HPV Infections—Classification, Pathogenesis, and Potential New Therapies
Abstract
:1. Introduction
2. A Brief Historical Overview of HPV Research
3. Classification of HPV
4. General Characteristics and Structure of HPV
HPV Genome
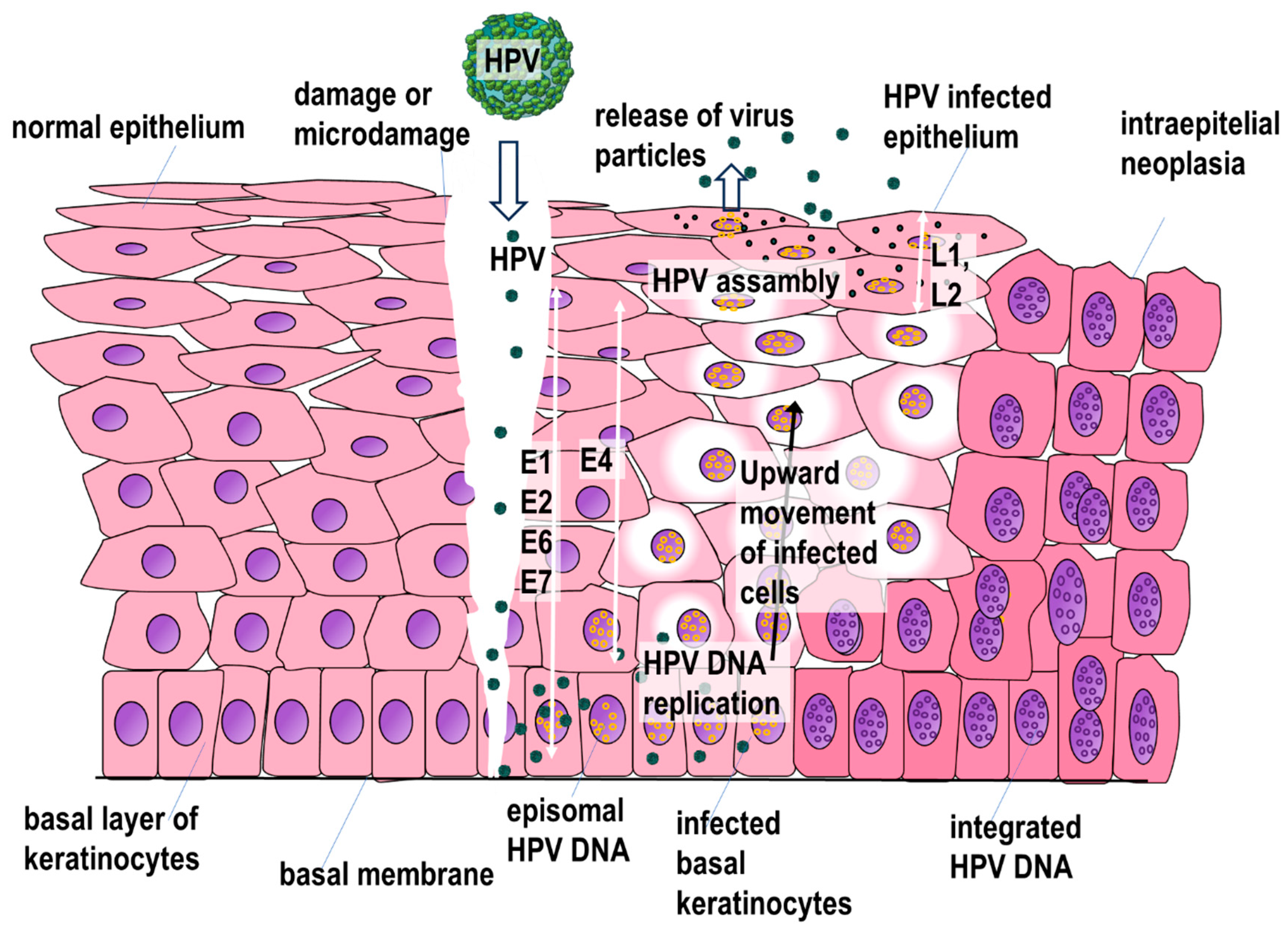
5. HPV Replication Cycle
6. Mechanisms of Precancerous and Neoplastic Lesions in HPV Infection
7. Immune Response and Immune Evasion by the Virus in HPV Infections
8. HPV’s Association with Various Diseases
9. Prophylactic Vaccines
10. Therapeutic Vaccines
11. Drugs Currently Used in the Treatment of HPV Infection
12. New Drug Therapy
13. Conclusions
- In order to make HPV systematics clearer, it would be necessary to merge and unify the information contained in the various databases;
- Current treatment is mainly based on:
- -
- Surgical procedures;
- -
- Topical or intralesional application of substances with antiproliferative and cytotoxic effects on infected cells (e.g., podophyllotoxin, bleomycin, 5-fluorouracil, cidofovir) or non-specific stimulation of the immune system to destroy HPV (e.g., imiquimod, intralesional immunotherapy). Some of the drugs used such as sinecatechins and vitamin D have both immunostimulating and antiproliferative effects;
- A number of therapeutic vaccines (specific immunotherapy) are undergoing clinical trials, some of which, such as MVA E2 and VGX-3100, have completed phase III clinical trials and are expected to be available soon;
- It is encouraging that prevention of infections with the most common mucosal HPV types is available. The efficacy of vaccines based on virus-like particles from the L1 protein is fully proven. Also noteworthy is the effect of prophylactic vaccines on reducing recurrence in women treated for CIN. These vaccines are increasingly used worldwide. However, in order to reduce the number of infections and their impact, it would be advisable to further increase the availability of vaccines and to disseminate more effectively information regarding their efficacy and safety;
- Despite a great deal of research, there are still no drugs available that specifically inhibit HPV replication. However, an increasing understanding of the HPV replication cycle and the structure and function of individual viral proteins should, in the future, allow the development of effective, specific pharmacotherapy. Research on such drugs is mostly still in the preclinical phase;
- The treatment of precancerous lesions, cancers and persistent/recurrent benign lesions caused by HPV is still an incompletely solved problem. The effectiveness of treatment may be enhanced by the inclusion of methods which are based on the stimulation of the immune system in the fight against infection.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIM2 | absent in melanoma 2 |
| BCG | Bacillus Calmette–Guérin |
| CIN | cervical intraepithelial neoplasia, and penile |
| CyPB | cyclophilin B |
| Dlg | Discs Large |
| DNMT1 | DNA methyltransferase |
| ds DNA | Double-stranded DNA |
| EGCG | epigallocatechin gallate |
| EGFR | epidermal growth factor receptor |
| EV | epidermodysplasia verruciformis |
| FGFR | fibroblast growth factor receptor |
| GAGs | glycosaminoglycans |
| HSPG | heparan sulfate proteoglycans |
| hTERT | human telomerase reverse transcriptase |
| ICTV | International Committee on Taxonomy of Viruses |
| IRHC | International Human Papillomavirus Reference Center |
| KLK8 | kallikrein-8 |
| L or S | lineage or sublineage |
| LCR | long control region |
| LLO | Listeria monocytogenes listeriolysin O |
| MMR | Measles, Mumps, and Rubella (vaccine) |
| NMSC | non-melanoma skin cancer |
| ORF | open reading frame |
| pAE | early polyadenylation sites |
| PaVe | The Papilloma Virus Episteme |
| PDGFR | platelet-derived growth factor receptor |
| PI3K | phosphatidylinositol-3-kinase |
| PIN | penile intraepithelial neoplasia |
| PML NBs | promyelocytic leukemia nuclear bodies |
| PPD | (tuberculin) purified protein derivative |
| PRR | pattern recognition receptors |
| PSD95 | Post Synaptic Density 95 |
| PTPN14 | Protein Tyrosine Phosphatase Non-Receptor Type 14 |
| PV | papillomaviruse |
| TB | Taxonomy Browser |
| VIN | vulvar intraepithelial neoplasia |
| ZO-1 | Zona Occludens 1 |
References
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report, 10 March 2023. [Google Scholar]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 19 April 2024).
- de Sanjosé, S.; Bruni, L.; Alemany, L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med. 2014, 43, e423–e428. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Tumban, E. Gardasil-9: A global survey of projected efficacy. Antivir. Res. 2016, 130, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Syrjänen, K. The history of papillomavirus research. Cent. Eur. J. Public Health 2008, 16 (Suppl. S16), S7–S13. [Google Scholar]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Nunes, E.M.; Talpe-Nunes, V.; Sichero, L. Epidemiology and biology of cutaneous human papillomavirus. Clinics 2018, 73 (Suppl. S1), e489s. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Eklund, C.; Dillner, J. Misclassifications in human papillomavirus databases. Virology 2021, 558, 57–66. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses; Book: Papillomaviridae 2024. Available online: https://ictv.global/report/chapter/papillomaviridae/papillomaviridae (accessed on 29 January 2024).
- Taxonomy Browser (Human Papillomavirus). Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Undef&name=Viruses&lvl=3&srchmode=1&keep=1&unlock (accessed on 28 January 2024).
- International Human Papillomavirus Reference Center. Available online: https://www.hpvcenter.se (accessed on 28 January 2024).
