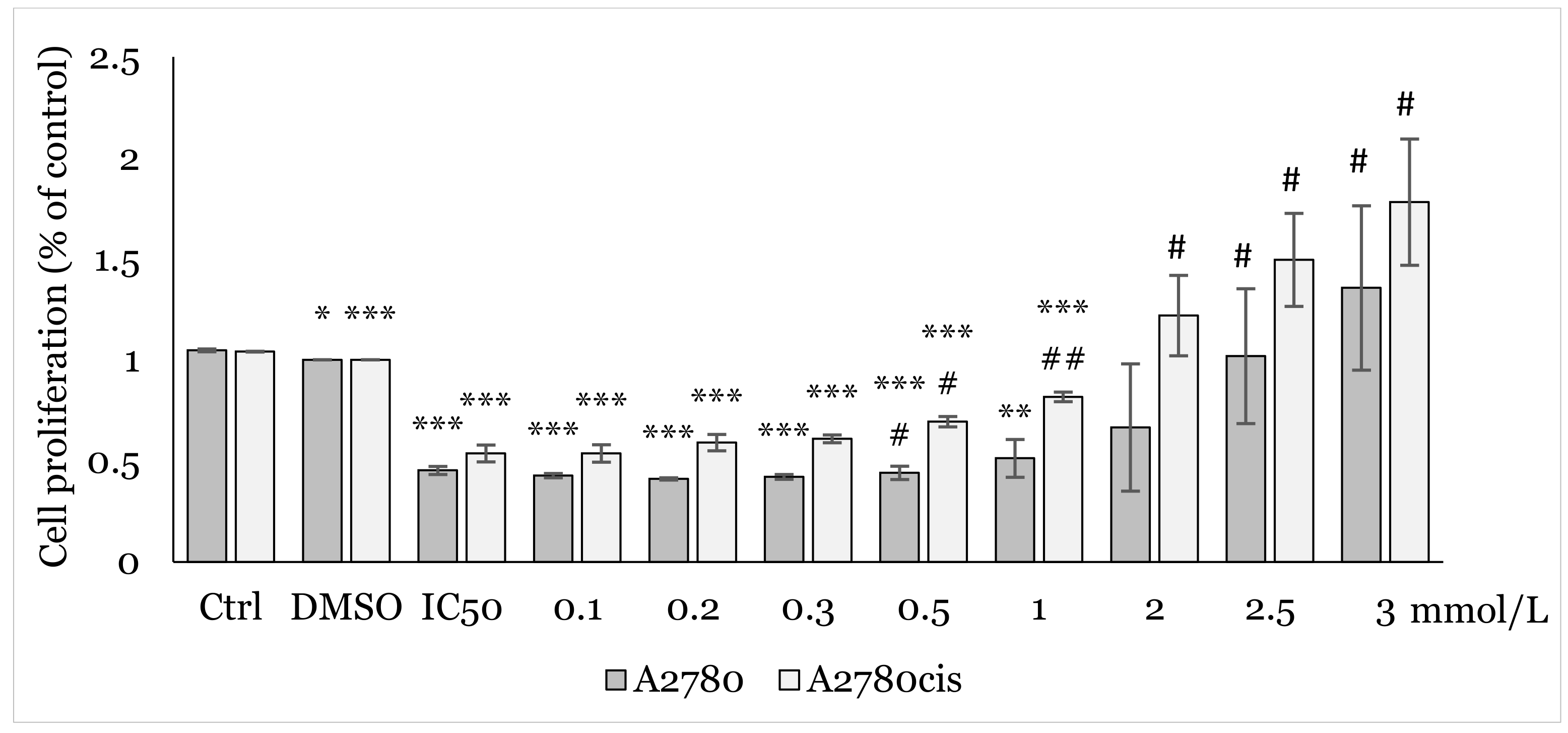
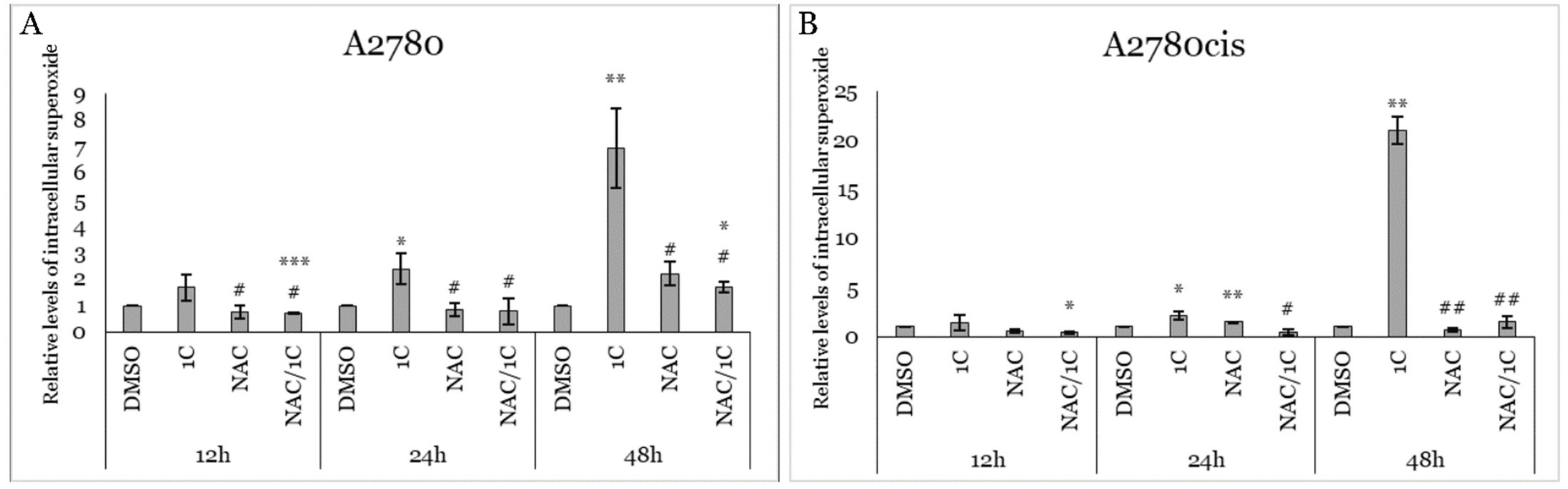
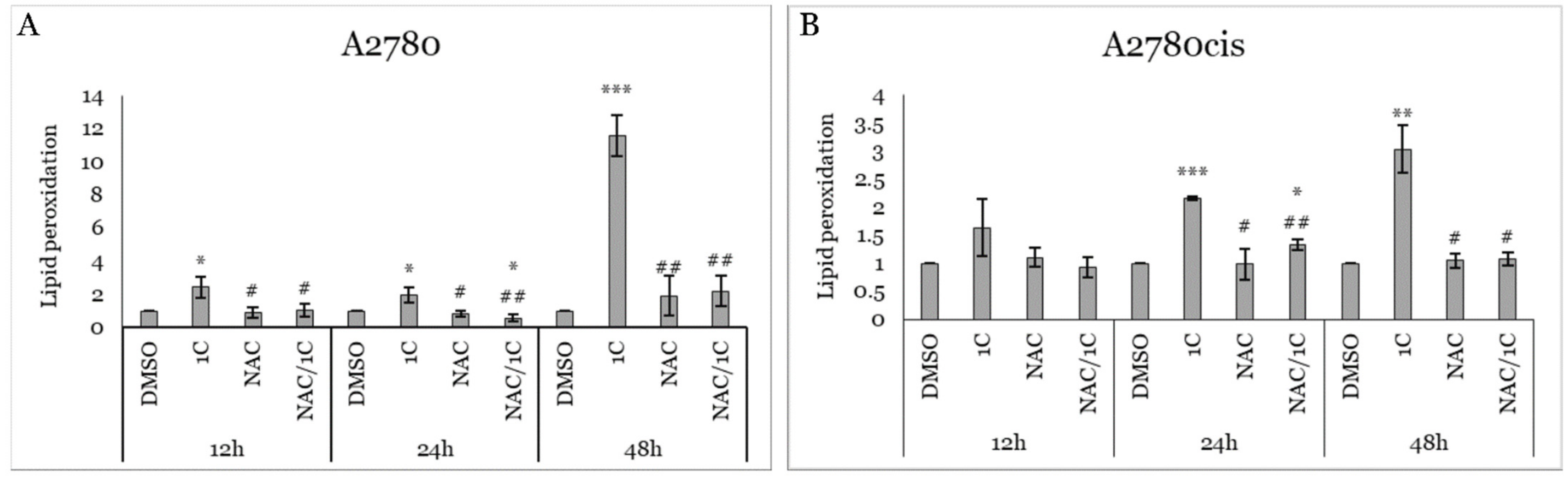
2.2. Effect of 1C and NAC on Oxidative Stress
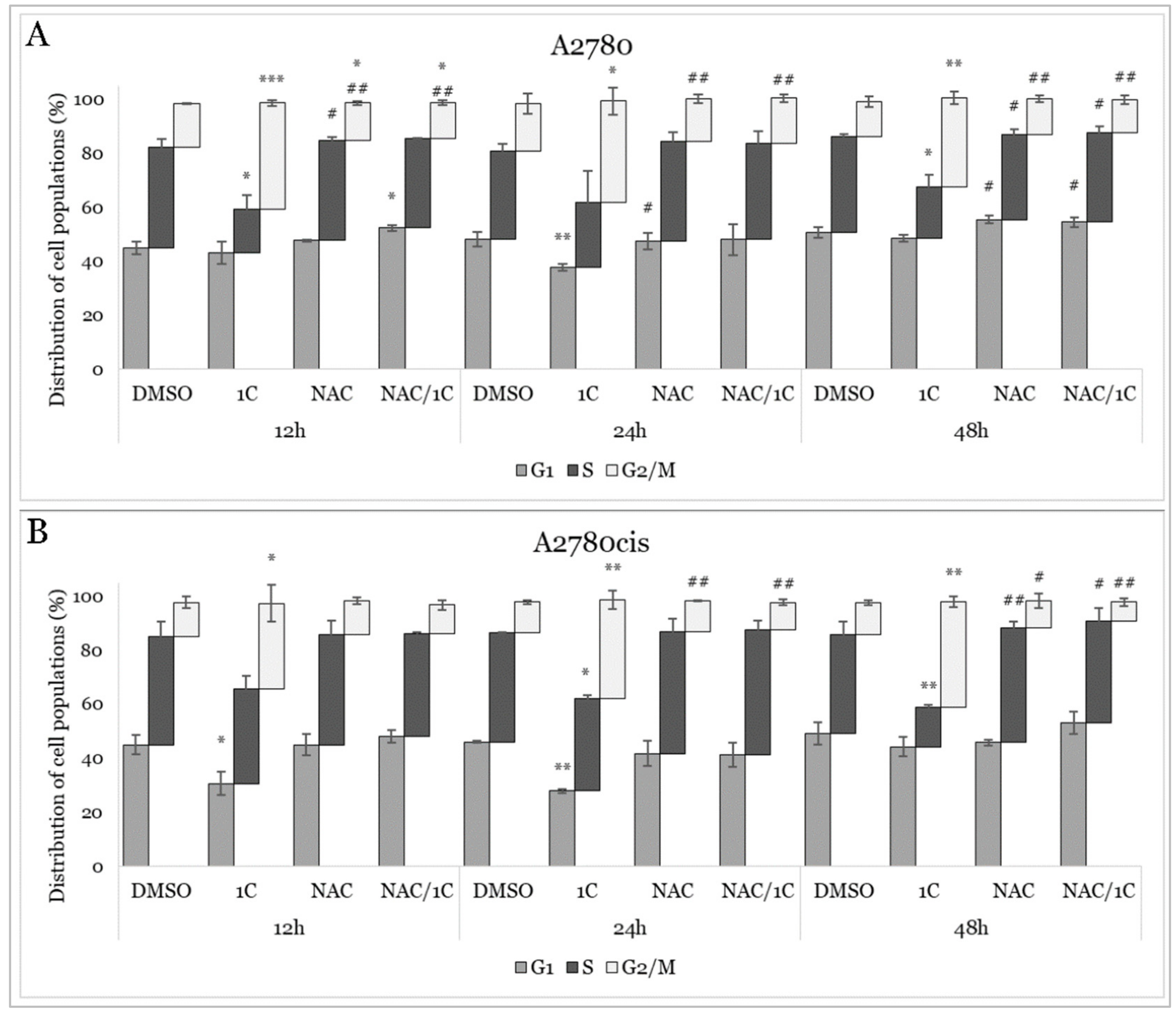
2.3. Cell Cycle Analysis
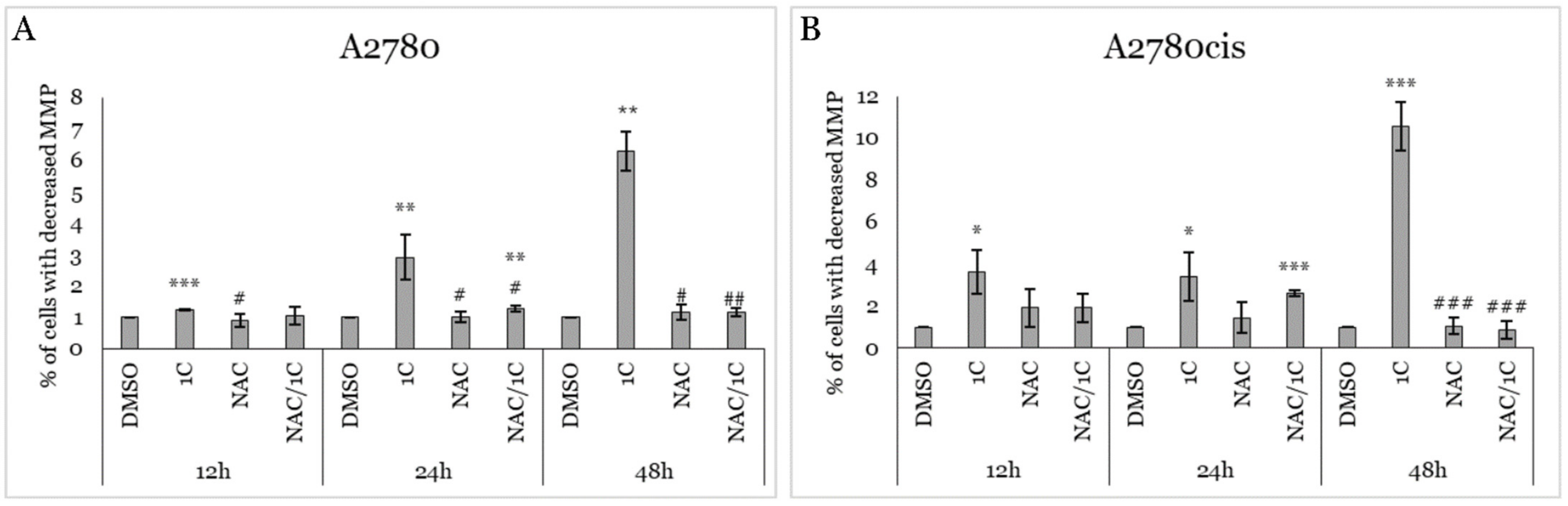
2.4. Impact of 1C, NAC, and Their Combination on MMP
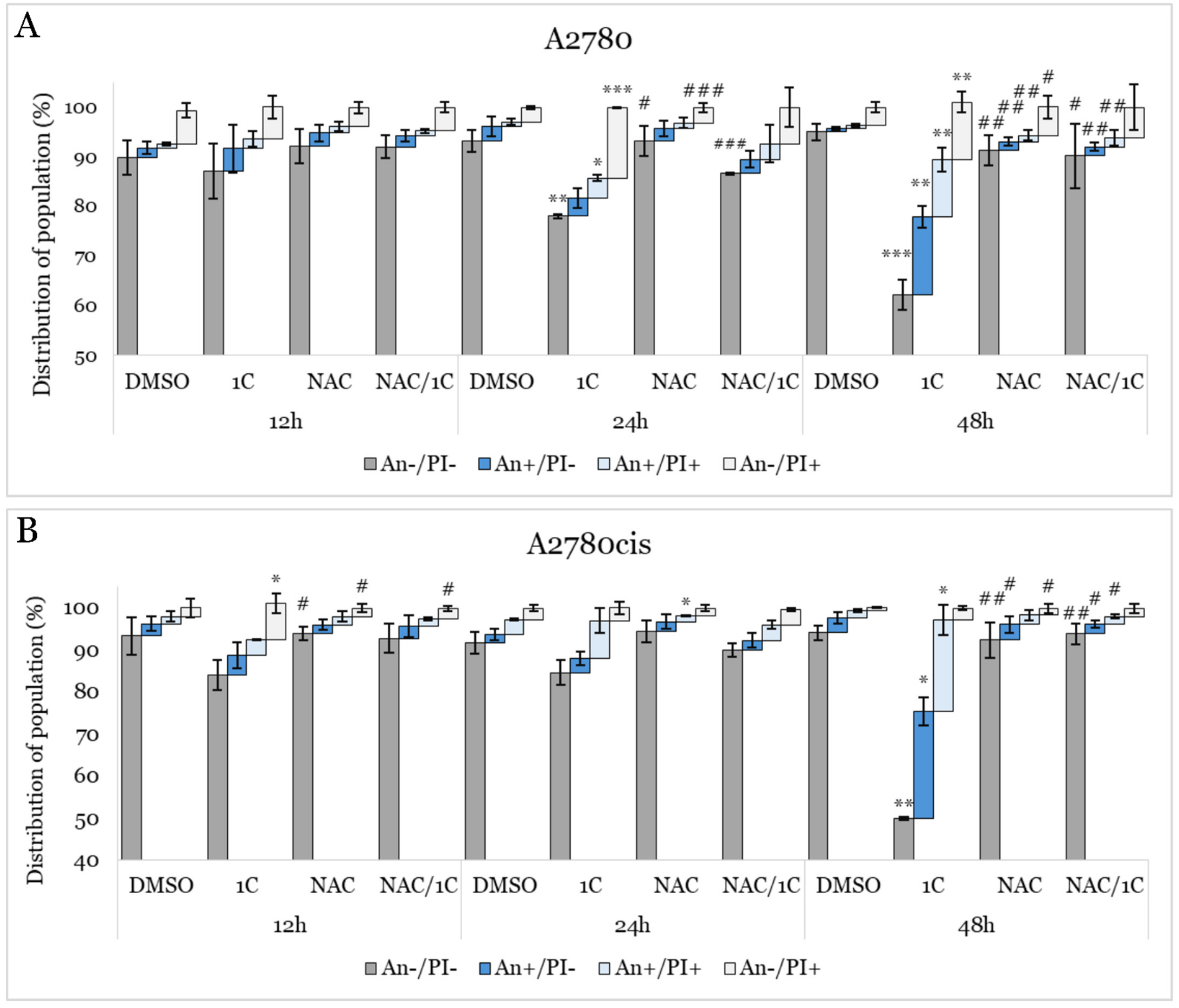
2.5. Apoptosis Detection
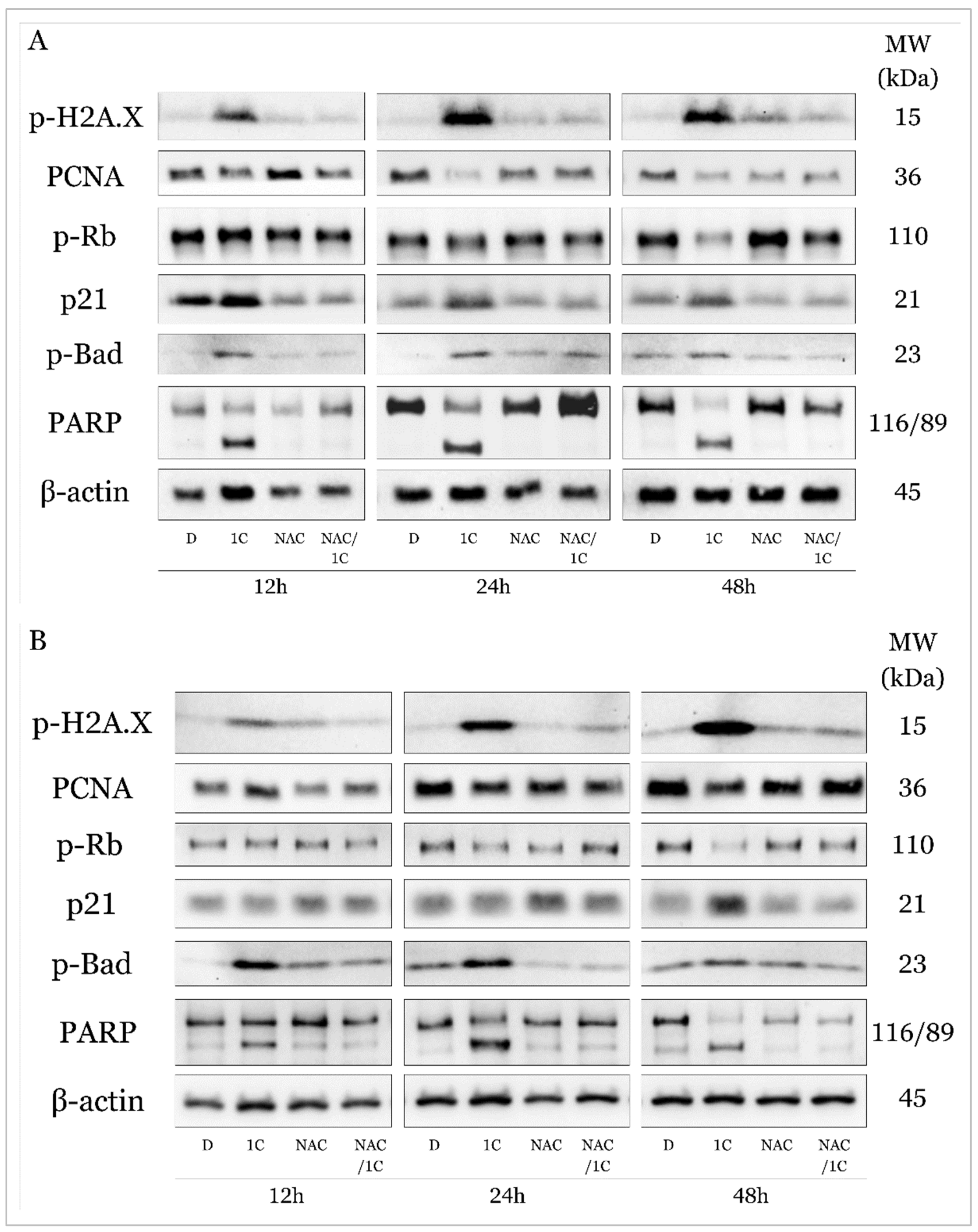
2.5.1. C modulates Expression of Cell Cycle and Apoptosis Regulating Proteins
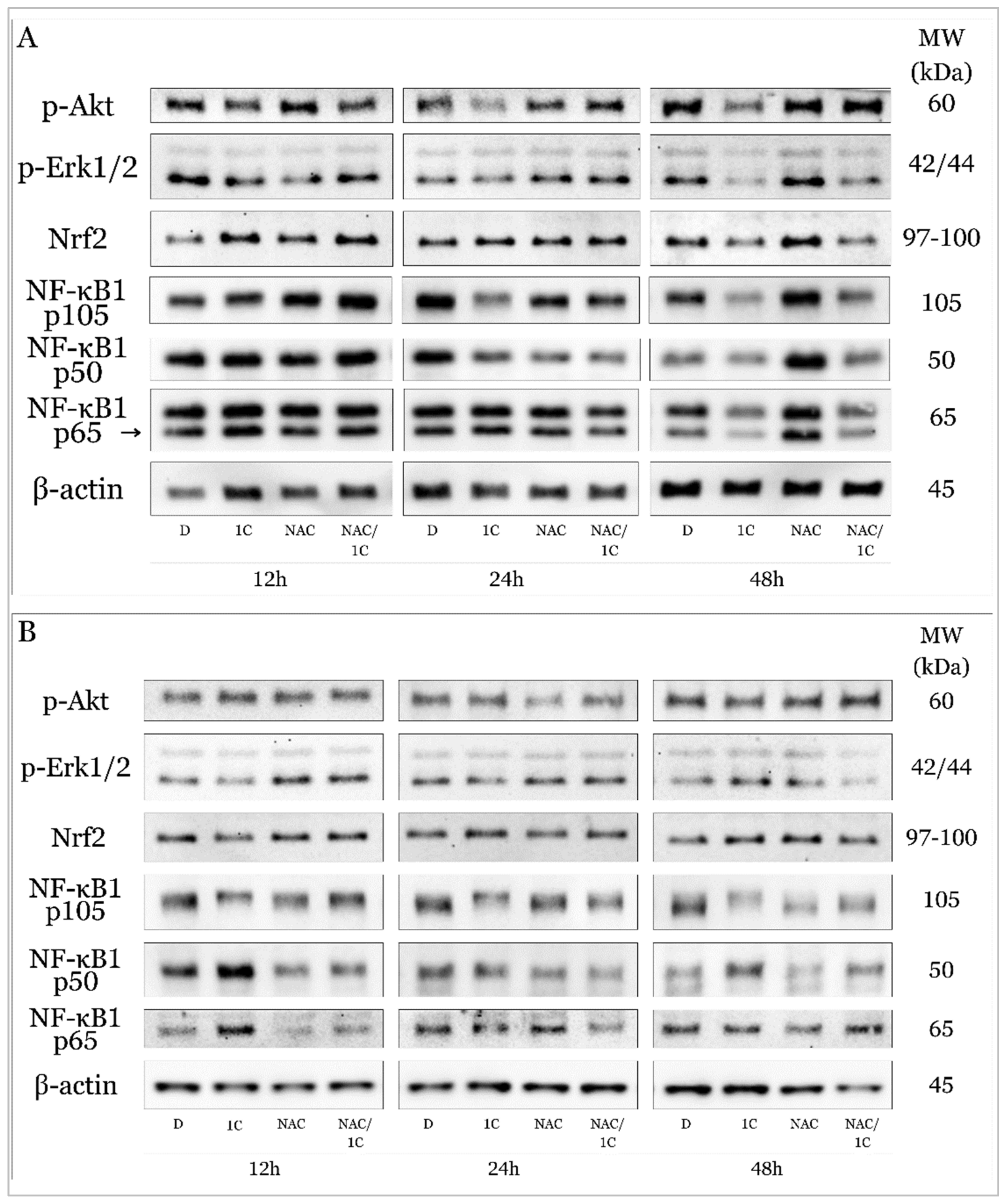
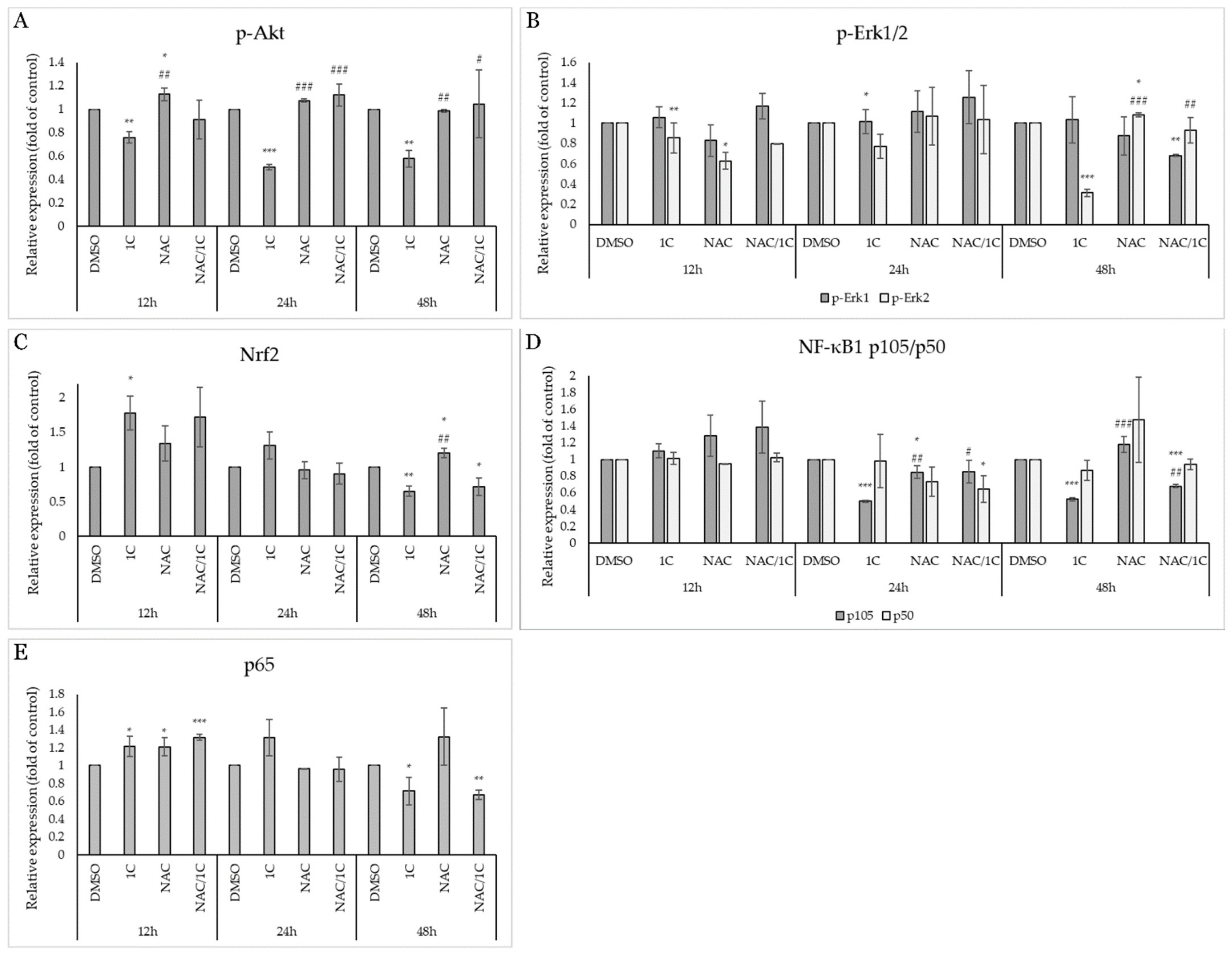
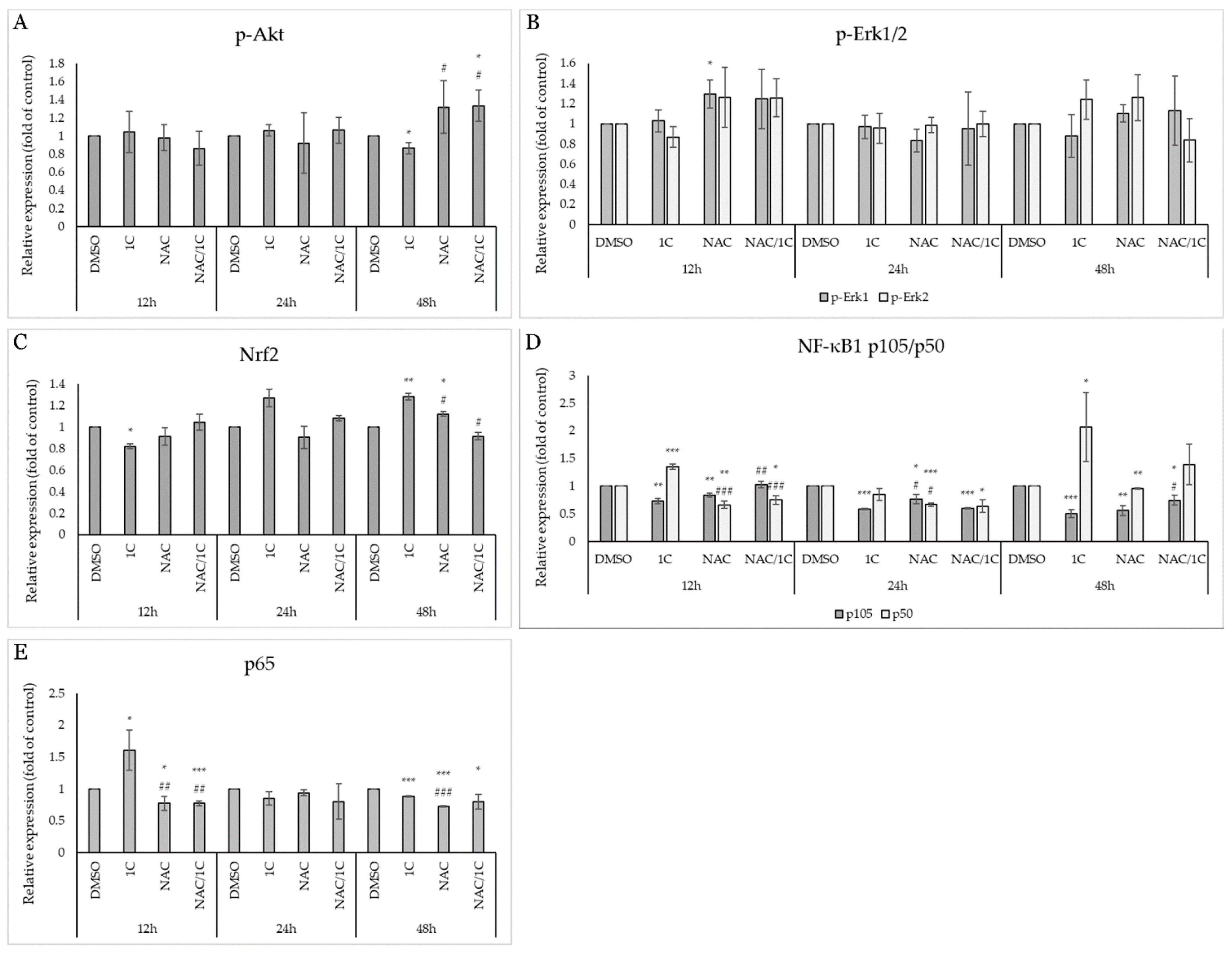
2.5.2. 1C Modulates Signaling Pathways Associated with Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Tested Compounds
4.2. Cell Culture
4.3. MTT Viability Assay
4.4. Flow Cytometry Analyses
4.4.1. Apoptosis Analysis
4.4.2. Analysis of Mitochondrial Membrane Potential Changes (MMP)
4.4.3. Cell Cycle Analysis
4.4.4. ROS Production Analysis
4.4.5. Lipid Peroxidation Analysis
4.4.6. Mitochondrial Superoxide Anion Production Analysis
4.5. Western Blot Analyses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Perol, D.; Gonzalez-Martin, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Maenpaa, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Majd, F.S.; Talebi, S.S.; Ahmad Abadi, A.N.; Poorolajal, J.; Dastan, D. Efficacy of a standardized herbal product from Pistacia atlantica subsp. Kurdica in type 2 diabetic patients with hyperlipidemia: A triple-blind randomized clinical trial. Complement. Ther. Clin. Pract. 2022, 48, 101613. [Google Scholar] [CrossRef] [PubMed]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2,3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar] [PubMed]
- Kubatka, P.; Mazurakova, A.; Koklesova, L.; Kuruc, T.; Samec, M.; Kajo, K.; Kotorova, K.; Adamkov, M.; Smejkal, K.; Svajdlenka, E.; et al. Salvia officinalis L. exerts oncostatic effects in rodent and in vitro models of breast carcinoma. Front. Pharmacol. 2024, 15, 1216199. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef] [PubMed]
- Usui-Kawanishi, F.; Kani, K.; Karasawa, T.; Honda, H.; Takayama, N.; Takahashi, M.; Takatsu, K.; Nagai, Y. Isoliquiritigenin inhibits NLRP3 inflammasome activation with CAPS mutations by suppressing caspase-1 activation and mutated NLRP3 aggregation. Genes Cells 2024, 29, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Lu, J.; Gao, C.; Liu, Q.; Yao, W.; Wang, T.; Wang, X.; Wang, Z. Isobavachalcone exhibits antifungal and antibiofilm effects against C. albicans by disrupting cell wall/membrane integrity and inducing apoptosis and autophagy. Front. Cell Infect. Microbiol. 2024, 14, 1336773. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Ali Shah, S.W.; Zahoor, M.; Ullah, R.; Alotaibi, A. Chalcones: The flavonoid derivatives synthesis, characterization, their antioxidant and in vitro/in vivo antidiabetic potentials. Heliyon 2023, 9, e22546. [Google Scholar] [CrossRef]
