Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes
Abstract
:1. Introduction
2. Results
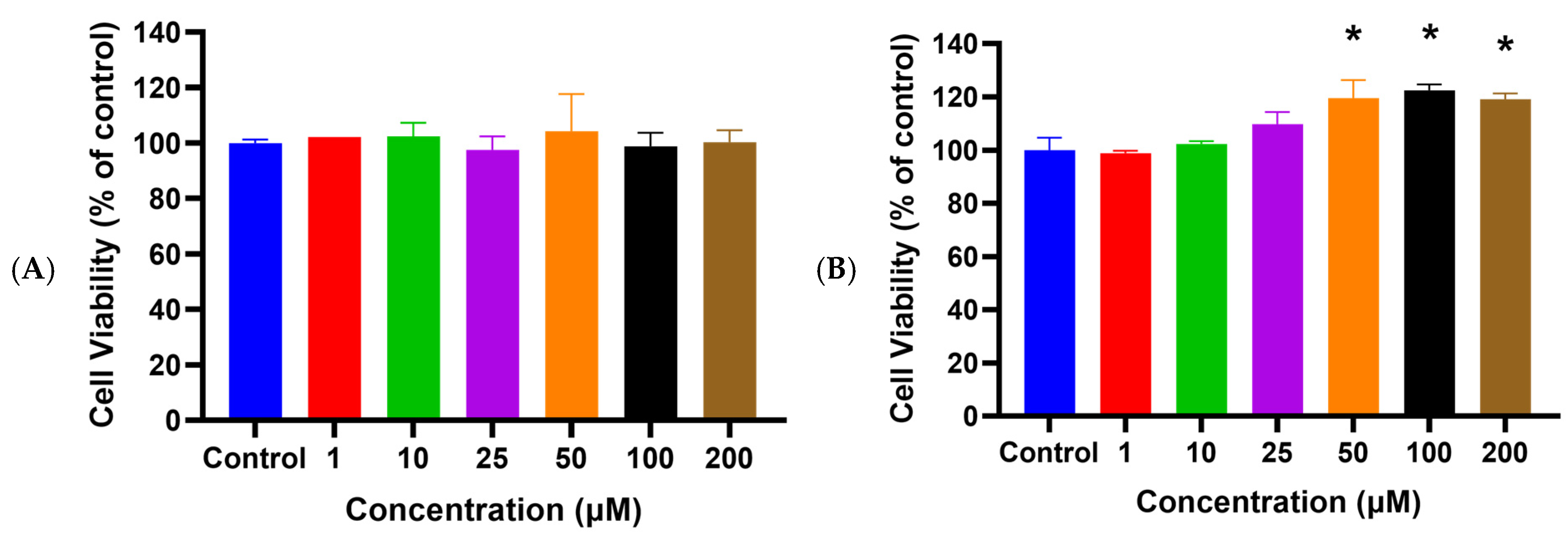
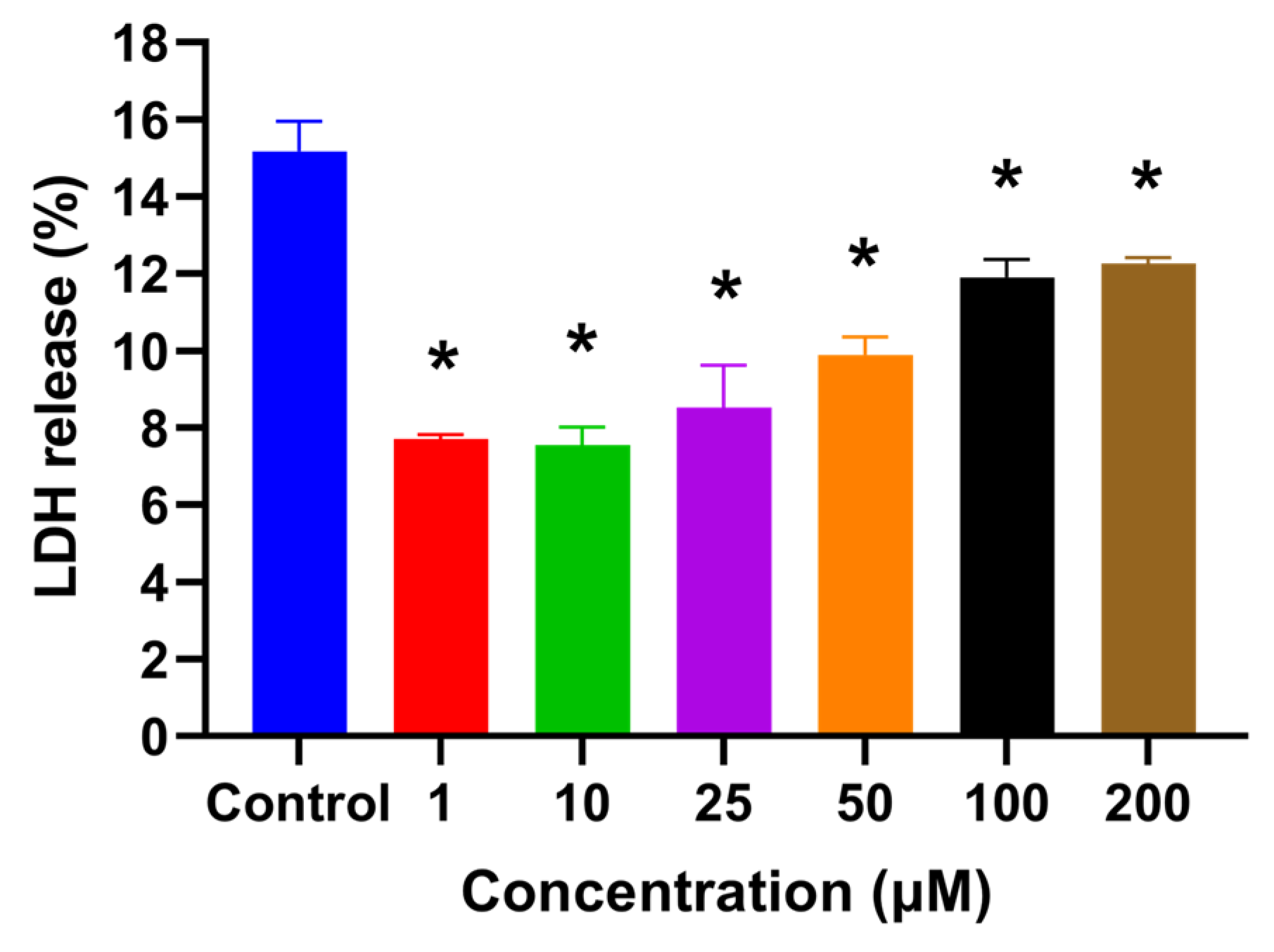
2.1. HSYA Exhibited No Cytotoxicity toward HaCaT Keratinocytes
2.2. HSYA Increased HaCaT Keratinocyte Viability after UVA Exposure
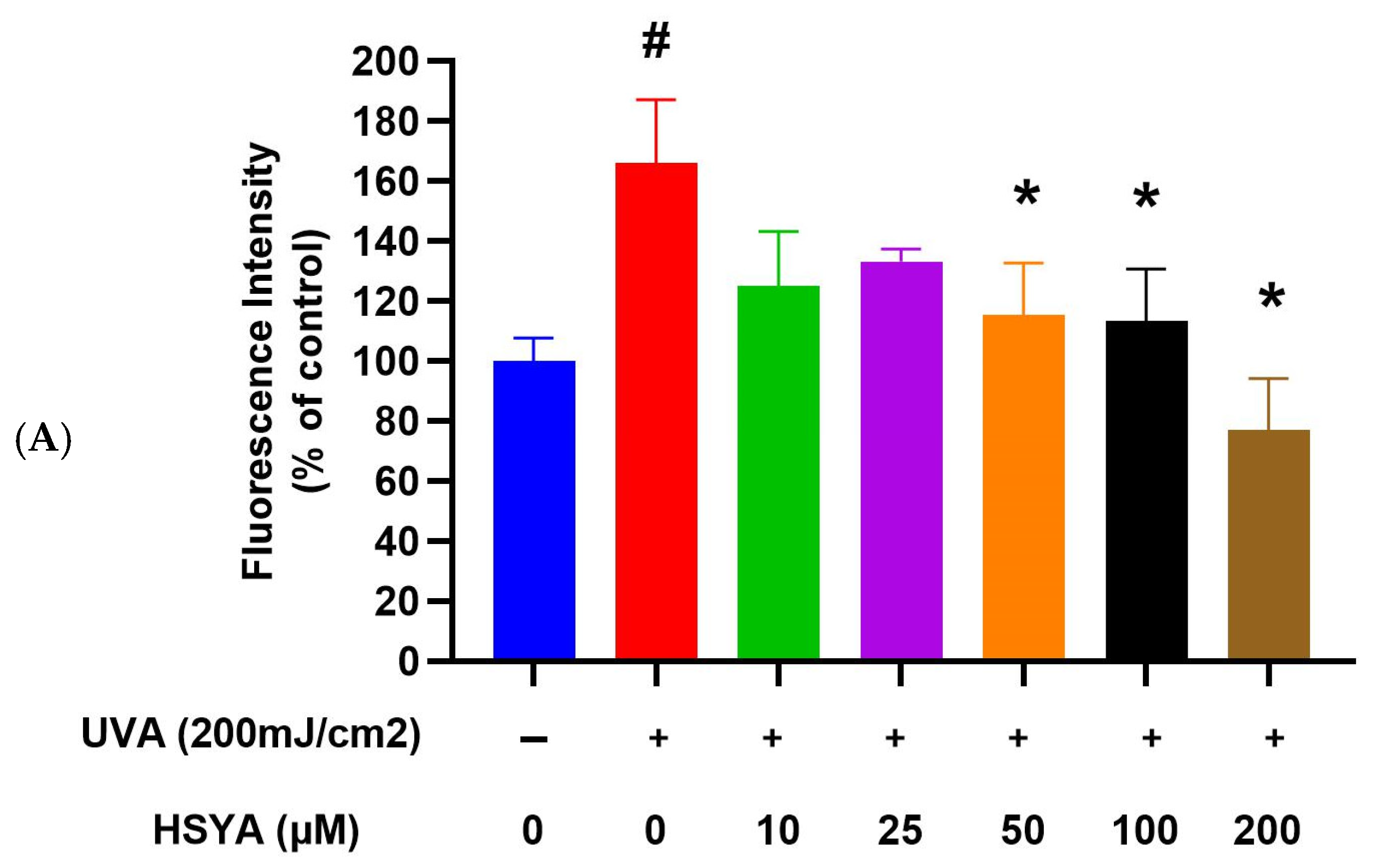
2.3. HSYA Reduced UVA-Induced Intracellular ROS Production in HaCaT Keratinocytes
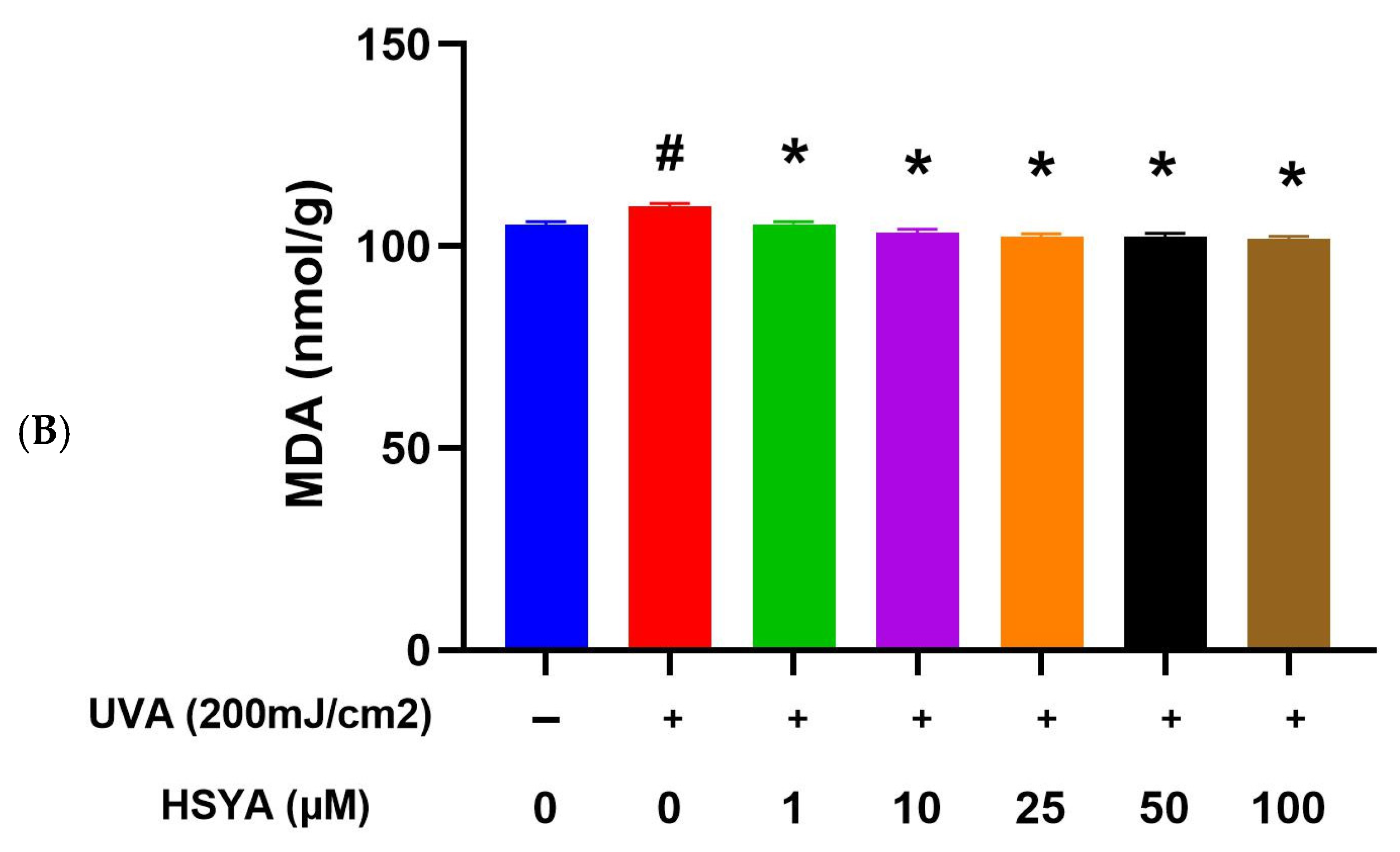
2.4. HSYA Inhibited UVA-Induced Lipid Peroxidation (LPO) in HaCaT Keratinocytes
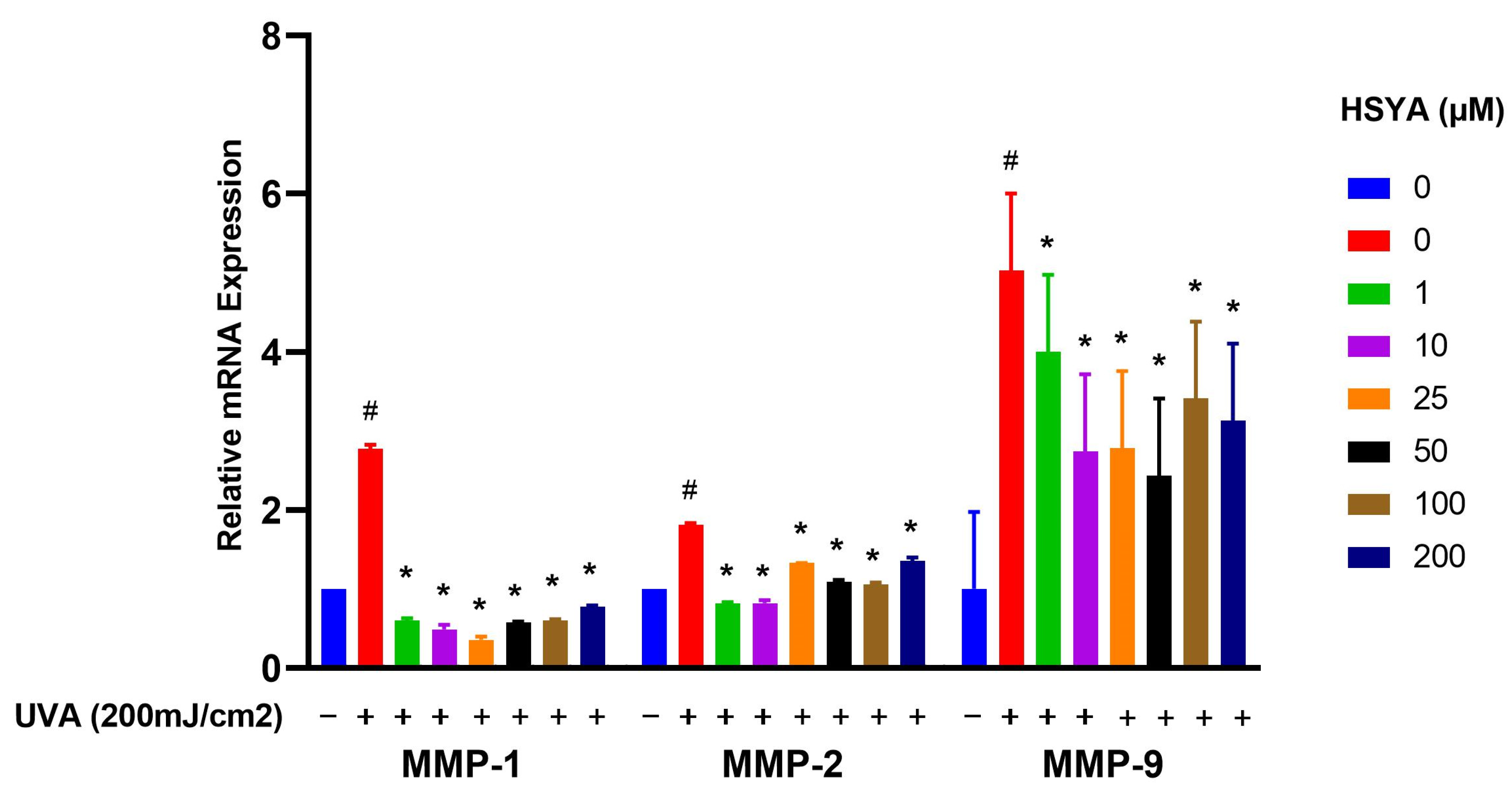
2.5. Effects on Expressions of MMP-1, MMP-2, MMP-9, and COX-2
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drug Treatment and UVA Irradiation
4.3. Cell Viability Assay
4.4. Lactate Dehydrogenase (LDH) Assay
4.5. Intracellular ROS Assay
4.6. Lipid Peroxidation (LPO) Assay
4.7. RNA Isolation and Quantification of Gene Expression
- (1)
- COX-2Forward primer: TTCAAATGAGATTGTGGGAAAATReverse primer: AGATCATCTCTGCCTGAGTATCTT
- (2)
- MMP-1Forward primer: CAGAGATGAAGTCCGGTTTTTCReverse primer: GGGGTATCCGTGTAGCACAT
- (3)
- MMP-2Forward primer: GGCCAAGTGGTCCGTGTGReverse primer: GAGGCCCCATAGAGCTCC
- (4)