- The Papillomavirus Episteme PaVE. Available online: https://pave.niaid.nih.gov/ (accessed on 29 January 2024).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2012. Biological agents. Volume 100 B. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization. Int. Agency Res. Cancer 2012, 100, 1–441. [Google Scholar]
- Siqueira, J.D.; Alves, B.M.; Prellwitz, I.M.; Furtado, C.; Meyrelles, A.R.; Machado, E.S.; Seuanez, H.N.; Soares, M.A.; Soares, E.A. Identification of novel human papillomavirus lineages and sublineages in HIV/HPVcoinfected pregnant women by next-generation sequencing. Virology 2016, 493, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Delius, H.; Hofmann, B. Primer-directed sequencing of human papillomavirus types. Curr. Top. Microbiol. Immunol. 1994, 186, 13–31. [Google Scholar] [CrossRef]
- Philipp, W.; Honoré, N.; Sapp, M.; Cole, S.T.; Streeck, R.E. Human papillomavirus type 42: New sequences, conserved genome organization. Virology 1992, 186, 331–334. [Google Scholar] [CrossRef]
- Delius, H.; Saegling, B.; Bergmann, K.; Shamanin, V.; de Villiers, E.M. The genomes of three of four novel HPV types, defined by differences of their L1 genes, show high conservation of the E7 gene and the URR. Virology 1998, 240, 359–365. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Lavergne, D.; McLaren, K.; Benton, E.C. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int. J. Cancer 1997, 73, 356–361. [Google Scholar] [CrossRef]
- Kohler, A.; Gottschling, M.; Forster, J.; Rowert-Huber, J.; Stockfleth, E.; Nindl, I. Genomic characterization of a novel human papillomavirus (HPV117) with a high viral load in a persisting wart. Virology 2010, 399, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, A.; Kocjan, B.J.; Potocnik, M.; Poljak, M. Characterization of a novel cutaneous human papillomavirus genotype HPV125. PLoS ONE 2011, 6, e22414. [Google Scholar] [CrossRef]
- Mitsuishi, T.; Ohsawa, I.; Kato, T.; Egawa, N.; Kiyono, T. Molecular cloning and characterisation of a novel type of human papillomavirus 160 isolated from a flat wart of an immunocompetent patient. PLoS ONE 2013, 8, e79592, Erratum in PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Tachezy, R.; Van Ranst, M.A.; Cruz, Y.; Burk, R.D. Analysis of short novel human papillomavirus sequences. Biochem. Biophys. Res. Commun. 1994, 204, 820–827. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Fu, L.; Terai, M.; Matsukura, T.; Herrero, R.; Burk, R.D. Codetection of a mixed population of candHPV62 containing wild-type and disrupted E1 open-reading frame in a 45-year-old woman with normal cytology. J. Infect. Dis. 2004, 190, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Burk, R.D. Complete nucleotide sequence and analysis of a novel human papillomavirus (HPV 84) genome cloned by an overlapping PCR method. Virology 2001, 279, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Burk, R.D. Identification and characterization of 3 novel genital human papillomaviruses by overlapping polymerase chain reaction: candHPV89, candHPV90, and candHPV91. J. Infect. Dis. 2002, 185, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, T.; Sugase, M. Relationships between 80 human papillomavirus genotypes and different grades of cervical intraepithelial neoplasia: Association and causality. Virology 2001, 283, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Menzo, S.; Monachetti, A.; Trozzi, C.; Ciavattini, A.; Carloni, G.; Varaldo, P.E.; Clementi, M. Identification of six putative novel human papillomaviruses (HPV) and characterization of candidate HPV type 87. J. Virol. 2001, 75, 11913–11919. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; McClowry, T.L.; Woods, K.; Fife, K.H. Nucleotide sequence and characterization of human papillomavirus type 83, a novel genital papillomavirus. Virology 1999, 260, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, J.; Forslund, O.; Dillner, J. Three novel papillomaviruses (HPV109, HPV112 and HPV114) and their presence in cutaneous and mucosal samples. Virology 2010, 397, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hirsch-Behnam, A.; Delius, H.; de Villiers, E.M. A comparative sequence analysis of two human papillomavirus (HPV) types 2a and 57. Virus Res. 1990, 18, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Tan, C.H.; Delius, H.; Bernard, H.U. Human papillomavirus type 2c is identical to human papillomavirus type 27. Virology 1994, 201, 397–398. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Miwa, Y.; Eizuru, Y. Candidate human papillomavirus (HPV) type 27b: Nucleotide sequence and heterogeneity with HPV 27. J. Med. Virol. 2005, 77, 113–115. [Google Scholar] [CrossRef]
- Lungu, O.; Crum, C.P.; Silverstein, S. Biologic properties and nucleotide sequence analysis of human papillomavirus type 51. J. Virol. 1991, 65, 4216–4225. [Google Scholar] [CrossRef]
- Kino, N.; Sata, T.; Sato, Y.; Sugase, M.; Matsukura, T. Molecular cloning and nucleotide sequence analysis of a novel human papillomavirus (type 82) associated with vaginal intraepithelial neoplasia. Clin. Diagn. Lab. Immunol. 2000, 7, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Narechania, A.; Chen, Z.; DeSalle, R.; Burk, R.D. Phylogenetic incongruence among oncogenic genital alpha human papillomaviruses. J. Virol. 2005, 79, 15503–15510. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Zhang, C.T.; Zhu, X.K.; Wang, Y.C. Detection of HPV types and neutralizing antibodies in women with genital warts in Tianjin City, China. Virol. Sin. 2010, 25, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tawheed, A.R.; Beaudenon, S.; Favre, M.; Orth, G. Characterization of human papillomavirus type 66 from an invasive carcinoma of the uterine cervix. J. Clin. Microbiol. 1991, 29, 2656–2660. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Danos, O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J. Mol. Biol. 1987, 193, 599–608. [Google Scholar] [CrossRef]
- Muñoz-Bello, J.O.; Carrillo-García, A.; Lizano, M. Epidemiology and molecular biology of HPV variants in cervical cancer: The state of the art in Mexico. Int. J. Mol. Sci. 2022, 23, 8566. [Google Scholar] [CrossRef]
- Chen, Z.; DeSalle, R.; Schiffman, M.; Herrero, R.; Burk, R.D. Evolutionary dynamics of variant genomes of human papillomavirus types 18, 45, and 97. J. Virol. 2009, 83, 1443–1455. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, L.; Herrero, R.; Schiffman, M.; Burk, R.D. Identification of a novel human papillomavirus (HPV97) related to HPV18 and HPV45. Int. J. Cancer 2007, 121, 193–198. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; Desalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and taxonomic classification of Alphapapillomavirus 7 complete genomes: HPV18, HPV39, HPV45, HPV59, HPV68 and HPV70. PLoS ONE 2013, 8, e72565. [Google Scholar] [CrossRef]
- Lurchachaiwong, W.; Junyangdikul, P.; Termrungruanglert, W.; Payungporn, S.; Sampatanukul, P.; Tresukosol, D.; Niruthisard, S.; Trivijitsilp, P.; Karalak, A.; Swangvaree, S.; et al. Whole-genome sequence analysis of human papillomavirus type 18 from infected thai women. Intervirology 2010, 53, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Volpers, C.; Streeck, R.E. Genome organization and nucleotide sequence of human papillomavirus type 39. Virology 1991, 181, 419–423. [Google Scholar] [CrossRef]
- Rho, J.; Roy-Burman, A.; Kim, H.; de Villiers, E.M.; Matsukura, T.; Choe, J. Nucleotide sequence and phylogenetic classification of human papillomavirus type 59. Virology 1994, 203, 158–161. [Google Scholar] [CrossRef]
- Longuet, M.; Beaudenon, S.; Orth, G. Two novel genital human papillomavirus (HPV) types, HPV68 and HPV70, related to the potentially oncogenic HPV39. J. Clin. Microbiol. 1996, 34, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Hansson, B.G. Human papillomavirus type 70 genome cloned from overlapping PCR products: Complete nucleotide sequence and genomic organization. J. Clin. Microbiol. 1996, 34, 802–809. [Google Scholar] [CrossRef]
- Chow, V.T.; Leong, P.W. Complete nucleotide sequence, genomic organization and phylogenetic analysis of a novel genital human papillomavirus type, HLT7474-S. J. Gen. Virol. 1999, 80, 2923–2929. [Google Scholar] [CrossRef]
- Burk, R.D.; Mirabello, L.; DeSalle, R. Distinguishing genetic drift from selection in papillomavirus evolution. Viruses 2023, 15, 1631. [Google Scholar] [CrossRef] [PubMed]
- Guerendiain, D.; Mühr, L.S.A.; Grigorescu, R.; Holden, M.T.G.; Cuschieri, K. Mapping HPV 16 sub-lineages in anal cancer and implications for disease outcomes. Diagnostics 2022, 12, 3222. [Google Scholar] [CrossRef]
- Mirabello, L.; Clarke, M.A.; Nelson, C.W.; Dean, M.; Wentzensen, N.; Yeager, M.; Cullen, M.; Boland, J.F.; NCI HPV Workshop; Schiffman, M.; et al. The intersection of HPV epidemiology, genomics and mechanistic studies of HPV mediated carcinogenesis. Viruses 2018, 10, 80. [Google Scholar] [CrossRef]
- Clifford, G.M.; Tenet, V.; Georges, D.; Alemany, L.; Pavón, M.A.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Bass, S.; et al. Human papillomavirus 16 sub-lineage dispersal and cervical cancer risk worldwide: Whole viral genome sequences from 7116 HPV16-positive women. Papillomavirus Res. 2019, 7, 67–74. [Google Scholar] [CrossRef]