- Lin, J.H.; Yang, K.T.; Ting, P.C.; Lee, W.S.; Lin, D.J.; Chang, J.C. Licochalcone a improves cardiac functions after ischemia-reperfusion via reduction of ferroptosis in rats. Eur. J. Pharmacol. 2023, 957, 176031. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Wu, M.; Yu, H.; Long, G.; Gui, Z.; Li, X.; Chen, H.; Jia, Z.; Xia, W. Isoliquiritin Ameliorates Cisplatin-Induced Renal Proximal Tubular Cell Injury by Antagonizing Apoptosis, Oxidative Stress and Inflammation. Front. Med. 2022, 9, 873739. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Mino, M.; Yamak, J.; Nguyen, V.; Lopez, D.; Pham, V.; Fazelpour, A.; Le, V.; Fu, D.; Tippin, M.; et al. Flavokawain A Reduces Tumor-Initiating Properties and Stemness of Prostate Cancer. Front. Oncol. 2022, 12, 943846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, L.; Xie, J.; Simoneau, A.R.; Uchio, E.; Zi, X. Chemoprevention of Urothelial Cell Carcinoma Tumorigenesis by Dietary Flavokawain A in UPII-Mutant Ha-ras Transgenic Mice. Pharmaceutics 2022, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, K.S.; Squarisi, I.S.; Acesio, N.O.; Nicolella, H.D.; Ozelin, S.D.; Reis Santos de Melo, M.; Guissone, A.P.P.; Fernandes, G.; Silva, L.M.; da Silva Filho, A.A.; et al. Licochalcone A, a licorice flavonoid: Antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J. Toxicol. Environ. Health A 2020, 83, 673–686. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Aparna, J.S.; Babu, A.; Paul, A.M.; Lankadasari, M.B.; Athira, S.R.; Kumar, S.S.; Vijayan, Y.; Namitha, N.N.; Mohammed, S.; et al. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules 2021, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Hseu, Y.C.; Thiyagarajan, V.; Lin, K.Y.; Way, T.D.; Korivi, M.; Liao, J.W.; Yang, H.L. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch. Toxicol. 2017, 91, 3341–3364. [Google Scholar] [CrossRef]
- Jin, H.; Seo, G.S.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharmacol. 2018, 841, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol protects against Azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhong, B.; Hou, Y.; Wang, M.; Guo, B.; Lin, L.; Zhou, Y.; Chen, X. Fighting cancer by triggering non-canonical mitochondrial permeability transition-driven necrosis through reactive oxygen species induction. Free Radic. Biol. Med. 2023, 202, 35–45. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; El-Shorbagy, H.M.; Hassaneen, H.M.; Abdelhamid, I.A.; Sabet, S.; Ibrahim, S.A. [1,2,4] Triazolo [3,4-a]isoquinoline chalcone derivative exhibits anticancer activity via induction of oxidative stress, DNA damage, and apoptosis in Ehrlich solid carcinoma-bearing mice. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, N.; Olszewski, M.; Jurasz, J.; Serocki, M.; Dzierzynska, M.; Cekala, K.; Wieczerzak, E.; Baginski, M. Novel chalcone-derived pyrazoles as potential therapeutic agents for the treatment of non-small cell lung cancer. Sci. Rep. 2022, 12, 3703. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Pinho, S.A.; Afonso, G.J.M.; Oliveira, P.J.; Cunha-Oliveira, T.; Saso, L. NRF2 and Mitochondrial Function in Cancer and Cancer Stem Cells. Cells 2022, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Buha, A.; Telkoparan-Akillilar, P.; Cevik, D.; Kouretas, D.; Veskoukis, A.; Skaperda, Z.; Tsatsakis, A.; Wallace, D.; Suzen, S.; et al. Potential Applications of NRF2 Modulators in Cancer Therapy. Antioxidants 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Saso, L. Inhibition of the NRF2/KEAP1 Axis: A Promising Therapeutic Strategy to Alter Redox Balance of Cancer Cells. Antioxid. Redox Signal. 2021, 34, 1428–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pan, Q. Butein Inhibits Oxidative Stress Injury in Rats with Chronic Heart Failure via ERK/Nrf2 Signaling. Cardiovasc. Ther. 2022, 2022, 8684014. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharmacother. 2022, 146, 112594. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Carradori, S.; Resende, D.; Saso, L.; Ricci, A.; Palmeira, A.; Cataldi, A.; Pinto, M.; Sousa, E. Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages. Int. J. Mol. Sci. 2022, 23, 13319. [Google Scholar] [CrossRef]
- Laphanuwat, P.; Kongpetch, S.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Licochalcone A Induces Cholangiocarcinoma Cell Death Via Suppression of Nrf2 and NF-kappaB Signaling Pathways. Asian Pac. J. Cancer Prev. 2022, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R.; et al. Cardamonin inhibits breast cancer growth by repressing HIF-1alpha-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, S.H.; Cho, S.; Lee, I.S.; Kang, B.Y.; Choi, H.J. 4-methoxychalcone enhances cisplatin-induced oxidative stress and cytotoxicity by inhibiting the Nrf2/ARE-mediated defense mechanism in A549 lung cancer cells. Mol. Cells 2013, 36, 340–346. [Google Scholar] [PubMed]
- Takac, P.; Kello, M.; Pilatova, M.B.; Kudlickova, Z.; Vilkova, M.; Slepcikova, P.; Petik, P.; Mojzis, J. New chalcone derivative exhibits antiproliferative potential by inducing G2/M cell cycle arrest, mitochondrial-mediated apoptosis and modulation of MAPK signalling pathway. Chem. Biol. Interact. 2018, 292, 37–49. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.W. The apple dihydrochalcone phloretin suppresses growth and improves chemosensitivity of breast cancer cells via inhibition of cytoprotective autophagy. Food Funct. 