- MMP-9Forward primer: CACTGTCCACCCCTCAGAGCReverse primer: GCCACTTGTCGGCGATAAGG
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S. Impact of UV Radiation on Genome Stability and Human Health. In Ultraviolet Light in Human Health, Diseases and Environment, 2nd ed.; Ahmad, S., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 996, pp. 207–219. [Google Scholar]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, S.H.; Youn, C.S.; Park, B.J.; Kim, K.H.; Park, K.C.; Cho, K.H.; Eun, H.C. Cutaneous photodamage in Koreans: Influence of sex, sun exposure, smoking, and skin color. Arch. Dermatol. 2001, 137, 1043–1051. [Google Scholar] [PubMed]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Md, J.J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. Systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154828. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Li, G.; Liu, Z.; Fu, F. Hydroxysafflor yellow A inhibits rat brain mitochondrial permeability transition pores by a free radical scavenging action. Pharmacology 2008, 82, 121–126. [Google Scholar] [CrossRef]
- Koyama, N.; Kuribayashi, K.; Seki, T.; Kobayashi, K.; Furuhata, Y.; Suzuki, K.; Arisaka, H.; Nakano, T.; Amino, Y.; Ishii, K. Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2006, 54, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-O.; Oh, J.-H.; Lee, S.-K.; Lee, J.-Y.; Choi, S.-W. Antioxidant properties and quantification of phenolic compounds from safflower (Carthamus tinctorius L.) seeds. Food Sci. Biotechnol. 2007, 16, 71–77. [Google Scholar]
- Zhao, J.; Liu, J.; Guo, Y.; Liu, Q.; Dai, Z.; Ma, S.; Lin, R. Chemical constituents from safflower injection and their bioactivity. Zhongguo Zhong Yao Za Zhi 2014, 39, 3102–3106. [Google Scholar]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2017, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Hölzle, E.; Neumann, N.; Hausen, B.; Przybilla, B.; Schauder, S.; Hönigsmann, H.; Bircher, A.; Plewig, G. Photopatch testing: The 5-year experience of the German, Austrian, and Swiss Photopatch Test Group. J. Am. Acad. Dermatol. 1999, 25, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Thomas, M. Photopatch and UV-irradiated patch testing in photosensitive dermatitis. Indian Dermatol. Online J. 2016, 7, 12–16. [Google Scholar]