- Farhadi, A.; Abuei, H.; Okhovat, M.A.; Geramizadeh, B.; Behzad-Behbahani, A.; Chong, P.P.; Nikouyan, N.; Namdari, S. Type distribution of human papillomaviruses in ThinPrep cytology samples and HPV16/18 E6 gene variations in FFPE cervical cancer specimens in Fars province, Iran. Cancer Cell Int. 2023, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, K.; Krammer, G.; Durst, M.; Suhai, S.; Rowekamp, W.G. Human papillomavirus type 16 DNA sequence. Virology 1985, 145, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, I.M.; Haddow, J.K.; Clements, J.B. A negative regulatory element in the human papillomavirus type 16 genome acts at the level of late mRNA stability. J. Virol. 1991, 65, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Chen, Z.; Reimers, L.; van Doorslaer, K.; Schiffman, M.; Desalle, R.; Herrero, R.; Yu, K.; Wacholder, S.; Wang, T.; et al. Sequence imputation of HPV16 genomes for genetic association studies. PLoS ONE 2011, 6, e21375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Terai, M.; Fu, L.; Herrero, R.; DeSalle, R.; Burk, R.D. Diversifying selection in human papillomavirus type 16 lineages based on complete genome analyses. J. Virol. 2005, 79, 7014–7023. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; Desalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS ONE 2011, 6, e20183. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Harari, A.; Schiffman, M.; Clifford, G.M.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Raine-Bennett, T.; Steinberg, M.; et al. Phylogenomic analysis of human Papillomavirus type 31 and cervical carcinogenesis: A study of 2093 viral genomes. Viruses 2021, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Goldsborough, M.D.; DiSilvestre, D.; Temple, G.F.; Lorincz, A.T. Nucleotide sequence of human papillomavirus type 31: A cervical neoplasia-associated virus. Virology 1989, 171, 306–311. [Google Scholar] [CrossRef]
- Cole, S.T.; Streeck, R.E. Genome organization and nucleotide sequence of human papillomavirus type 33, which is associated with cervical cancer. J. Virol. 1986, 58, 991–995. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, C.; Feng, S.; Liu, C.; Li, Y.; Yang, Y.; Gao, J.; Li, H.; Meng, S.; Li, L.; et al. Detection of HPV types and neutralizing antibodies in Gansu province, China. J. Med. Virol. 2009, 81, 693–702. [Google Scholar] [CrossRef]
- Kirii, Y.; Iwamoto, S.; Matsukura, T. Human papillomavirus type 58 DNA sequence. Virology 1991, 185, 424–427. [Google Scholar] [CrossRef]
- Kirii, Y.; Matsukura, T. Nucleotide sequence and phylogenetic classification of human papillomavirus type 67. Virus Genes 1998, 17, 117–121. [Google Scholar] [CrossRef]
- Bee, K.J.; Gradissimo, A.; Chen, Z.; Harari, A.; Schiffman, M.; Raine-Bennett, T.; Castle, P.E.; Clarke, M.; Wentzensen, N.; Burk, R.D. Genetic and epigenetic variations of HPV52 in cervical precancer. Int. J. Mol. Sci. 2021, 22, 6463. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, J.A.; Garland, S.M.; McCloskey, J.; Hillman, R.J.; Tabrizi, S.N. Human papillomavirus type 6 and 11 genetic variants found in 71 oral and anogenital epithelial samples from Australia. PLoS ONE 2013, 8, e63892, Erratum in: PLoS ONE 2015, 10, e0117962. [Google Scholar] [CrossRef]
- Schwarz, E.; Durst, M.; Demankowski, C.; Lattermann, O.; Zech, R.; Wolfsperger, E.; Suhai, S.; zur Hausen, H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983, 2, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Seedat, R.Y.; Combrinck, C.E.; Bester, P.A.; Lee, J.Y.; Burt, F.J. Determination of the complete genome and functional analysis of HPV6 isolate VBD19/10 from a patient with aggressive recurrent respiratory papillomatosis. Epidemiol. Infect. 2016, 144, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Jelen, M.M.; Chen, Z.; Kocjan, B.J.; Burt, F.J.; Chan, P.K.; Chouhy, D.; Combrinck, C.E.; Coutlée, F.; Estrade, C.; Ferenczy, A.; et al. Global genomic diversity of human papillomavirus 6 based on 724 isolates and 190 complete genome sequences. J. Virol. 2014, 88, 7307–7316. [Google Scholar] [CrossRef]
- Jelen, M.M.; Chen, Z.; Kocjan, B.J.; Hošnjak, L.; Burt, F.J.; Chan, P.K.S.; Chouhy, D.; Combrinck, C.E.; Estrade, C.; Fiander, A.; et al. Global genomic diversity of human papillomavirus 11 based on 433 isolates and 78 complete genome sequences. J. Virol. 2016, 90, 5503–5513. [Google Scholar] [CrossRef]
- Kovelman, R.; Bilter, G.K.; Roman, A.; Brown, D.R.; Barbosa, M.S. Human papillomavirus type 6: Classification of clinical isolates and functional analysis of E2 proteins. J. Gen. Virol. 1999, 80, 2445–2451. [Google Scholar] [CrossRef]
- Burk, R.D.; Chen, Z.; Harari, A.; Smith, B.C.; Kocjan, B.J.; Maver, P.J.; Poljak, M. Classification and nomenclature system for human Alphapapillomavirus variants: General features, nucleotide landmarks and assignment of HPV6 and HPV11 isolates to variant lineages. Acta Dermatovenerol. Alp. Pannonica Adriat. 2011, 20, 113–123. [Google Scholar]
- Kocjan, B.J.; Jelen, M.M.; Maver, P.J.; Seme, K.; Poljak, M. Pre-vaccination genomic diversity of human papillomavirus genotype 6 (HPV 6): A comparative analysis of 21 full-length genome sequences. Infect. Genet. Evol. 2011, 11, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.J.; Cook, J.C.; Joyce, J.G.; Brown, D.R.; Schultz, L.D.; George, H.A.; Rosolowsky, M.; Fife, K.H.; Jansen, K.U. Sequence determination of human papillomavirus type 6a and assembly of virus-like particles in Saccharomyces cerevisiae. Virology 1995, 209, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Kahn, T.; Turazza, E.; Ojeda, R.; Bercovich, A.; Stremlau, A.; Lichter, P.; Poustka, A.; Grinstein, S.; zur Hausen, H. Integration of human papillomavirus type 6a DNA in a tonsillar carcinoma: Chromosomal localization and nucleotide sequence of the genomic target region. Cancer Res. 1994, 54, 1305–1312. [Google Scholar] [PubMed]
- Metcalfe, L.; Chen, S.L.; Mounts, P. Structural analysis of human papillomavirus type 6c isolates from condyloma acuminatum and juvenile-onset and adult-onset laryngeal papillomata. Virus Genes 1989, 3, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Dartmann, K.; Schwarz, E.; Gissmann, L.; zur Hausen, H. The nucleotide sequence and genome organization of human papilloma virus type 11. Virology 1986, 151, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Maver, P.J.; Kocjan, B.J.; Seme, K.; Potocnik, M.; Gale, N.; Poljak, M. Prevaccination genomic diversity of human papillomavirus genotype 11: A study on 63 clinical isolates and 10 full-length genome sequences. J. Med. Virol. 2011, 83, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Conde-Ferráez, L.; Ek-Hernández, G.E.; Canché-Pech, J.R.; Gómez-Carballo, J.G.; Kantún-Moreno, N.E.; González-Losa, M.D.R. Genomic characterization of human papillomavirus type 13, associated to multifocal epithelial hyperplasia, in a Mayan community. Infect. Genet. Evol. 2021, 91, 104595. [Google Scholar] [CrossRef] [PubMed]
- Van Ranst, M.; Fuse, A.; Fiten, P.; Beuken, E.; Pfister, H.; Burk, R.D.; Opdenakker, G. Human papillomavirus type 13 and pygmy chimpanzee papillomavirus type 1: Comparison of the genome organizations. Virology 1992, 190, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Volter, C.; He, Y.; Delius, H.; Roy-Burman, A.; Greenspan, J.S.; Greenspan, D.; de Villiers, E.M. Novel HPV types present in oral papillomatous lesions from patients with HIV infection. Int. J. Cancer 1996, 66, 453–456. [Google Scholar] [CrossRef]
- Zachow, K.R.; Ostrow, R.S.; Faras, A.J. Nucleotide sequence and genome organization of human papillomavirus type 5. Virology 1987, 158, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Yabe, Y.; Sakai, A.; Hitsumoto, T.; Kato, H.; Ogura, H. A subtype of human papillomavirus 5 (HPV5b) and its subgenomic segment amplified in a carcinoma: Nucleotide sequences and genomic organizations. Virology 1991, 183, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.G.; Iftner, T.; Weninger, J.; Pfister, H. Epidermodysplasia verruciformis-associated human papillomavirus 8: Genomic sequence and comparative analysis. J. Virol. 1986, 58, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kremsdorf, D.; Favre, M.; Jablonska, S.; Obalek, S.; Rueda, L.A.; Lutzner, M.A.; Blanchet-Bardon, C.; Van Voorst Vader, P.C.; Orth, G. Molecular cloning and characterization of the genomes of nine newly recognized human papillomavirus types associated with epidermodysplasia verruciformis. J. Virol. 1984, 52, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Favre, M.; Jablonska, S.; Obalek, S.; Orth, G. Characterization of a new type of human papillomavirus (HPV) related to HPV5 from a case of actinic keratosis. Virology 1986, 154, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, T.; Adachi, A.; Ishibashi, M. Genome organization and taxonomic position of human papillomavirus type 47 inferred from its DNA sequence. Virology 1990, 177, 401–405. [Google Scholar] [CrossRef]
- Vasiljevic, N.; Hazard, K.; Eliasson, L.; Ly, H.; Hunziker, A.; de Villiers, E.M.; Norrild, B.; Dillner, J.; Forslund, O. Characterization of two novel cutaneous human papillomaviruses, HPV93 and HPV96. J. Gen. Virol. 2007, 88, 1479–1483. [Google Scholar] [CrossRef]
- de Villiers, E.M.; Gunst, K. Characterization of seven novel human papillomavirus types isolated from cutaneous tissue, but also present in mucosal lesions. J. Gen. Virol. 2009, 90, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef]
- Bottalico, D.; Chen, Z.; Dunne, A.; Ostoloza, J.; McKinney, S.; Sun, C.; Schlecht, N.F.; Fatahzadeh, M.; Herrero, R.; Schiffman, M.; et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2011, 204, 787–792. [Google Scholar] [CrossRef]
- Hopfl, R.; Bens, G.; Wieland, U.; Petter, A.; Zelger, B.; Fritsch, P.; Pfister, H. Human papillomavirus DNA in non-melanoma skin cancers of a renal transplant recipient: Detection of a new sequence related to epidermodysplasia verruciformis associated types. J. Investig. Dermatol. 1997, 108, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Scheurlen, W.; Gissmann, L.; Gross, G.; zur Hausen, H. Molecular cloning of two new HPV types (HPV 37 and HPV 38) from a keratoacanthoma and a alignant melanoma. Int. J. Cancer 1986, 37, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Santos, L.; Neves, F. Characterization of a new genotype of Betapapillomavirus HPV 17 through L1, E7, E7 and LCR sequences. Acta Virol. 2018, 62, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, N.; Hazard, K.; Dillner, J.; Forslund, O. Four novel human betapapillomaviruses of species 2 preferentially found in actinic keratosis. J. Gen. Virol. 2008, 89, 2467–2474. [Google Scholar] [CrossRef]