2021, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Yuan, X.; Li, Y.; Hou, G.; Liu, X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/beta-catenin signal pathway. Investig. New Drugs 2020, 38, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Lusvarghi, S.; Hsiao, S.H.; Liu, T.C.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. Licochalcone A Selectively Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. J. Nat. Prod. 2020, 83, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Peng, Y.; Lin, F.; Singh, R.K.; Mahato, R.I. Micellar Delivery of miR-34a Modulator Rubone and Paclitaxel in Resistant Prostate Cancer. Cancer Res. 2017, 77, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Zigova, M.; Miskufova, V.; Budovska, M.; Michalkova, R.; Mojzis, J. Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules 2024, 29, 1773. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Koh, D.; Lim, Y.; Shin, S.Y.; Lee, Y.H. Overcoming multidrug resistance by activating unfolded protein response of the endoplasmic reticulum in cisplatin-resistant A2780/CisR ovarian cancer cells. BMB Rep. 2020, 53, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.N.; Jung, E.; Koh, D.; Lim, Y.; Lee, Y.H.; Shin, S.Y. A synthetic chalcone derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139), triggers reactive oxygen species-induced apoptosis independently of p53 in A549 lung cancer cells. Chem. Biol. Interact. 2019, 298, 72–79. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, C.; Liu, G.; Ma, H.; Jiang, M.; Li, P.; Lu, Q.; Li, L.; Qi, H. Isobavachalcone inhibits acute myeloid leukemia: Potential role for ROS-dependent mitochondrial apoptosis and differentiation. Phytother. Res. 2021, 35, 3337–3350. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chiang, Y.C.; Vudhya Gowrisankar, Y.; Lin, K.Y.; Huang, S.T.; Shrestha, S.; Chang, G.R.; Yang, H.L. The In Vitro and In Vivo Anticancer Properties of Chalcone Flavokawain B through Induction of ROS-Mediated Apoptotic and Autophagic Cell Death in Human Melanoma Cells. Cancers 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, T.; Zhang, L.; Wang, X.; Dong, H.; Li, L.; Fu, D.; Li, Y.; Zi, X.; Liu, H.M.; et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci. Rep. 2017, 7, 9873. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med. Chem. 2022, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Suzuki-Karasaki, Y. Mitochondrial superoxide mediates mitochondrial and endoplasmic reticulum dysfunctions in TRAIL-induced apoptosis in Jurkat cells. Free Radic. Biol. Med. 2013, 61, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Li, K.T.; Fayyaz, S.; Chang, Y.T.; Ismail, M.; Liaw, C.C.; Yuan, S.S.; Tang, J.Y.; Chang, H.W. Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol. 2015, 36, 5743–5752. [Google Scholar] [CrossRef] [PubMed]