- He, Y.; Liu, Q.; Li, Y.; Yang, X.; Wang, W.; Li, T.; Zhang, W.; Cui, Y.; Wang, C.; Lin, R. Protective effects of hydroxysafflor yellow A (HSYA) on alcohol-induced liver injury in rats. J. Physiol. Biochem. 2015, 71, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Z.; Shi, X.-G.; Feng, X.-X.; Li, W.-J.; Liu, W.-H.; Chen, Z.-W.; Xie, J.-H.; Lai, X.-P.; Zhang, S.-X.; Zhang, X.-J.; et al. Inhibitory effect of hydroxysafflor yellow A on mouse skin photoaging induced by Ultraviolet Irradiation. Rejuvenation Res. 2013, 16, 404–413. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Bi, Z.-G. UVB-irradiated human keratinocytes and interleukin-1alpha indirectly increase MAP kinase/AP-1 activation and MMP-1 production in UVA-irradiated dermal fibroblasts. Chin. Med. J. 2006, 119, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Buechner, N.; Schroeder, P.; Jakob, S.; Kunze, K.; Maresch, T.; Calles, C.; Krutmann, J.; Haendeler, J. Changes of MMP-1 and collagen type Iα1 by UVA, UVB and IRA are differentially regulated by Trx-1. Exp. Gerontol. 2008, 43, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Kozak, I.; Klisenbauer, D.; Juhas, T. UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol. Res. 2003, 52, 229–234. [Google Scholar] [CrossRef]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, D.; Sun, X.; Liu, W.; Wang, L.; Li, X.; Man, X.; Fu, Q. Effects of hydroxysafflor yellow A on proliferation and collagen synthesis of rat vascular adventitial fibroblasts induced by angiotensin II. Int. J. Clin. Exp. Pathol. 