- Bottalico, D.; Chen, Z.; Kocjan, B.J.; Seme, K.; Poljak, M.; Burk, R.D. Characterization of human papillomavirus type 120: A novel betapapillomavirus with tropism for multiple anatomical niches. J. Gen. Virol. 2012, 93, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, B.J.; Poljak, M.; Seme, K.; Potocnik, M.; Fujs, K.; Babic, D.Z. Distribution of human papillomavirus genotypes in plucked eyebrow hairs from Slovenian males with genital warts Infect. Genet. Evol. 2005, 5, 255–259. [Google Scholar] [CrossRef]
- Kovanda, A.; Kocjan, B.J.; Luzar, B.; Bravo, I.G.; Poljak, M. Characterization of novel cutaneous human papillomavirus genotypes HPV150 and HPV151. PLoS ONE 2011, 6, e22529. [Google Scholar] [CrossRef]
- Kocjan, B.J.; Hosnjak, L.; Seme, K.; Poljak, M. Complete genome sequence of a novel human Betapapillomavirus, HPV159. Genome Announc. 2013, 1, e00298-13. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, B.J.; Steyer, A.; Sagadin, M.; Hosnjak, L.; Poljak, M. Novel human papillomavirus type 174 from a cutaneous squamous cell carcinoma. Genome Announc. 2013, 1, e00445-13. [Google Scholar] [CrossRef]
- Marković, I.; Hosnjak, L.; Seme, K.; Poljak, M. Molecular characterization of human papillomavirus type 159 (HPV159). Viruses 2021, 13, 1668. [Google Scholar] [CrossRef]
- Dutta, S.; Robitaille, A.; Rollison, D.E.; Tommasino, M.; Gheit, T. Complete genome sequence of a novel human Betapapillomavirus isolated from a skin sample. Genome Announc. 2017, 5, e01642-16. [Google Scholar] [CrossRef] [PubMed]
- Bolatti, E.M.; Chouhy, D.; Hošnjak, L.; Casal, P.E.; Kocjan, B.J.; Bottai, H.; Stella, E.J.; Sanchez, A.; Bussy, R.F.; Poljak, M.; et al. Natural history of human papillomavirus infection of sun-exposed healthy skin of immunocompetent individuals over three climatic seasons and identification of HPV209, a novel betapapillomavirus. J. Gen. Virol. 2017, 98, 1334–1348, Erratum in: J. Gen. Virol. 2017, 98, 2205–2206. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, R.N.; Robitaille, A.; Dutta, S.; Rollison, D.E.; Tommasino, M.; Gheit, T. Isolation of a novel beta-2 human papillomavirus from skin. Microbiol. Resour. Announc. 2019, 8, e01628-18. [Google Scholar] [CrossRef] [PubMed]
- Chouhy, D.; Gorosito, M.; Sanchez, A.; Serra, E.C.; Bergero, A.; Fernandez Bussy, R.; Giri, A.A. New generic primer system targeting mucosal/genital and cutaneous human papillomaviruses leads to the characterization of HPV 115, a novel Betapapillomavirus species 3. Virology 2010, 397, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Antonsson, A.; Higgins, G.; Ly, H.; Delius, H.; Hunziker, A.; de Villiers, E.M. Nucleotide sequence and phylogenetic classification of candidate human papilloma virus type 92. Virology 2003, 312, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Gheit, T.; Feng, Q.; Stern, J.E.; Lin, J.; Cherne, S.; Tommasino, M. Prevalence and correlates of β- and γ-human papillomavirus detection in oral samples from mid-adult women. J. Infect. Dis. 2019, 219, 1067–1075. [Google Scholar] [CrossRef]
- Egawa, K.; Delius, H.; Matsukura, T.; Kawashima, M.; de Villiers, E.M. Two novel types of human papillomavirus, HPV 63 and HPV 65:comparisons of their clinical and histological features and DNA sequences to other HPV types. Virology 1993, 194, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Dang, J.; Bzhalava, D.; Stern, J.; Edelstein, Z.R.; Koutsky, L.A.; Kiviat, N.B.; Feng, Q. Characterization of three novel human papillomavirus types isolated from oral rinse samples of healthy individuals. J. Clin. Virol. 2014, 59, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bolatti, E.M.; Chouhy, D.; Casal, P.E.; Perez, G.R.; Stella, E.J.; Sanchez, A.; Gorosito, M.; Bussy, R.F.; Giri, A.A. Characterization of novel human papillomavirus types 157, 158 and 205 from healthy skin and recombination analysis in genus gamma-Papillomavirus. Infect. Genet. Evol. 2016, 42, 20–29. [Google Scholar] [CrossRef]
- Muller, M.; Kelly, G.; Fiedler, M.; Gissmann, L. Human papillomavirus type 48. J. Virol. 1989, 63, 4907–4908. [Google Scholar] [CrossRef]
- Arroyo Muhr, L.S.; Bzhalava, D.; Lagheden, C.; Eklund, C.; Johansson, H.; Forslund, O.; Dillner, J.; Hultin, E. Does human papillomavirus-negative condylomata exist? Virology 2015, 485, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Favre, M.; Obalek, S.; Jablonska, S.; Orth, G. Human papillomavirus (HPV) type 50, a type associated with epidermodysplasia verruciformis (EV) and only weakly related to other EV-specific HPVs. J. Virol. 1989, 63, 4910. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, T.; Iwasaki, T.; Kawashima, M. Molecular cloning of a novel human papillomavirus (type 60) from a plantar cyst with characteristic pathological changes. Virology 1992, 190, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Kullander, J.; Handisurya, A.; Forslund, O.; Geusau, A.; Kirnbauer, R.; Dillner, J. Cutaneous human papillomavirus 88: Remarkable differences in viral load. Int. J. Cancer 2008, 122, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Nobre, R.J.; Herraez-Hernandez, E.; Fei, J.W.; Langbein, L.; Kaden, S.; Grone, H.J.; de Villiers, E.M. E7 oncoprotein of novel human papillomavirus type 108 lacking the E6 gene induces dysplasia in organotypic keratinocyte cultures. J. Virol. 2009, 83, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schiffman, M.; Herrero, R.; Desalle, R.; Burk, R.D. Human papillomavirus (HPV) types 101 and 103 isolated from cervicovaginal cells lack an E6 open reading frame (ORF) and are related to gamma-papillomaviruses. Virology 2007, 360, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Murahwa, A.T.; Meiring, T.L.; Mbulawa, Z.Z.A.; Williamson, A.L. Discovery, characterisation and genomic variation of six novel Gammapapillomavirus types from penile swabs in South Africa. Papillomavirus Res. 2019, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Latsuzbaia, A.; Arbyn, M.; Dutta, S.; Fischer, M.; Gheit, T.; Tapp, J.; Tommasino, M.; Weyers, S.; Mossong, J. Complete genome sequence of a novel human Gammapapillomavirus isolated from a cervical swab in Luxembourg. Genome Announc. 2018, 6, e00114-18. [Google Scholar] [CrossRef] [PubMed]
- Ekström, J.; Bzhalava, D.; Svenback, D.; Forslund, O.; Dillner, J. High throughput sequencing reveals diversity of Human Papillomaviruses in cutaneous lesions. Int. J. Cancer 2011, 129, 2643–2650. [Google Scholar] [CrossRef]
- Kohler, A.; Gottschling, M.; Manning, K.; Lehmann, M.D.; Schulz, E.; Kruger-Corcoran, D.; Stockfleth, E.; Nindl, I. Genomic characterization of ten novel cutaneous human papillomaviruses from keratotic lesions of immunosuppressed patients. J. Gen. Virol. 2011, 92, 1585–1594. [Google Scholar] [CrossRef]
- Li, J.; Cai, H.; Xu, Z.; Wang, Q.; Hang, D.; Shen, N.; Liu, M.; Zhang, C.; Abliz, A.; Ke, Y. Nine complete genome sequences of cutaneous human papillomavirus genotypes isolated from healthy skin of individuals living in rural he nan province, China. J. Virol. 2012, 86, 11936. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Sultana, F.; Roy, R.; Dey, S.; Naskar, S.; Dam, A.; Bhowmick, A.K.; Begum, R.; Mandal, S.S.; Mandal, R.K.; et al. Prevalence of novel gamma HPV types 223 and 225 in oral cavity and skin of Indian normal and neoplastic participants. J. Med. Virol. 2023, 95, e29019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, Y.; Deng, Q.; Cai, H.; Ke, Y. Identification and characterization of eleven novel human gamma-papillomavirus isolates from healthy skin, found at low frequency in a normal population. PLoS ONE 2013, 8, e77116. [Google Scholar] [CrossRef]
- Li, L.; Barry, P.; Yeh, E.; Glaser, C.; Schnurr, D.; Delwart, E. Identification of a novel human gammapapillomavirus species. J. Gen. Virol. 2009, 90, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Bzhalava, D.; Ekstrom, J.; Hultin, E.; Dillner, J.; Forslund, O. Metagenomic sequencing of ‘HPVnegative’ condylomas detects novel putative HPV types. Virology 2013, 440, 1–7. [Google Scholar] [CrossRef]
- Murahwa, A.T.; Meiring, T.L.; Mbulawa, Z.Z.A.; Williamson, A.L. Complete genome sequences of four novel human Gammapapillomavirus types, HPV219, HPV220, HPV221, and HPV222, isolated from penile skin swabs from South African men. Genome Announc. 2018, 6, e00584-18. [Google Scholar] [CrossRef]
- Egawa, N.; Kawai, K.; Egawa, K.; Honda, Y.; Kanekura, T.; Kiyono, T. Molecular cloning and characterization of a novel human papillomavirus, HPV 126, isolated from a flat wart-like lesion with intracytoplasmic inclusion bodies and a peculiar distribution of Ki-67 and p53. Virology 2012, 422, 99–104. [Google Scholar] [CrossRef]
- Ure, A.E.; Forslund, O. Characterization of human papillomavirus type 154 and tissue tropism of Gammapapillomaviruses. PLoS ONE 2014, 9, e89342. [Google Scholar] [CrossRef]
- Schowalter, R.M.; Pastrana, D.V.; Pumphrey, K.A.; Moyer, A.L.; Buck, C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010, 7, 509–515. [Google Scholar] [CrossRef]
- Ostrbenk, A.; Kocjan, B.J.; Hosnjak, L.; Li, J.; Deng, Q.; Sterbenc, A.; Poljak, M. Identification of a novel human papillomavirus, type HPV199, isolated from a nasopharynx and anal canal, and complete genomic characterization of papillomavirus species Gamma-12. PLoS ONE 2015, 10, e0138628. [Google Scholar] [CrossRef]