- Santarsiero, A.; Pappalardo, I.; Rosa, G.M.; Pisano, I.; Superchi, S.; Convertini, P.; Todisco, S.; Scafato, P.; Infantino, V. Mitochondrial Role in Intrinsic Apoptosis Induced by a New Synthesized Chalcone in Hepatocellular Carcinoma Cells. Biomedicines 2022, 10, 3120. [Google Scholar] [CrossRef] [PubMed]
- Travnicek, Z.; Vanco, J.; Belza, J.; Zoppellaro, G.; Dvorak, Z. Dinuclear copper(II) complexes with a bridging bis(chalcone) ligand reveal considerable in vitro cytotoxicity on human cancer cells and enhanced selectivity. J. Inorg. Biochem. 2024, 252, 112481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Wang, X.; Zhu, Q.; Liu, L.; Qiu, S.; Zou, L.; Liu, K.; Li, G.; Miao, H.; et al. Isoliquiritigenin induces HMOX1 and GPX4-mediated ferroptosis in gallbladder cancer cells. Chin. Med. J. 2023, 136, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, Q.; Xia, Z.; Wang, H.; Jiang, F.; Lu, Y. Novel Chalcone-Phenazine Hybrids Induced Ferroptosis in U87-MG Cells through Activating Ferritinophagy. Chem. Biodivers. 2023, 20, e202201117. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.C.; Joughin, B.A.; van de Kooij, B.; Lim, D.C.; Lauffenburger, D.A.; Yaffe, M.B. ROS and Oxidative Stress Are Elevated in Mitosis during Asynchronous Cell Cycle Progression and Are Exacerbated by Mitotic Arrest. Cell Syst. 2019, 8, 163–167 e2. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, S.; Long, J.; Su, J.; Wu, L.; Tao, J.; Zhou, J.; Zhang, J.; Chen, X.; Peng, C. A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products. Cancer Cell Int. 2020, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Toettcher, J. Cell Cycle Arrest after DNA Damage. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 249–254. [Google Scholar]
- Michalkova, R.; Kello, M.; Kudlickova, Z.; Gazdova, M.; Mirossay, L.; Mojzisova, G.; Mojzis, J. Programmed Cell Death Alterations Mediated by Synthetic Indole Chalcone Resulted in Cell Cycle Arrest, DNA Damage, Apoptosis and Signaling Pathway Modulations in Breast Cancer Model. Pharmaceutics 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.R. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 2001, 10, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231–254. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, J.; Wen, Z.; Chen, X.; Yu, J.; Yuan, D.; Xu, B.; Luo, H.; Zhu, J. A novel 3′,5′-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCdelta/JNK signal in prostate cancer. Aging 2020, 12, 9103–9124. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Q.; Wang, Z. Anti-tumor effects of isoliquiritigenin in Bcl-2/Bax and PCNA expression of T24 human bladder cancer cells. Arch. Med. Sci. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Su, D.; Lv, C. Hydroxysafflor yellow A inhibits the proliferation, migration, and invasion of colorectal cancer cells through the PPARgamma/PTEN/Akt signaling pathway. Bioengineered 2021, 12, 11533–11543. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, M.; Liu, Y.; Zhang, M.; Yang, G. Tetramethoxychalcone, a chalcone derivative, suppresses proliferation, blocks cell cycle progression, and induces apoptosis of human ovarian cancer cells. PLoS ONE 2014, 9, e106206. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chiang, Y.C.; Vudhya Gowrisankar, Y.; Lin, K.Y.; Huang, S.T.; Shrestha, S.; Chang, G.R.; Yang, H.L. Correction: Hseu, Y.-C. et al. The In Vitro and In Vivo Anticancer Properties of Chalcone Flavokawain B through Induction of ROS-Mediated Apoptotic and Autophagic Cell Death in Human Melanoma Cells. Cancers 2020, 12, 2936. Cancers 2021, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.W.; Lee, M.J.; Lee, M.H.; Yoon, G.; Cho, S.S.; Chae, J.I.; Shim, J.H. The 3-deoxysappanchalcone induces ROS-mediated apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway in human esophageal cancer cells. Phytomedicine 2021, 86, 153564. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.W.; Choi, J.S.; Lee, M.H.; Oh, H.N.; Cho, S.S.; Yoon, G.; Liu, K.; Chae, J.I.; Shim, J.H. Retrochalcone Echinatin Triggers Apoptosis of Esophageal Squamous Cell Carcinoma via ROS- and ER Stress-Mediated Signaling Pathways. Molecules 2019, 24, 4055. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Li, H.H.; Li, M.; Wang, S.; Jiang, X.R.; Li, Y.; Ping, G.F.; Cao, Q.; Liu, X.; Fang, W.H.; et al. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Prolif. 2015, 48, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Lee, M.S.; Wu, C.R.; Cho, H.J.; Lin, K.Y.; Lai, G.H.; Wang, S.Y.; Kuo, Y.H.; Kumar, K.J.; Yang, H.L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Bardhi, E.; Ruscito, I.; Papadia, A.; Farooqi, A.A.; Marchetti, C.; Bogani, G.; Ceccacci, I.; Mueller, M.D.; Benedetti Panici, P. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd. 2017, 77, 1095–1103. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Navaei, Z.N.; Khalili-Tanha, G.; Zangouei, A.S.; Abbaszadegan, M.R.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of Cisplatin response in tumor cells. Oncol. Res. 2021, 29, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.J.; Wang, J.; Zhou, J.Y.; Wu, G.S. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2010, 394, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat. Res. 2020, 819–820, 111690. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Joo, S.H.; Lee, J.Y.; Kwak, A.W.; Kim, K.T.; Cho, S.S.; Yoon, G.; Choi, Y.H.; Park, J.W.; Shim, J.H. Licochalcone C Inhibits the Growth of Human Colorectal Cancer HCT116 Cells Resistant to Oxaliplatin. Biomol. Ther. 2024, 32, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.Y.; Wu, G.S. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007, 67, 11933–11941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; Lin, Y.; Jiang, X.; Jin, L.; Ye, P.; Lu, Y.; Pei, R.; Jiang, L. Licochalcone A induces mitochondria-dependent apoptosis and interacts with venetoclax in acute myeloid leukemia. Eur. J. Pharmacol. 2024, 968, 176418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, S.O.; Kwak, A.W.; Chae, S.B.; Cho, S.S.; Yoon, G.; Kim, K.T.; Choi, Y.H.; Lee, M.H.; Joo, S.H.; et al. 3-Deoxysappanchalcone Inhibits Cell Growth of Gefitinib-Resistant Lung Cancer Cells by Simultaneous Targeting of EGFR and MET Kinases. Biomol. Ther. 2023, 31, 446–455. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, L.Y.; Xu, N.; An, Q. Hydroxysafflor yellow A inhibited lipopolysaccharide-induced non-small cell lung cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT/mTOR and ERK/MAPK signaling pathways. Thorac. Cancer 2019, 10, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Silva, M.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, L.; Minetti, A.; Realini, G.; Pio, E.; Giustarini, D.; Rossi, R.; Rocchio, C.; Franci, L.; Salvini, L.; Catona, O.; et al. NRF2 activation by cysteine as a survival mechanism for triple-negative breast cancer cells. Oncogene 2024, 43, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jin, J.M.; Liang, X.H.; Yu, M.Z.; Yang, C.; Huang, F.; Wu, H.; Zhang, B.B.; Fei, X.Y.; Wang, Z.T.; et al. Helichrysetin inhibits gastric cancer growth by targeting c-Myc/PDHK1 axis-mediated energy metabolism reprogramming. Acta Pharmacol. Sin. 2022, 43, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Liu, H.; Wu, P. Echinatin mitigates H2O2-induced oxidative damage and apoptosis in lens epithelial cells via the Nrf2/HO-1 pathway. Adv. Clin. Exp. Med. 2021, 30, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- No, J.H.; Kim, Y.B.; Song, Y.S. Targeting nrf2 signaling to combat chemoresistance. J. Cancer Prev. 2014, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-kappaB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.L.; Baltimore, D. Two pathways to NF-kappaB. Mol. Cell 2002, 10, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Papierska, K.; Krajka-Kuzniak, V.; Kleszcz, R.; Stefanski, T.; Kurczab, R.; Kubicki, M. The synthesis of novel thioderivative chalcones and their influence on NF-kappaB, STAT3 and NRF2 signaling pathways in colorectal cancer cells. Sci. Rep. 2022, 12, 14915. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, Y.; Zhang, B.; He, L.; Li, W.; Zhang, W.; Li, C.; Luo, L.; Umar, T.; Feng, H.; et al. 2′-Hydroxychalcone Induces Autophagy and Apoptosis in Breast Cancer Cells via the Inhibition of the NF-kappaB Signaling Pathway: In Vitro and In Vivo Studies. Nutrients 2024, 1, 514. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Noori, S.; Aminzade, Z.; Bayanati, M.; Alemi, M.; Zarghi, A. Attenuation of Inflammatory Responses in Breast and Ovarian Cancer Cells by a Novel Chalcone Derivative and Its Increased Potency by Curcumin. Mediat. Inflamm. 2023, 2023, 5156320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Luo, Y.H.; Piao, X.J.; Zhang, Y.; Feng, Y.C.; Li, J.Q.; Xu, W.T.; Zhang, Y.; Zhang, T.; Wang, S.N.; et al. Mechanisms underlying isoliquiritigenin-induced apoptosis and cell cycle arrest via ROS-mediated MAPK/STAT3/NF-kappaB pathways in human hepatocellular carcinoma cells. Drug Dev. Res. 2019, 80, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Godwin, P.; Baird, A.M.; Heavey, S.; Barr, M.P.; O’Byrne, K.J.; Gately, K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front. Oncol. 2013, 3, 120. [Google Scholar] [CrossRef] [PubMed]




| Primary Antibody | Mr (Kda) | Origin | Dilution | Catologue No. | Manufacturer |
|---|---|---|---|---|---|
| β-Actin (8H10D10) | 45 | mouse | 1:1000 | #3700 | Cell Signaling Technology®, Danvers, MA, USA |
| Phospho/Akt (Ser473) (D9E) XP® | 60 | rabbit | #4060 | ||
| Phospho-Bad (Ser112) (40A9) | 23 | rabbit | #5284 | ||
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® | 42/44 | rabbit | #4370 | ||
| Phospho-Histone H2A.X (Ser139) (20E3) | 15 | rabbit | #9718 | ||
| Phospho-Rb (Ser807/811) (D20B12) XP® | 110 | rabbit | #8516 | ||
| p21 Waf1/Cip1 (12D1) | 21 | rabbit | #2947 | ||
| PARP (46D11) | 116/89 | rabbit | #9532 | ||
| PCNA (D3H8P) XP® Rabbit mAb | 36 | rabbit | #13110 | ||
| NRF2 (D1Z9C) XP® | 97–100 | rabbit | #12721 | ||
| NF-κB1 p105/p50 (D7H5M) | 105/50 | rabbit | #12540 | ||
| NF-κB p65 (D14E12) | 65 | rabbit | #8242 | ||
| Secondary antibody | |||||
| anti-rabbit-IgG HRP linked | - | goat | #7074 | ||
| anti-mouse-IgG HRP linked | - | goat | #7075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salanci, Š.; Vilková, M.; Martinez, L.; Mirossay, L.; Michalková, R.; Mojžiš, J. The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation. Int. J. Mol. Sci. 2024, 25, 7541. https://doi.org/10.3390/ijms25147541
Salanci Š, Vilková M, Martinez L, Mirossay L, Michalková R, Mojžiš J. The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation. International Journal of Molecular Sciences. 2024; 25(14):7541. https://doi.org/10.3390/ijms25147541
Chicago/Turabian StyleSalanci, Šimon, Mária Vilková, Lola Martinez, Ladislav Mirossay, Radka Michalková, and Ján Mojžiš. 2024. "The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation" International Journal of Molecular Sciences 25, no. 14: 7541. https://doi.org/10.3390/ijms25147541
APA StyleSalanci, Š., Vilková, M., Martinez, L., Mirossay, L., Michalková, R., & Mojžiš, J. (2024). The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation. International Journal of Molecular Sciences, 25(14), 7541. https://doi.org/10.3390/ijms25147541