2014, 7, 5772. [Google Scholar] [PubMed]
- Lang, R.; Stern, M.M.; Smith, L.; Liu, Y.; Bharadwaj, S.; Liu, G.; Baptista, P.M.; Bergman, C.R.; Soker, S.; Yoo, J.J. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials 2011, 32, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Quyen, B.T.; Choi, H.-K.; Kang, K.W. Pin1 is required for ultraviolet A-stimulated cyclooxygenase-2 induction in mouse epidermal cells. Cancer Lett. 2013, 335, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Eggert, S. Current problems in dermatology. In Current Problems in Dermatology Challenges in Sun Protection, 2nd ed.; Christian, S., Uli, O., Eds.; S. Karger, A.G.: Basel, Switzerland, 2021; Volume 55, pp. 53–61. [Google Scholar]
- Pentland, A.P.; Schoggins, J.W.; Scott, G.A.; Khan, K.N.M.; Han, R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis 1999, 20, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Nechalioti, P.-M.; Georgopoulou, K.E.; Goniotakis, G.; Schulze, A.V.R.; Zafiriou, E.; Kouretas, D. Systemic oxidative stress parameters in skin cancer patients and patients with benign lesions. Stresses 2023, 3, 785–812. [Google Scholar] [CrossRef]
- Karampinis, E.; Aloizou, A.-M.; Zafiriou, E.; Bargiota, A.; Skaperda, Z.; Kouretas, D.; Roussaki-Schulze, A.-V. Non-melanoma skin cancer and vitamin D: The “lost sunlight” paradox and the oxidative stress explanation. Antioxidants 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-C.; Chiu, W.-C.; Loe, P.Y.; Chien, Y.-W. Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes. Int. J. Mol. Sci. 2024, 25, 7573. https://doi.org/10.3390/ijms25147573
Yu S-C, Chiu W-C, Loe PY, Chien Y-W. Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes. International Journal of Molecular Sciences. 2024; 25(14):7573. https://doi.org/10.3390/ijms25147573
Chicago/Turabian StyleYu, Szu-Chieh, Wan-Chun Chiu, Pei Yu Loe, and Yi-Wen Chien. 2024. "Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes" International Journal of Molecular Sciences 25, no. 14: 7573. https://doi.org/10.3390/ijms25147573
APA StyleYu, S.-C., Chiu, W.-C., Loe, P. Y., & Chien, Y.-W. (2024). Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes. International Journal of Molecular Sciences, 25(14), 7573. https://doi.org/10.3390/ijms25147573