- Bolatti, E.M.; Hosnjak, L.; Chouhy, D.; Re-Louhau, M.F.; Casal, P.E.; Bottai, H.; Kocjan, B.J.; Stella, E.J.; Gorosito, M.D.; Sanchez, A.; et al. High prevalence of Gammapapillomaviruses (Gamma-PVs) in pre-malignant cutaneous lesions of immunocompetent individuals using a new broad-spectrum primer system, and identification of HPV210, a novel Gamma-PV type. Virology 2018, 525, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Sturegård, E.; Johansson, H.; Ekström, J.; Hansson, B.G.; Johnsson, A.; Gustafsson, E.; Dillner, J.; Forslund, O. Human papillomavirus typing in reporting of condyloma. Sex. Transm. Dis. 2013, 40, 123–129, Erratum in Sex. Transm. Dis. 2013, 40, 760. [Google Scholar] [CrossRef] [PubMed]
- Hosnjak, L.; Kocjan, B.J.; Pirš, B.; Seme, K.; Poljak, M. Characterization of two novel Gammapapillomaviruses, HPV179 and HPV184, isolated from common warts of a renal-transplant recipient. PLoS ONE 2015, 10, e0119154. [Google Scholar] [CrossRef] [PubMed]
- Hosnjak, L.; Kocjan, B.J.; Pirš, B.; Seme, K.; Poljak, M. The genetic diversity of human papillomavirus types from the species Gammapapillomavirus 15: HPV135, HPV146, and HPV179. PLoS ONE 2021, 16, e0249829. [Google Scholar] [CrossRef] [PubMed]
- Chouhy, D.; Bolatti, E.M.; Piccirilli, G.; Sanchez, A.; Fernandez Bussy, R.; Giri, A.A. Identification of human papillomavirus type 156, the prototype of a new human gammapapillomavirus species, by a generic and highly sensitive PCR strategy for long DNA fragments. J. Gen. Virol. 2013, 94, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Forslund, O. Complete genome sequences of three novel human papillomavirus types, 175, 178, and 180. Genome Announc. 2014, 2, e00443-14. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Mühr, L.S.; Hultin, E.; Bzhalava, D.; Eklund, C.; Lagheden, C.; Ekström, J.; Johansson, H.; Forslund, O.; Dillner, J. Human papillomavirus type 197 is commonly present in skin tumors. Int. J. Cancer 2015, 136, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Danos, O.; Katinka, M.; Yaniv, M. Human papillomavirus 1a complete DNA sequence: A novel type of genome organization among papovaviridae. EMBO J. 1982, 1, 231–236. [Google Scholar] [CrossRef]
- Sterbenc, A.; Hosnjak, L.; Chouhy, D.; Bolatti, E.M.; Oštrbenk, A.; Seme, K.; Kocjan, B.J.; Luzar, B.; Giri, A.A.; Poljak, M. Molecular characterization, tissue tropism, and genetic variability of the novel Mupapillomavirus type HPV204 and phylogenetically related types HPV1 and HPV63. PLoS ONE 2017, 12, e0175892. [Google Scholar] [CrossRef]
- Kocjan, B.J.; Šterbenc, A.; Hošnjak, L.; Chouhy, D.; Bolatti, E.; Giri, A.A.; Poljak, M. Genome announcement: Complete genome sequence of a novel Mupapillomavirus, HPV204. Acta Dermatovenerol. Alp. Pannonica Adriat. 2015, 24, 21–23. [Google Scholar] [CrossRef]
- Hirt, L.; Hirsch-Behnam, A.; de Villiers, E.M. Nucleotide sequence of human papillomavirus (HPV) type 41: An unusual HPV type without a typical E2 binding site consensus sequence. Virus Res. 1991, 18, 179–189. [Google Scholar] [CrossRef]
- Buck, C.B.; Cheng, N.; Thompson, C.D.; Lowy, D.R.; Steven, A.C.; Schiller, J.T.; Trus, B.L. Arrangement of L2 within the papillomavirus capsid. J. Virol. 2008, 82, 5190–5197. [Google Scholar] [CrossRef]
- Wang, J.W.; Roden, R.B. L2, the minor capsid protein of papillomavirus. Virology 2013, 445, 175–186. [Google Scholar] [CrossRef]
- Finnen, R.L.; Erickson, K.D.; Chen, X.S.; Garcea, R.L. Interactions between papillomavirus L1 and L2 capsid proteins. J. Virol. 2003, 77, 4818–4826. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, J.; Skwarczynski, M.; Stephenson, R.J.; Toth, I.; Hussein, W.M. Peptide-based nanovaccines in the treatment of cervical cancer: A review of recent advances. Int. J. Nanomed. 2022, 17, 869–900. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Xue, J.; Vesper, B.J.; Radosevich, J.A. Proteins encoded by the human papillomavirus genome and their functions. In HPV and Cancer; Radosevich, J.A., Ed.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Egawa, N.; Shiraz, A.; Crawford, R.; Saunders-Wood, T.; Yarwood, J.; Rogers, M.; Sharma, A.; Eichenbaum, G.; Doorbar, J. Dynamics of papillomavirus in vivo disease formation & susceptibility to high-level disinfection-implications for transmission in clinical settings. EBioMedicine 2021, 63, 103177. [Google Scholar] [CrossRef]
- Meyers, C.; Milici, J.; Robison, R. UVC radiation as an effective disinfectant method to inactivate human papillomaviruses. PLoS ONE 2017, 12, e0187377. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Yu, L.; Majerciak, V.; Zheng, Z.M. HPV16 and HPV18 genome structure, expression, and post-transcriptional regulation. Int. J. Mol. Sci. 2022, 23, 4943. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. S1), 2–23. [Google Scholar] [CrossRef]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV entry into cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Briones-Herrera, A.; Pedraza-Chaverri, J. Regulation of autophagy by high- and low-risk human papillomaviruses. Rev. Med. Virol. 2021, 31, e2169. [Google Scholar] [CrossRef]
- Cruz, L.; Meyers, C. Differential dependence on host cell glycosaminoglycans for infection of epithelial cells by high-risk HPV types. PLoS ONE 2013, 8, e68379. [Google Scholar] [CrossRef]
- Mikuličić, S.; Strunk, J.; Florin, L. HPV16 entry into epithelial cells: Running a gauntlet. Viruses 2021, 13, 2460. [Google Scholar] [CrossRef]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef]
- Graham, S.V. Keratinocyte differentiation-dependent human papillomavirus gene regulation. Viruses 2017, 9, 245. [Google Scholar] [CrossRef]
- Moody, C. Mechanisms by which HPV induces a replication competent environment in differentiating keratinocytes. Viruses 2017, 9, 261. [Google Scholar] [CrossRef]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent human papillomavirus infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, L.; Yang, R.; Xu, T. Advances in molecular mechanism of HPV16 E5 oncoprotein carcinogenesis. Arch. Biochem. Biophys. 2023, 745, 109716. [Google Scholar] [CrossRef]
- Graham, S.V. Human papillomavirus: Gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol. 2010, 5, 1493–1506. [Google Scholar] [CrossRef]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer. 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Fu, L.; Van Doorslaer, K.; Chen, Z.; Ristriani, T.; Masson, M.; Travé, G.; Burk, R.D. Degradation of p53 by human Alphapapillomavirus E6 proteins shows a stronger correlation with phylogeny than oncogenicity. PLoS ONE 2010, 5, e12816. [Google Scholar] [CrossRef]
- Hatterschide, J.; Bohidar, A.E.; Grace, M.; Nulton, T.J.; Kim, H.W.; Windle, B.; Morgan, I.M.; Munger, K.; White, E.A. PTPN14 degradation by high-risk human papillomavirus E7 limits keratinocyte differentiation and contributes to HPV-mediated oncogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 7033–7042. [Google Scholar] [CrossRef]
- Ibeanu, O.A. Molecular pathogenesis of cervical cancer. Cancer Biol. Ther. 2011, 11, 295–306. [Google Scholar] [CrossRef]
- Katzenellenbogen, R. Telomerase induction in HPV infection and oncogenesis. Viruses 2017, 9, 180. [Google Scholar] [CrossRef]
- Ganti, K.; Broniarczyk, J.; Manoubi, W.; Massimi, P.; Mittal, S.; Pim, D.; Szalmas, A.; Thatte, J.; Thomas, M.; Tomaić, V.; et al. The human papillomavirus E6 PDZ binding motif: From life cycle to malignancy. Viruses 2015, 7, 3530–3551. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer. 2018, 42, 161–174. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and cutaneous human papillomavirus Infections and cancer biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 drive alternative carcinogenic pathways in HPV positive cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017, 231, 21–33. [Google Scholar] [CrossRef]
- Beachler, D.C.; Jenkins, G.; Safaeian, M.; Kreimer, A.R.; Wentzensen, N. Natural acquired immunity against subsequent genital human papillomavirus infection: A systematic review and meta-analysis. J. Infect. Dis. 2016, 213, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- Tang, J.; Li, M.; Zhao, C.; Shen, D.; Liu, L.; Zhang, X.; Wei, L. Therapeutic DNA vaccines against HPV-related malignancies: Promising leads from clinical trials. Viruses 2022, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.R.; Gelmini, G.F.; Roxo, V.S.; de Carvalho, N.S.; Bicalho, M.D.G. Is there a role played by HLA-E, if any, in HPV immune evasion? Scand. J. Immunol. 2020, 91, 12850. [Google Scholar] [CrossRef]
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV vaccines. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- Kamolratanakul, S.; Pitisuttithum, P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef]
- Rochefort, J.; Karagiannidis, I.; Baillou, C.; Belin, L.; Guillot-Delost, M.; Macedo, R.; Le Moignic, A.; Mateo, V.; Soussan, P.; Brocheriou, I.; et al. Defining biomarkers in oral cancer according to smoking and drinking status. Front. Oncol. 2023, 12, 1068979. [Google Scholar] [CrossRef] [PubMed]
- Westrich, J.A.; Vermeer, D.W.; Colbert, P.L.; Spanos, W.C.; Pyeon, D. The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol. Carcinog. 2020, 59, 794–806. [Google Scholar] [CrossRef]
- Winer, R.L.; Kiviat, N.B.; Hughes, J.P.; Adam, D.E.; Lee, S.K.; Kuypers, J.M.; Koutsky, L.A. Development and duration of human papillomavirus lesions, after initial infection. J. Infect. Dis. 2005, 191, 731–738. [Google Scholar] [CrossRef]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral infections, the microbiome, and probiotics. Front. Cell Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef]
- Bukowska, E.; Mlynarczyk-Bonikowska, B.; Malejczyk, M.; Przedpełska, G.; Walter De Walthoffen, S.; Mlynarczyk, G.; Majewski, S. Human papillomavirus (HPV) coinfection with other sexually transmitted infections in patients of the Department of Dermatology and Venereology at the Medical University of Warsaw. Dermatol. Rev. 2020, 107, 138–147. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG 2020, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, B.; Malejczyk, M.; Muszyński, J.; Majewski, S. The occurrence of human papillomavirus--HPV in the biopsies from colon polyps and cancer. Med. Dosw. Mikrobiol. 2009, 61, 191–196. [Google Scholar]
- Bristow, I. Paediatric cutaneous warts and verrucae: An update. Int. J. Environ. Res. Public Health 2022, 19, 16400. [Google Scholar] [CrossRef]
- Skubic, L.; Breznik, V.; Poljak, M. Different skin wart types, different human papillomavirus types? A narrative review. Acta Dermatovenerol. Alp. Pannonica Adriat. 2023, 32, 165–171. [Google Scholar] [CrossRef]
- Yuan, H.; Li, R.; Lv, J.; Yi, G.; Sun, X.; Zhao, N.; Zhao, F.; Xu, A.; Kou, Z.; Wen, H. Epidemiology of human papillomavirus on condyloma acuminatum in Shandong Province, China. Hum. Vaccines Immunother. 2023, 19, 2170662. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Li, X.; Yu, M.; Chen, Q. Multifocal epithelial hyperplasia confined to the interdental papilla of an adult Chinese man: A rare case report and literature review. BMC Oral Health 2023, 23, 699. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Corchado-Cobos, R.; García-Sancha, N.; Revelles, L.; Revilla, D.; Ugalde, T.; Román-Curto, C.; Pérez-Losada, J.; Cañueto, J. Viruses and skin cancer. Int. J. Mol. Sci. 2021, 22, 5399. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Yamaguchi, R.; Kuriyama, Y. Recent advances in cutaneous HPV infection. J. Dermatol. 2023, 50, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Malagón, T.; Drolet, M.; Boily, M.C.; Franco, E.L.; Jit, M.; Brisson, J.; Brisson, M. Cross-protective efficacy of two human papillomavirus vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Schiller, J.T. Human papillomavirus vaccines. J. Infect. Dis. 2021, 224 (Suppl. S2), S367–S378. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, R.A. Addressing HPV vaccine myths: Practical information for healthcare providers. Hum. Vaccines Immunother. 2019, 15, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Stefanos, R.; Lewis, R.M.; Querec, T.D.; Gargano, J.W.; Unger, E.R.; Markowitz, L.E. High impact of quadrivalent human papillomavirus vaccine across racial/ethnic groups: National Health and Nutrition Examination Survey, 2003-2006 and 2015-2018. Hum. Vaccines Immunother. 2024, 20, 2308378. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.L. Recent developments in human papillomavirus (HPV) vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, C.; Zhao, Y.; Li, J.; Wei, L. Immunogenicity, efficacy, and safety of human papillomavirus vaccine: Data from China. Front. Immunol. 2023, 14, 1112750. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and therapeutic HPV vaccines: Current scenario and perspectives. Front. Cell Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef]
- Huber, B.; Wang, J.W.; Roden, R.B.S.; Kirnbauer, R. RG1-VLP and other L2-based, broad-spectrum HPV vaccine candidates. J. Clin. Med. 2021, 10, 1044. [Google Scholar] [CrossRef]
- Goldstone, S.E. Human papillomavirus (HPV) vaccines in adults: Learnings from long-term follow-up of quadrivalent HPV vaccine clinical trials. Hum. Vaccines Immunother. 2023, 19, 2184760. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, B. Can prophylactic HPV vaccination reduce the recurrence of cervical lesions after surgery? review and prospect. Infect. Agent Cancer 2023, 18, 66. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, J.M.; Morrow, M.P. Vaccines for HPV-associated diseases. Mol. Asp. Med. 2023, 94, 101224. [Google Scholar] [CrossRef]
- Yan, F.; Cowell, L.G.; Tomkies, A.; Day, A.T. Therapeutic vaccination for HPV-mediated cancers. Curr. Otorhinolaryngol. Rep. 2023, 11, 44–61. [Google Scholar] [CrossRef]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef]
- Rosales, R.; Rosales, C. Immune therapy for human papillomaviruses-related cancers. World J. Clin. Oncol. 2014, 5, 1002–1019. [Google Scholar] [CrossRef]
- Cabo Beltran, O.R.; Rosales Ledezma, R. MVA E2 therapeutic vaccine for marked reduction in likelihood of recurrence of respiratory papillomatosis. Head Neck 2019, 41, 657–665. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hur, S.Y.; Kim, T.J.; Hong, S.R.; Lee, J.K.; Cho, C.H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A phase II, prospective, randomized, multicenter, open-label study of GX-188E, an HPV DNA vaccine, in patients with cervical intraepithelial neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Roden, R.B.S.; Ferrall, L.; Akin, M.; Blomer, A.; Wu, T.C.; Chang, Y.N. Safety run-in of intramuscular pNGVL4a-Sig/E7(detox)/HSP70 DNA and TA-CIN protein vaccination as treatment for HPV16+ ASC-US, ASC-H, or LSIL/CIN1. Cancer Prev. Res. 2023, 16, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Adachi, K.; Tomio, K.; Eguchi-Kojima, S.; Tsuruga, T.; Uchino-Mori, M.; Taguchi, A.; Komatsu, A.; Nagamatsu, T.; Oda, K.; et al. A placebo-controlled, double-blind randomized (phase IIB) trial of oral administration with HPV16 E7-expressing Lactobacillus, GLBL101c, for the treatment of cervical intraepithelial neoplasia grade 2 (CIN2). Vaccines 2021, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Kobayashi, O.; Ikeda, Y.; Yahata, H.; Iwata, T.; Satoh, T.; Akiyama, A.; Maeda, D.; Hori-Hirose, Y.; Uemura, Y.; et al. Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3. JNCI Cancer Spectr. 2023, 7, pkad101. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Ouh, Y.T.; Sung, M.H.; Park, H.G.; Kim, T.J.; Cho, C.H.; Park, J.S.; Lee, J.K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef]
- Speetjens, F.M.; Welters, M.J.P.; Slingerland, M.; van Poelgeest, M.I.E.; de Vos van Steenwijk, P.J.; Roozen, I.; Boekestijn, S.; Loof, N.M.; Zom, G.G.; Valentijn, A.R.P.M.; et al. Intradermal vaccination of HPV-16 E6 synthetic peptides conjugated to an optimized Toll-like receptor 2 ligand shows safety and potent T cell immunogenicity in patients with HPV-16 positive (pre-) malignant lesions. J. Immunother. Cancer. 2022, 10, e005016. [Google Scholar] [CrossRef] [PubMed]
- Kenter, G.G.; Welters, M.J.; Valentijn, A.R.; Lowik, M.J.; Berends-van der Meer, D.M.; Vloon, A.P.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- Akhatova, A.; Chan, C.K.; Azizan, A.; Aimagambetova, G. The efficacy of therapeutic DNA vaccines expressing the human papillomavirus E6 and E7 oncoproteins for treatment of cervical cancer: Systematic review. Vaccines 2021, 10, 53. [Google Scholar] [CrossRef]
- Aggarwal, C.; Saba, N.F.; Algazi, A.; Sukari, A.; Seiwert, T.Y.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Esser, M.T.; et al. Safety and efficacy of MEDI0457 plus Durvalumab in patients with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma. Clin. Cancer Res. 2023, 29, 560–570. [Google Scholar] [CrossRef]
- Morris, V.K.; Jazaeri, A.; Westin, S.N.; Pettaway, C.; George, S.; Huey, R.W.; Grinsfelder, M.; Shafer, A.; Johnson, B.; Vining, D.; et al. Phase II trial of MEDI0457 and Durvalumab for patients with recurrent/metastatic human papillomavirus-associated cancers. Oncologist 2023, 28, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Carmona, T.; Arango-Bravo, E.; Serrano-Olvera, J.A.; Flores-de La Torre, C.; Cruz-Esquivel, I.; Villalobos-Valencia, R.; Morán-Mendoza, A.; Castro-Eguiluz, D.; Cetina-Pérez, L. ADXS11-001 LM-LLO as specific immunotherapy in cervical cancer. Hum. Vaccines Immunother. 2021, 17, 2617–2625. [Google Scholar] [CrossRef]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Ekhlassi, E.; Downing, C.; Guidry, J.; Lee, M.; Tyring, S.K. Advancements in pharmacotherapy for noncancerous manifestations of HPV. J. Clin. Med. 2015, 4, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; van der Burg, S.H.; Hampson, I.N.; Broker, T.R.; Fiander, A.; Lacey, C.J.; Kitchener, H.C.; Einstein, M.H. Therapy of human papillomavirus-related disease. Vaccine 2012, 30 (Suppl. S5), F71–F82. [Google Scholar] [CrossRef] [PubMed]
- García-Oreja, S.; Álvaro-Afonso, F.J.; García-Álvarez, Y.; García-Morales, E.; Sanz-Corbalán, I.; Lázaro Martínez, J.L. Topical treatment for plantar warts: A systematic review. Dermatol. Ther. 2021, 34, e14621. [Google Scholar] [CrossRef] [PubMed]
- Majewski, S.; Marczak, M.; Mlynarczyk, B.; Benninghoff, B.; Jablonska, S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int. J. Dermatol. 2005, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ni, G.; Wang, T.; Mounsey, K.; Cavezza, S.; Pan, X.; Liu, X. Genital warts treatment: Beyond imiquimod. Hum. Vaccines Immunother. 2018, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Herzum, A.; Ciccarese, G.; Occella, C.; Gariazzo, L.; Pastorino, C.; Trave, I.; Viglizzo, G. Treatment of pediatric anogenital warts in the era of HPV-vaccine: A literature review. J. Clin. Med. 2023, 12, 4230. [Google Scholar] [CrossRef]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a standardized green tea catechin preparation for viral warts and human papilloma virus-related and unrelated cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef]
- Lau, W.C.; Lau, C.B.; Frangos, J.E.; Nambudiri, V.E. Intralesional cidofovir for the management of refractory cutaneous verrucae: A review of applications and opportunities. Ther. Adv. Infect. Dis. 2023, 10, 20499361231165862. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, R.M.; Ashry, W.M.O.; Elsaie, M.L. Comparative study between intralesional injection of MMR, BCG, and candida albicans antigen in treatment of multiple recalcitrant warts. J. Cosmet. Dermatol. 2022, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Elsayed Ghaly, N.; El-Ashmawy, A.A.; Abou Zeid, M.; E Shaker, E.S. Efficacy and safety of intralesional injection of vitamin D3 versus tuberculin PPD in the treatment of plantar warts: A comparative controlled study. J. Cosmet. Dermatol. 2021, 20, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, J.; Hwang, S.S.; Yam, W.K.; Rahman, T.; Wezen, X.C. In-silico discovery of inhibitors against human papillomavirus E1 protein. J. Biomol. Struct. Dyn. 2023, 41, 5583–5596. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675. [Google Scholar] [CrossRef] [PubMed]
- Toots, M.; Ustav, M., Jr.; Männik, A.; Mumm, K.; Tämm, K.; Tamm, T.; Ustav, E.; Ustav, M. Identification of several high-risk HPV inhibitors and drug targets with a novel high-throughput screening assay. PLoS Pathog. 2017, 13, e1006168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Egawa, N.; Shiraz, A.; Katakuse, M.; Okamura, M.; Griffin, H.M.; Doorbar, J. The reservoir of persistent human papillomavirus infection; strategies for elimination using anti-viral therapies. Viruses 2022, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, L.F.; Wasson, C.W.; Swinscoe, G.; Kealy, D.; Foster, R.; Griffin, S.; Macdonald, A. Alkyl-imino sugars inhibit the pro-oncogenic ion channel function of human papillomavirus (HPV) E5. Antivir. Res. 2018, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bertagnin, C.; Messa, L.; Pavan, M.; Celegato, M.; Sturlese, M.; Mercorelli, B.; Moro, S.; Loregian, A. A small molecule targeting the interaction between human papillomavirus E7 oncoprotein and cellular phosphatase PTPN14 exerts antitumoral activity in cervical cancer cells. Cancer Lett. 2023, 571, 216331. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Y.; Wu, C.; Si, L.; Wang, Q.; Sui, L.; Jiang, S. The underlying mechanism of 3-hydroxyphthalic anhydride-modified bovine beta-lactoglobulin to block human papillomavirus entry into the host cell. Front. Microbiol. 2019, 10, 2188. [Google Scholar] [CrossRef]
- Valencia-Reséndiz, D.G.; Villegas, A.; Bahena, D.; Palomino, K.; Cornejo-Bravo, J.M.; Quintanar, L.; Palomino-Vizcaino, G.; Alvarez-Salas, L.M. Non-functionalized gold nanoparticles inhibit human papillomavirus (HPV) infection. Int. J. Mol. Sci. 2022, 23, 7552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Moreno, R.; Lambert, P.F.; DiMaio, D. Cell-penetrating peptide inhibits retromer-mediated human papillomavirus trafficking during virus entry. Proc. Natl. Acad. Sci. USA 2020, 117, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Zine El Abidine, A.; Gómez-Martinez, R.A.; Bondu, V.; Sterk, R.T.; Surviladze, Z.; Ozbun, M.A. Protamine sulfate is a potent inhibitor of human papillomavirus infection in vitro and in vivo. Antimicrob. Agents Chemother. 2022, 66, e0151321. [Google Scholar] [CrossRef] [PubMed]
- Khamjan, N.A.; Beigh, S.; Algaissi, A.; Megha, K.; Lohani, M.; Darraj, M.; Kamli, N.; Madkhali, F.; Dar, S.A. Natural and synthetic drugs and formulations for intravaginal HPV clearance. J. Infect. Public Health 2023, 16, 1471–1480. [Google Scholar] [CrossRef]
- Nadile, M.; Retsidou, M.I.; Gioti, K.; Beloukas, A.; Tsiani, E. Resveratrol against cervical cancer: Evidence from in vitro and in vivo studies. Nutrients 2022, 14, 5273. [Google Scholar] [CrossRef]
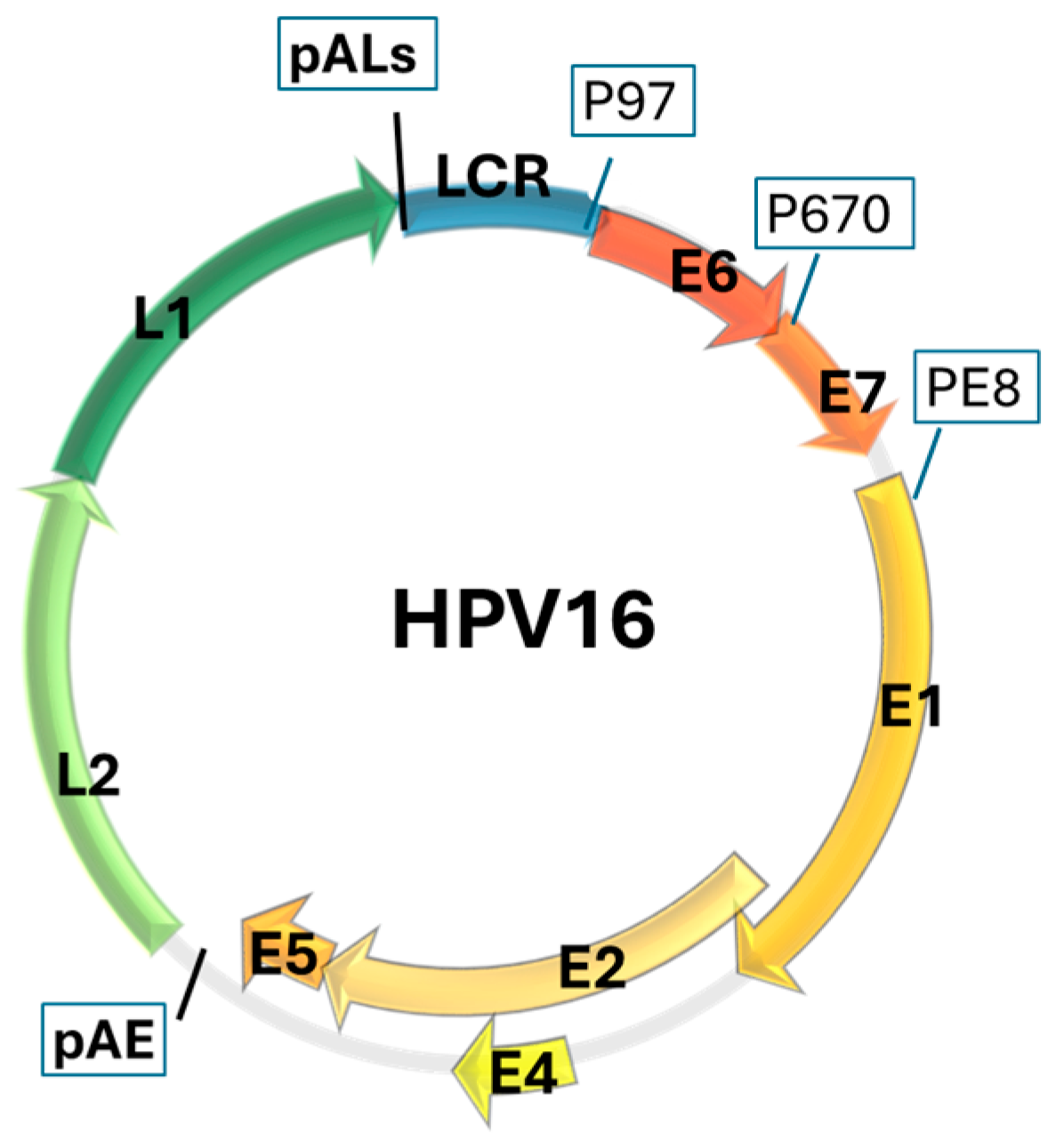
| Viruses (Superkingdom); Monodnaviria (Clade); Shotokuvirae (Kingdom); Cossaviricota (Phylum); Papovaviricetes (Class); Zurhausenvirales (Order); Papillomaviridae (Family); Firstpapillomavirinae (Subfamily) | |
|---|---|
| genus | Alphapapillomavirus |
| species | Alphapapillomavirus 1 [17,18,19] |
| type | HPV32, 42 |
| species | Alphapapillomavirus 2 [18,20,21,22,23,24] |
| type | HPV3, 10, 28, 29, 77, 78, 94, 117, 125, 160; the TB [13] database also includes HPVXS2 |
| species | Alphapapillomavirus 3 [17,25,26,27,28,29,30,31,32,33] |
| type | HPV61 sublineage A1, A2, lineage B, C, HPV62, 72, 81, 83, 84, 86, 87, 89, 102, 114 |
| species | Alphapapillomavirus 4 [18,34,35,36] |
| type | HPV2, lineage 2a and 2c; HPV27, lineage b; HPV57, lineage b and c |
| species | Alphapapillomavirus 5 [18,26,37,38,39] |
| type | HPV26 lineage A, HPV51 sublineage A1 to A4, B1, B2, HPV69 sublineage A1 to A4, HPV82 sublineage A1 to A3, B1, B2, C1 to C5 |
| species | Alphapapillomavirus 6 [18,26,40,41] |
| type | HPV30: sublineage A1 to A3 and lineage B, HPV53: lineage A, B, C, and sublineage D1 to D4 |
| HPV56: sublineage A1, A2, and lineage B; HPV66: lineage A and sublineage B1, B2 | |
| species | Alphapapillomavirus 7 [18,26,39,42,43,44,45,46,47,48,49,50,51,52] |
| type | HPV18 sublineage A1 and A2 (Asian-Amerindian), A3 to A5 (European), B1 to B3 (African), and lineage C (African) |
| HPV39 sublineage A1, A2, and lineage B; HPV45 sublineage A1 to A3, B1, B2 | |
| HPV59 sublineage A1 to A3, B, and lineage B | |
| HPV68 lineage a, sublineage A1, A2, lineage B, and b, sublineage C1, C2, sublineage D1, D2, lineage E, and sublineage F1, F2 | |
| HPV70 lineage A and B; HPV85 lineage A; HPV97 lineage A | |
| species | Alphapapillomavirus 8 [17,18,29] |
| type | HPV7; HPV40; HPV43; HPV91 |
| species | Alphapapillomavirus 9 [18,26,43,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] |
| type | HPV16 sublineage A1 to A3 (European), A4 (Asian), B1 (African-1, Afr1a), B2 African-1, Afr1b), B3 and B4, C1 (African-2, Afr2a) AF472509, C2, C3, C4, D1 (North American, NA1), D2 (Asian-American, AA2), D3 (Asian-American AA1), and D4 |
| HPV31: sublineage A1, A2, B1, B2, C1 to C4; HPV33: sublineage A1 to A3, B1, C1 | |
| HPV35: sublineage A1, A2; HPV52: A1, A2, B1, B2, B3, C1, C2, D1, E1 | |
| HPV58: sublineage A1 to A3, B1, B2, C1, D1, D2; HPV67: sublineage A1, A2, B1 | |
| species | Alphapapillomavirus 10 [17,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| type | HPV6: lineage A, 6a (classified as sublineage B3), 6b (classified as lineage A, 6c, 6e, 6vc (classified as sublineage B1), and sublineage B1, B2, B3, B4, B5 |
| HPV11: sublineage A1, A2, A3 (isolate C185), A4 (isolate LT4), and lineage B; HPV13; HPV44; HPV74 | |
| The TB database [13] also includes HPV55 | |
| species | Alphapapillomavirus 11 [18,26,86] |
| type | HPV34 sublineage A1, A2, lineage B, and sublineage C1, C2 |
| HPV73 sublineage A1, A2, and lineage B | |
| In addition, the TB database [13] includes HPV177 | |
| species | Alphapapillomavirus 13 [26,39] |
| type | HPV54 lineage A, B, C |
| species | Alphapapillomavirus 14 [29,30,39] |
| type | HPV71; HPV90; HPV106 |
| genus | Betapapillomavirus |
| species | Betapapillomavirus 1 [18,87,88,89,90,91,92,93,94,95,96,97] |
| type | HPV5: lineage b; HPV8, 12; HPV14: lineage D; HPV19, 20, 21, 24, 25, 36, 47, 93, 98, 99, 105, 118, 124, 143, 152 |
| In addition, the [13] database includes HPVRTRX7, HPVV001/Slovenia/2010 | |
| In addition, the [14] database includes HPV195, 196, 206, which in ICTV [12] appear as unclassified Betapapillomavirus HPVmRTRX7 | |
| species | Betapapillomavirus 2 [18,20,90,98,99,100,101,102,103,104,105,106,107,108,109] |
| type | HPV9, 15, 17, 22, 23, 37; HPV38: lineage b; HPV80, 100, 104, 107, 110, 111, 113, 120, 122, 145, 151, 174 |
| In addition, the [14] database includes HPV182, 198, 209, 227 | |
| In addition, the [13] database includes HPVFA75/KI88-03 | |
| species | Betapapillomavirus 3 [18,110] |
| type | HPV49, 75, 76, 115 |
| species | Betapapillomavirus 4 [111] |
| type | HPV92 |
| Betapapillomavirus 5 [93,102,112] | |
| HPV96, 150 | |
| In addition, the [13,14] databases include HPV185 | |
| genus | Gammapapillomavirus |
| species | Gammapapillomavirus 1 [113,114,115] |
| type | HPV4, 65, 95,173, 205 |
| species | Gammapapillomavirus 2 [116,117] |
| type | HPV48 {NC_001690}; HPV200 |
| species | Gammapapillomavirus 3 [118] |
| type | HPV50 |
| In addition, the [13,14] databases include HPV188 | |
| species | Gammapapillomavirus4 |
| type | HPV60 [119] |
| species | Gammapapillomavirus5 |
| type | HPV88 [120] |
| species | Gammapapillomavirus 6 [121,122,123,124] |
| type | HPV101, 103, 108 |
| In addition, the [14] database includes HPV214, 226 | |
| species | Gammapapillomavirus 7 [33,95,96,125,126,127,128] |
| type | HPV109, 123, 134,138,139,149, 155, 170 |
| In addition, the [13,14] databases include HPV186, 189, 193 | |
| Furthermore, the [14] database includes HPV203, 225, 229 | |
| species | Gammapapillomavirus 8 [33,95,123,127,129] |
| type | HPV112, 119, 147,164, 168 |
| Furthermore, the [13,14] databases include HPV176 | |
| In addition, the [14] database includes HPV211, 224, which in ICTV [12] appear as unclassified Betapapillomavirus HPVmICB1. | |
| species | Gammapapillomavirus 9 [123,126,130] |
| type | HPV116, 129 |
| In addition, the [14] database includes HPV 215, 216 | |
| species | Gammapapillomavirus 10 [95,126,131,132] |
| type | HPV121,130, 133, 142, 180 |
| In addition, the [13,14] databases include HPV191 | |
| Furthermore, the [14] database includes HPV221, 231 | |
| species | Gammapapillomavirus 11 [96,114,117,127,133,134] |
| type | HPV126, 136, 140, 141, 154, 169, 171, 202 |
| In addition, the [13,14] databases include HPV181 | |
| Furthermore, the [13] database includes HPV230 | |
| species | Gammapapillomavirus 12 [115,126,127,135,136,137] |
| type | HPV127, 132, 148, 157, 158, 165, 199 |
| In addition, the [13,14] databases include HPV210 | |
| species | Gammapapillomavirus 13 [123,126,132,138] |
| type | HPV128, 153 |
| In addition, the [14] database includes HPV213, 219 | |
| species | Gammapapillomavirus 14 [126] |
| type | HPV131 |
| species | Gammapapillomavirus 15 [96,114,139,140] |
| type | HPV135, 146, 179 |
| In addition, the [13,14] databases include HPV192 a | |
| In addition, the [14] database includes HPV230 | |
| species | Gammapapillomavirus 16 [96] |
| type | HPV137 |
| species | Gammapapillomavirus 17 [96,123,132] |
| type | HPV144 |
| In addition, the [14] database includes HPV212, 220 | |
| species | Gammapapillomavirus 18 [141] |
| type | HPV156 |
| species | Gammapapillomavirus 19 [127,132] |
| type | HPV161, 162, 166 |
| In addition, the [14] database includes HPV222 | |
| species | Gammapapillomavirus 20 [127] |
| type | HPV163 |
| In addition, the [13,14] databases include HPV 183 a | |
| Furthermore, the [14] database includes HPV194 | |
| species | Gammapapillomavirus 21 [129] |
| type | HPV167 |
| species | Gammapapillomavirus 22 [114] |
| type | HPV172 |
| In addition, the [14] database includes HPV223 | |
| species | Gammapapillomavirus 23 [131] |
| type | HPV175 |
| species | Gammapapillomavirus 24 [142,143] |
| type | HPV178, 197; in addition, the [14] database includes HPV190, 208 |
| species | Gammapapillomavirus 25 [139] |
| type | HPV184 |
| species | Gammapapillomavirus26 |
| type | HPV187 |
| species | Gammapapillomavirus 27 [117] |
| type | HPV201; in addition, the [14] database includes HPV228 |
| genus | Mupapillomavirus |
| species | Mupapillomavirus 1 [144] |
| type | HPV1; in the [13] database, it appears under the name HPV1a |
| species | Mupapillomavirus 2 [113] |
| type | HPV63 |
| species | Mupapillomavirus 3 [145,146] |
| type | HPV204 |
| genus | Nupapillomavirus |
| species | Nupapillomavirus 1 [147] |
| type | HPV41 |
| Type of Skin/Mucosal Lesions | HPV Types | References |
|---|---|---|
| plantar warts | usually HPV2, 27, 57, 63, furthermore HPV 1, 4, 10, 41, 65, 88, 95, 60, 65, 66 | [195,196] |
| common warts | HPV 27, 57,2,1,4 | [159,196] |
| flat warts | HPV3, 10, 26, 27, 28, 29, 77, 78, 94, 114, 41 | [196] |
| genital warts (Condyloma acuminatum) | usually (90%) HPV 6 and 11, less commonly HPV 2, 16, 18, 30–33, 35, 39, 41–45, 51–56, and 59 | [159,197] |
| cervical intraepithelial neoplasia, and cancer | HPV 16 (mostly), HPV18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 (carcinogenic), 68 (probably carcinogenic), 26, 53, 66, 67, 69, 70, 73, and 82 (possibly carcinogenic) | [16] |
| focal epithelial hyperplasia | mainly HPV13 and 32, but infection or co-infection with other HPVs including HPV6, 11, 16, 18, 31, 39, 40, 51, 52, 55, 58, 66, 68, 69, 71, 74, and 90 has also been reported | [198] |
| warts and probably NMSC in EV and immunocompromised patients; in the general population usually asymptomatic but also may be associated with NMSC | HPV5, 8 (possibly carcinogenic) HPV9, 12, 14, 15, 17, 19–25, 36–38, 47, 49, 75, 76, 80, 92, 93, 96, 98–100, 104, 105, 107, 110, 111, 113, 115, 118, 120, 122, 124, 143, 145 | [199] |
| Vaccine (Manufacturer) | HPV Types Included | Adjuvant | Method of Producing HPV Proteins | Registration Year | Vaccine Schedule |
|---|---|---|---|---|---|
| Gardasil® (Merck & Co., Rahway, NJ, USA) | 6 (20 µg), 11 (40 µg), 16 (40 µg), 18 (20 µg) quadrivalent | Amorphous aluminium hydroxyphosphate sulphate (225 µg Al) | Saccharomyces cerevisiae (yeast) expressing L1 | 2006 | I.M. 9–14 years: 0, 6 months from the age of 15 0, 2, 6 months |
| Cervarix® (GlaxoSmithKline, Tsim Sha Tsui, Hong Kong) | 16 (20 µg), 18 (20 µg), bivalent | AS04: 3-O-deacylo-4′-monofosforylolipid A (MPL) 50 μg, adsorbed on aluminium hydroxide (0.5 mg Al3+) | Utilizing a baculovirus expression system, with the use of insect Hi-5 Rix4446 cells derived from Trichoplusia ni. | 2007 | I.M. 9–14 years: 0, 6 months from the age of 15 0, 1, 6 months |
| Gardasil9® (Merck & Co.) | 6 (30 µg), 11 (40 µg), 16 (60 µg), 18 (40 µg), 31 (20 µg), 33 (20 µg), 45 (20 µg), 52 (20 µg), 58 (20 µg) nonavalent | Amorphous aluminium hydroxy phosphate sulphate (0.5 mg Al) | Saccharomyces cerevisiae (yeast) expressing L1 | 2014 | I.M. 9–14 years: 0, 6 months from the age of 15 0, 2, 6 months |
| Cecolin® (Xiamen Innovax Biotechnology, Xiamen, China) | 16 (40 µg), 18 (20 µg) bivalent | Aluminium hydroxide (208 μg Al) | Escherichia coli (bacteria) expressing L1 | 2020 | I.M. 9–14 years: 0, 6 or 0, 1, 6 months from the age of 15 0, 1, 6 months |
| Walvax recombinant HPV vaccine (Hanghai Zerun Biotechnology, Shanghai, China; Subsidiary of Walvax Biotechnology, Shanghai, China) | 16 (20 µg), 18 (40 µg) bivalent | Aluminium phosphate (225 µg Al) | Pichia pastoris expressing L1 (yeast) | 2022 | I.M. 9–14 years: 0, 6 or 0, 2, 6 months from the age of 15 0, 2, 6 months |
| Cervavac® (Serum Institute of India, Pune, India) | 6 (≥20 µg), 11 (≥40 µg), 16 (≥40 µg), 18 (≥20 µg) quadrivalent | Aluminium (Al3+) ≥ 1.25 mg | Hansenula polymorpha expressing L1 (yeast) | 2022 | I. M. 9–14 years: 0, 6 months from the age of 15 0, 2, 6 months |
| Vaccine Name (Manufacturer If Available) | Antigen/Other Vaccine Components | Type of Vaccine | Number of Administrations, Method of Delivery, | Research Stage | Results | References |
|---|---|---|---|---|---|---|
| TG4001 Tipapkinogen Sovacivec | DNA -modified HPV 16 E6 and E7 and human IL-2 in vaccinia virus Ankara (MVA) | DNA, virus-vectored | 3 subcutaneous injections | phase II | CIN 2/3 lesions caused by HR HPV ceased in 24% of the 129 women who received the vaccine and in 10% of the 64 women receiving placebo. The result was statistically significant. The vaccine was well tolerated. | [215] |
| MVA E2 | Cross-reactive E2 (bovine papilloma virus) in vaccinia virus Ankara (MVA) | DNA, virus-vectored | 6 site-specific injections | phase III | In 1051 of 1176 women with CIN (89%) and in all 180 PIN men who received the vaccine, the lesions resolved completely. 81% of the women tested had eliminated oncogenic HPV. | [216] |
| phase II | In 13 (43%) of the 29 patients studied, respiratory papillomatosis resolved completely; in the others, the lesions recurred between 8–12 months after vaccination, but resolved without further recurrence after re-administration. | [217] | ||||
| VGX-3100 (Inovio Pharmaceuticals) | Modified E6 and E7 HPV-16 and -18 in two synthetic plasmids/pVAX | DNA plasmid | 3 IM injections with electroporation | phase II NCT01304524 | Regression of CIN 2/3 lesions confirmed by histopathological examination in 53 (49.5%) of 107 patients receiving the vaccine versus 11(30.6%) of 35 patients receiving placebo. | [218] |
| phase III NCT03721978 | In total, in the REVEAL 1 and 2 studies, cessation of CIN lesions and elimination of the virus was found in 68 of 272 (25.0%) women receiving the vaccine and in 13 of 132 (9.8%) receiving placebo. | [213] | ||||
| GX-188E Tirvalimogene teraplasmid | Modified E6 and E7 HPV-16 and -18 in Plasmid/pGX27 | DNA plasmid | 3 IM injections with electroporation | Phase II | 64 patients with CIN, histopathologically confirmed resolution of lesions in 52% (33/64) of patients after 20 weeks from the first administration of the vaccine and in 67% (35/52) after 36 weeks. HPV was eliminated in 73% and 77% of patients who had resolved lesions after 20 and 36 weeks, respectively. | [219] |
| pBI-11 | pNGVL4a-Sig/E7(detox)/HSP70 plasmid encoding HPV16 L2E7E6 fusion protein | DNA plasmid | 2 doses of pBI-11 + 1 dose of TA-CIN, I.M. | Phase II | HPV eradicated and lesions resolved after 6 months in 5 (45%) and after 12 months in 7 (64%) of 11 HPV16+ vaccinated women with ASC-US, ASC-H, or LSIL/CIN. Patients additionally vaccinated with the protein vaccine TA-CIN (recombinant HPV-16 L2 E6 E7). | [220] |
| GLBL101c | Heat-attenuated recombinant Lactobacillus casei expressing modified HPV16 E7 | live-bacteria-vectored | 4 rounds of oral vaccination, daily for 5 days at weeks 1, 2, 4, and 8 | Phase II | No statistically significant differences in resolution of CIN 2/3 lesions between the 20 patients receiving the vaccine and the 20 patients receiving placebo. However, complete resolution of lesions in 2 patients in the vaccine group and none in the placebo group. | [221] |
| IGMKK16E7 | Lacticaseibacillus paracasei expressing on cell surface, full-length HPV-16 E7 | live-bacteria-vectored | 4 rounds of oral vaccination daily at weeks 1, 2, 4, and 8. | Phase II | CIN 2/3 lesions resolved in 13 (31.7%) of 41 patients receiving the high dose of vaccine and in 5 (12.5%) of 40 patients receiving placebo. For patients infected only with HPV16, the incidence of resolution of lesions was 40.0% (12 of 30) in those receiving the vaccine and 11.5% (3 of 26) in those receiving placebo. | [222] |
| BLS-M07 | Lactobacillus casei expressing on cell surface, full-length HPV-16 E7 | live-bacteria-vectored | 4 rounds of oral vaccination- daily for 5 days at weeks 1, 2, 4, and 8. | Phase I/IIa | Phase I—19 patients with HPV16 and CIN 3 infection—safe, immunogenic; phase IIa—lesions resolved in 6 of 8 patients studied. | [223] |
| HPV-16 E6 synthetic peptides | HPV-16 E6 synthetic peptides conjugated to Amplivant (optimized Toll-like receptor 2 ligand) | peptide | 3 administrations, intradermally | Phase I | 25 patients with HPV-16 positive (pre-)malignant lesions. Safe, immunogenicity increases with increasing dosage, but mild side effects (flu-like symptoms) are more frequent. | [224] |
| ISA 101 | Overlapping long peptides 9 from the E6 HPV 16 protein and 4 from E7 in incoplete | peptide | 3 or 4 administrations subcutaneously at 3-week intervals | Phase II | After 3 months response in 12 of 20 patients and complete regression of lesions in 5 of 20 patients. After 12 months, response in 15 of 19 patients with VIN 2/3 receiving the vaccine, complete regression of lesions in 9 of 15. | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlynarczyk-Bonikowska, B.; Rudnicka, L. HPV Infections—Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024, 25, 7616. https://doi.org/10.3390/ijms25147616
Mlynarczyk-Bonikowska B, Rudnicka L. HPV Infections—Classification, Pathogenesis, and Potential New Therapies. International Journal of Molecular Sciences. 2024; 25(14):7616. https://doi.org/10.3390/ijms25147616
Chicago/Turabian StyleMlynarczyk-Bonikowska, Beata, and Lidia Rudnicka. 2024. "HPV Infections—Classification, Pathogenesis, and Potential New Therapies" International Journal of Molecular Sciences 25, no. 14: 7616. https://doi.org/10.3390/ijms25147616
APA StyleMlynarczyk-Bonikowska, B., & Rudnicka, L. (2024). HPV Infections—Classification, Pathogenesis, and Potential New Therapies. International Journal of Molecular Sciences, 25(14), 7616. https://doi.org/10.3390/ijms25147616






